Aspergillus fumigatus is an opportunistic pathogen that causes 90% of invasive aspergillosis (IA) due to Aspergillus genus, with a 50–95% mortality rate. It has been postulated that certain virulence factors are characteristic of A. fumigatus, but the “non-classical” virulence factors seem to be highly variable. Overall, published studies have demonstrated that the virulence of this fungus is multifactorial, associated with its structure, its capacity for growth and adaptation to stress conditions, its mechanisms for evading the immune system and its ability to cause damage to the host. In this review we intend to give a general overview of the genes and molecules involved in the development of IA. The thermotolerance section focuses on five genes related with the capacity of the fungus to grow at temperatures above 30°C (thtA, cgrA, afpmt1, kre2/afmnt1, and hsp1/asp f 12). The following sections discuss molecules and genes related to interaction with the host and with the immune responses. These sections include β-glucan, α-glucan, chitin, galactomannan, galactomannoproteins (afmp1/asp f 17 and afmp2), hydrophobins (rodA/hyp1 and rodB), DHN-melanin, their respective synthases (fks1, rho1–4, ags1–3, chsA-G, och1–4, mnn9, van1, anp1, glfA, pksP/alb1, arp1, arp2, abr1, abr2, and ayg1), and modifying enzymes (gel1–7, bgt1, eng1, ecm33, afpigA, afpmt1-2, afpmt4, kre2/afmnt1, afmnt2–3, afcwh41 and pmi); several enzymes related to oxidative stress protection such as catalases (catA, cat1/catB, cat2/katG, catC, and catE), superoxide dismutases (sod1, sod2, sod3/asp f 6, and sod4), fatty acid oxygenases (ppoA–C), glutathione tranferases (gstA–E), and others (afyap1, skn7, and pes1); and efflux transporters (mdr1–4, atrF, abcA–E, and msfA–E). In addition, this review considers toxins and related genes, such as a diffusible toxic substance from conidia, gliotoxin (gliP and gliZ), mitogillin (res/mitF/asp f 1), hemolysin (aspHS), festuclavine and fumigaclavine A–C, fumitremorgin A–C, verruculogen, fumagillin, helvolic acid, aflatoxin B1 and G1, and laeA. Two sections cover genes and molecules related with nutrient uptake, signaling and metabolic regulations involved in virulence, including enzymes, such as serine proteases (alp/asp f 13, alp2, and asp f 18), metalloproteases (mep/asp f 5, mepB, and mep20), aspartic proteases (pep/asp f 10, pep2, and ctsD), dipeptidylpeptidases (dppIV and dppV), and phospholipases (plb1–3 and phospholipase C); siderophores and iron acquisition (sidA–G, sreA, ftrA, fetC, mirB–C, and amcA); zinc acquisition (zrfA–H, zafA, and pacC); amino acid biosynthesis, nitrogen uptake, and cross-pathways control (areA, rhbA, mcsA, lysF, cpcA/gcn4p, and cpcC/gcn2p); general biosynthetic pathway (pyrG, hcsA, and pabaA), trehalose biosynthesis (tpsA and tpsB), and other regulation pathways such as those of the MAP kinases (sakA/hogA, mpkA–C, ste7, pbs2, mkk2, steC/ste11, bck1, ssk2, and sho1), G-proteins (gpaA, sfaD, and cpgA), cAMP-PKA signaling (acyA, gpaB, pkaC1, and pkaR), His kinases (fos1 and tcsB), Ca2+ signaling (calA/cnaA, crzA, gprC and gprD), and Ras family (rasA, rasB, and rhbA), and others (ace2, medA, and srbA). Finally, we also comment on the effect of A. fumigatus allergens (Asp f 1–Asp f 34) on IA. The data gathered generate a complex puzzle, the pieces representing virulence factors or the different activities of the fungus, and these need to be arranged to obtain a comprehensive vision of the virulence of A. fumigatus. The most recent gene expression studies using DNA-microarrays may be help us to understand this complex virulence, and to detect targets to develop rapid diagnostic methods and new antifungal agents.
Aspergillus fumigatus es un patógeno oportunista que causa el 90% de las aspergilosis invasoras (AI) con un 50–95% de mortalidad. Se ha postulado la existencia de factores de virulencia característicos, pero en A. fumigatus existe una gran variabilidad de factores de virulencia «no clásicos». Todos los estudios han demostrado que la virulencia de este hongo es multifactorial, asociada a su estructura, su capacidad de crecimiento y adaptación a condiciones de estrés, sus mecanismos de evasión del sistema inmune y su capacidad de causar daños en un huésped. En esta revisión se pretende dar una visión general de los genes y moléculas que intervienen en el desarrollo de la AI. La sección de termotolerancia incluye cinco genes relacionados con la capacidad de que el hongo crezca a más de 30°C (thtA, cgrA, afpmt1, kre2/afmnt1 y hsp1/asp f 12). En las siguientes secciones se discuten las moléculas y los genes relacionados con la interacción con el huésped y con la respuesta inmune. Estas secciones incluyen el β-glucano, el α-glucano, la quitina, el galactomanano, galactomanoproteinas (afmp1/asp f 17 y afmp2), hidrofobinas (rodA/hyp1 y rodB), la DHN-melanina, sus respectivas enzimas sintasas (fks1, rho1-4, ags1-3, chsA-G, och1-4, mnn9, van1, anp1, glfA, pksP/alb1, arp1, arp2, abr1, abr2 y ayg1) y enzimas modificantes (gel1-7, bgt1, eng1, ecm33, afpigA, afpmt1-2, afpmt4, kre2/afmnt1, afmnt2-3, afcwh41 y pmi), varias enzimas relacionadas con la protección del estrés oxidativo como catalasas (catA, cat1/catB, cat2/katG, catC y catE), superóxido dismutasas (sod1-2, sod3/asp f 6 y sod4), oxigenasas de ácidos grasos (ppoA-C), glutatión transferasas (gstA-E) y otros (afyap1, skn7 y pes1), y los transportadores de moléculas (mdr1-4, atrF, abcA-E y msfA-E). Esta revisión también incluye las toxinas y los genes relacionados, como la sustancia difusible de conidios, la gliotoxina (gliP y gliZ), la mitogilina (asp f 1/mitF/res), la hemolisina (aspHS), la festuclavina y la fumigaclavina A-C, la fumitremorgina, el verruculógeno, la fumagilina, el ácido helvólico, las aflatoxinas B1 y G1, y laeA. Dos secciones incluyen los genes y moléculas relacionadas con la absorción de nutrientes, la señalización y las regulaciones metabólicas implicadas en la virulencia, incluyendo enzimas, como las serin-proteasas (alp/asp f 13, alp2 y asp f 18), metaloproteasas (mep/asp f 5, mepB y mep20), aspártico-proteasas (pep/asp f 10, pep2 y ctsD), dipeptidilpeptidasas (dppIV y dppV) y fosfolipasas (plb1-3 y fosfolipasa C); sideróforos y la adquisición de hierro (sidA-G sreA, ftrA, fetC, mirB-C y amcA); adquisición de zinc (zrfA-H, zafA, y pacC); biosíntesis de aminoácidos, absorción de nitrógeno, y regulación por Cross-pathway Control (areA, rhbA, mcsA, lysF, cpcA/gcn4p y cpcC/gcn2p); vías de biosíntesis generales (pyrG, hcsA, y pabaA) y biosíntesis de trehalosa (tpsA y tpsB); otras vías de regulación, como MAP quinasas (sakA/hogA, mpkA-C, ste7, pbs2, mkk2, steC/ste11, bck1, ssk2 y sho1), proteínas G (gpaA, sfaD y cpgA), AMPc-PKA (acyA, gpaB, pkaC1 y pkaR), histidin-quinasas (fos1 y tcsB), señalización de Ca2+(calA/cnaA, crzA, gprC y gprD), familia Ras (rasA, rasB y rhbA), y otros (ace2, medA, y srbA). Por último, también se comentan los efectos de los alérgenos de A. fumigatus (Asp f 1 a Asp f 34) en la AI. Los datos obtenidos generan un complejo rompecabezas, cuyas piezas serían factores de virulencia o diferentes actividades del hongo, que se deben reunir para obtener una visión conjunta de la virulencia de A. fumigatus. Los estudios de expresión mediante microarrays de ADN podrían ser útiles para entender esta compleja virulencia, y para detectar dianas para desarrollar métodos rápidos de diagnóstico y nuevos agentes antifúngicos.
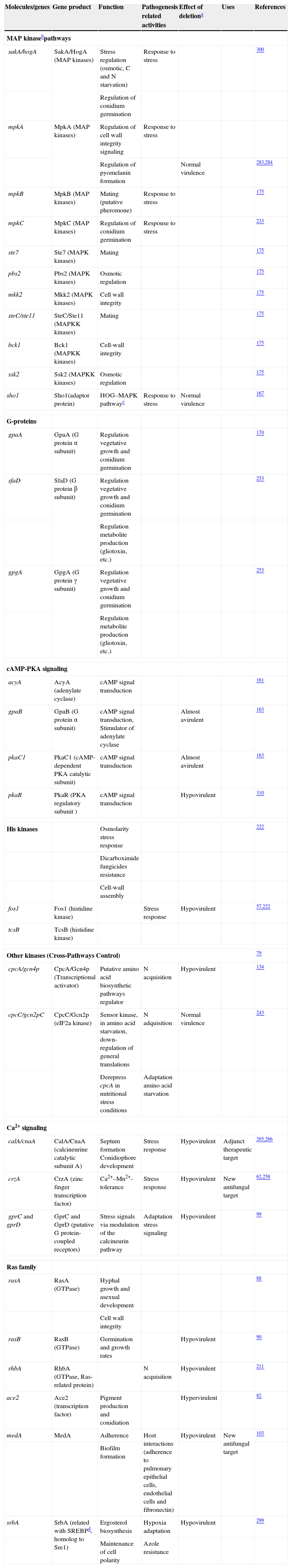
Aspergillus fumigatus is a well adapted saprophytic mold that produces large number of small airborne spores that can survive a wide range of environmental conditions, and accordingly are abundant in soil and decaying organic matter. Due to the 10,000–15,000l of air we inhale each day, humans are continuously in contact with these asexual spores17 and it is estimated that an individual inhales several hundred conidia per day.154 It is remarkable that, despite this constant exposure, most humans do not develop any illness attributable to these spores. In immunocompetent hosts, these spores do not normally cause harm because they are eliminated by pulmonary defense mechanisms.17 However, in immunocompromised individuals, with altered or weakened immune responses, inhaled conidia are able to develop pulmonary mycoses known as aspergillosis. Aspergillosis can be regarded as a broad spectrum of diseases, each related to a spectrum of abnormal immune responses of the host.217 Among them invasive aspergillosis (IA) stands out, with mortality rates greater than 50%, reaching 95% in certain situations17,173 (Fig. 1). The higher mortality observed in the infections by A. fumigatus appears to be due to a weakened immune response, to the virulence of the microorganism itself and also, probably, to delays in establishing a diagnosis, which can prevent the success of treatments.72 About 40 of the 250 species of Aspergillus have been reported to be human pathogens,126 but although the spores of A. fumigatus are a small proportion of all the airborne spores within a hospital (0.3%), this fungus causes approximately 90% of the systemic infections due to Aspergillus.40 Given this, it has been postulated that A. fumigatus has characteristic virulence factors.
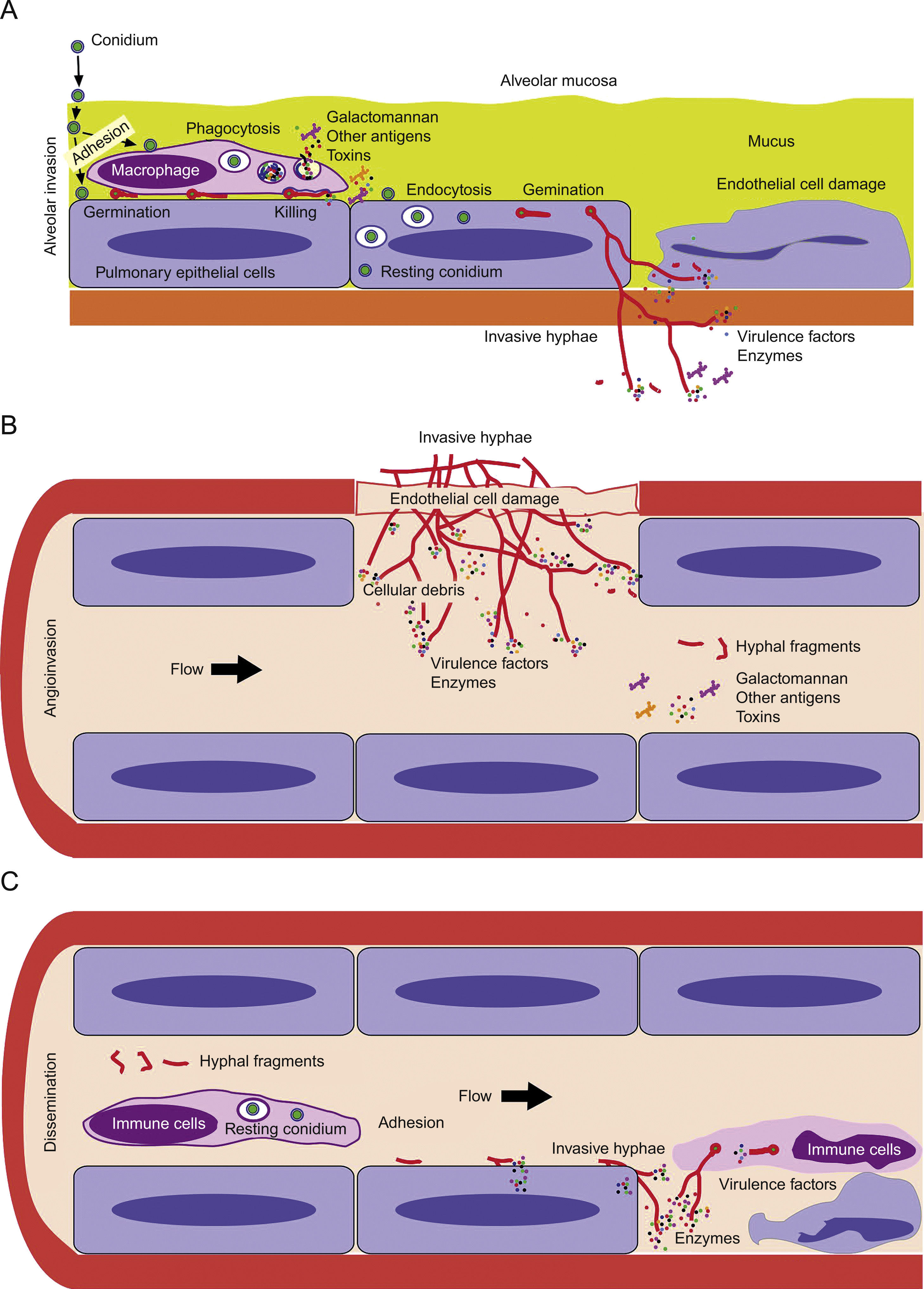
Model of invasive aspergillosis development. (A) First step of colonization and invasion of pulmonary epithelium. (B) Invasion of blood capillaries and haematogenous dissemination of hyphal fragments, galactomannan and other molecules. (C) Dissemination and first step of invasion of deep organs.
The genome sequence of A. fumigatus has been made available recently.201 About 9,630 predicted protein-coding genes have been described of which one-third have unknown functions.85 During infection, fungi encounter dynamic changes in host conditions to which they must continually adapt to survive. This adaptation often requires substantial metabolic reprogramming,91,263 with the simultaneous expression of virulence factors that mediate host cell damage.51 A successful invasion strategy can involve large-scale alterations in protein expression and/or cellular differentiation209. The degree of host immunosuppression needed to develop IA could reduce the requirement for adaptive responses for infection in A. fumigatus. The importance of A. fumigatus infections is reflected by a number of reviews that have been published in the last few years concerning its biology and pathology, and the great effort being made to identify virulence factors.13,19,21,40,41,69,72,112,133,153–155,183,193,208,232,272,285 All these studies have demonstrated that the virulence of this fungus is multifactorial and is due to a combination of biological characteristics of the fungus and the immune status of the patient. Some of these characteristics include the small size of its conidia (2–3μm in diameter) which allows them to reach the human pulmonary alveoli, its thermotolerance and resistance to oxidative stress, and its high growth rate and nutritional versatility, among others. Other fungi share some of these features, but A. fumigatus possibly possesses a unique combination of different traits that make it the primary pathogenic mold in the world.208
With the use of sequenced genomes, we begin to be able to dissect some complex networks of fungal gene interactions such as metabolic regulation, autophagy, and sexuality. Although it is currently unclear, some authors have suggested that these networks have certain effects on the adaptive response of A. fumigatus to infections. Autophagy helps organisms to survive periods of nutrient stress by providing a source of recycled intracellular nutrients to fuel essential cellular functions.209 In Saccharomyces cerevisiae, approximately 30 genes have been identified and collectively termed autophagy-related genes (ATGs)127,128 and many of these are highly conserved across eukaryotes. In A. fumigatus, autophagy is required for conidiation, hyphal foraging, and maintenance of metal-ion homeostasis (all related to nutrient deficiency).235,236 Nutrient deprivation is one of the antimicrobial mechanisms of the host, however, the number of secreted hydrolases encoded by the genome of A. fumigatus,201,239 may allow this fungus to obtain nutrients from mammalian tissues without activating the autophagic network209. The fact that other pathogenic fungi use this mechanism to adapt to the host makes autophagy a putative virulence factor that should be considered carefully in the future.
A. fumigatus, like many other clinically important fungal species, has traditionally been considered an asexual organism. However, the teleomorph phase of this fungus has been discovered and named Neosartorya fumigata.204 The genome sequence of this fungus has made it clear that it occurs in two idiomorphs, MAT1-1 and MAT1-2, and strains of the two opposite mating types occur at the same frequency and are found in close proximity to each other.204 Successful mating was obtained between unrelated, clinical isolates of A. fumigatus, and requires the presence of both mating-type idiomorphs.270 Others authors showed the need for the expression of MAT1-1 and MAT1-2, as well as the expression of genes that encode factors involved in this process, such as genes encoding for sex pheromones and pheromone receptors.80,212 For example, the nsdD gene, a conserved regulator of cleistothecium development, could be related to hyphal fusion and hence heterokaryon formation.270 Heterothallism has now been discovered in four species of Aspergillus that affect human health or have an economic impact, namely A. fumigatus, A. parasiticus, A. flavus, and N. udagawae, but these fungi appear to have relatively low levels of fertility compared to other heterothallic or homothallic species of Aspergillus and require unusually precise environmental parameters to complete their sexual cycle.142 There are different interpretations of this low fertility. Some authors favor the hypothesis that while fertility of these species is on the decline, this is compensated by their proficiency to reproduce asexually in a wider range of environmental conditions.142 Other authors believe that the maintenance of all the machinery required for sex and the limitation of their access to sexual reproduction, has enabled the pathogenic fungi to proliferate rapidly in their environmental niche, but also to undergo genetic exchange, via sexual reproduction, in response to stressful conditions, for example, new environments, different host organisms, or changes in the human host, such as antimicrobial therapy.200 Highly dynamic changes in A. fumigatus populations have been observed within a clinical setting, with new populations found in just a few months,11 and coinfections with different related species of the Aspergillus genus have already been reported.207 These data imply that there may be coinfections with different mating type strains, and surprisingly the possibility that mating could occur in hosts during fungal infection. The presence of a sexual cycle in A. fumigatus would have significant medical implications. Some data suggest a possible association between one idiomorph, the MAT1-1 mating type, and A. fumigatus invasiveness that might contribute to increased virulence and/or resistance to antifungal agents.7 The study of sexual reproduction of this fungus and its possible relationship with virulence will remain a topic of interest in the coming years.6
The intention in this review is to give a general overview of the genes and molecules which have been associated with fungal virulence in the literature, the activities which they can perform and the importance that they could have in the development of IA.
Genes and molecules related to A. fumigatus virulenceVirulence factors are defined as pathogen determinants that cause damage to the infected host.50 This definition includes genes the deletion of which reduces virulence of the reference strain without affecting normal growth, excluding therefore, genes encoding biosynthetic proteins.208 Other genes related to A. fumigatus virulence, like catalases or secreted proteases, do not fit with this definition due to the redundancy of their gene families, and the difficulty of developing disruption of all the genes of a family in a single strain. Nevertheless, all of the genes that help and promote the growth of A. fumigatus in its environmental niche are also implicated in the pathogenesis of aspergillosis in the human host, and hence have to be considered as possible targets for new antifungal agents.13
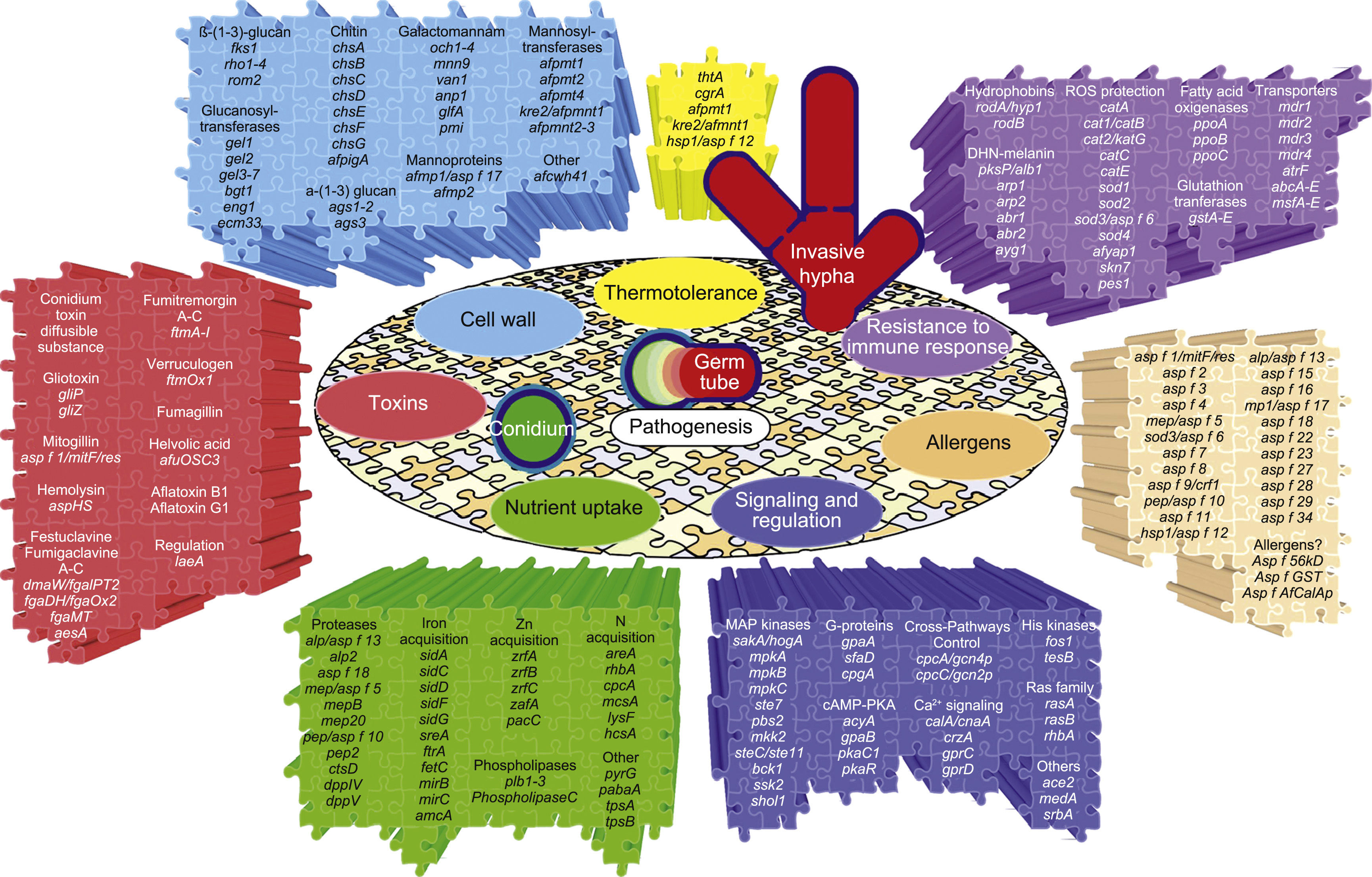
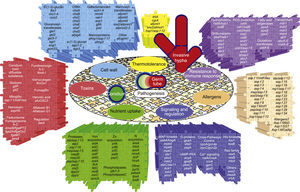
The genes and molecules related to A. fumigatus virulence can be classified according to the process they are involved in, e.g., thermotolerance; cell wall composition and maintenance; resistance to the immune response; toxins; nutrient uptake during invasive growth; signaling, metabolism regulation and response to stress conditions; and allergens.
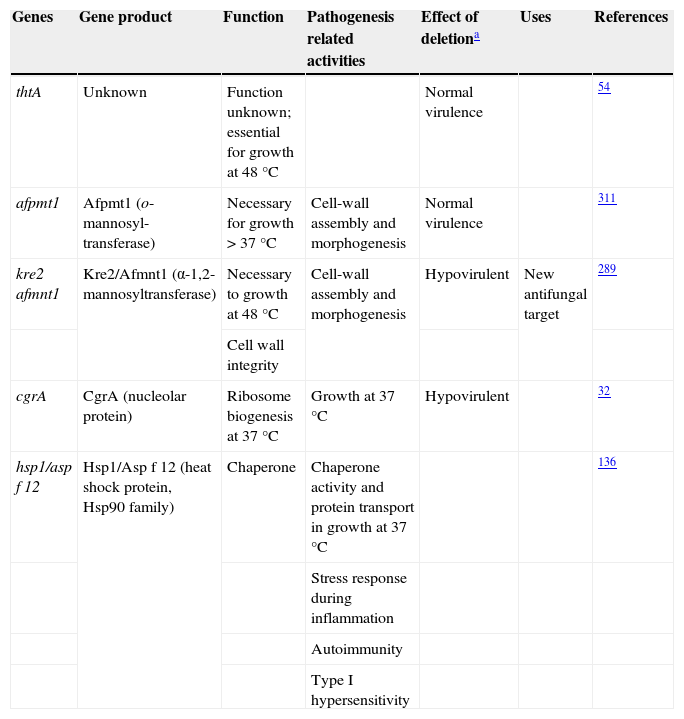
ThermotoleranceA. fumigatus is a thermophilic fungus able to grow at 55°C and survive at more than 75°C,26,241 an essential ability to thrive in decaying organic matter and to infect mammalian hosts. Therefore, genes related to thermotolerance may also contribute to the virulence of this mold.31 Until now, only four genes studied have been found to be necessary for thermotolerance (Table 1). The thtA gene is essential for A. fumigatus growth at 48°C but does not contribute to the pathogenicity of the species.54 Similarly, the afpmt1 gene codes for an o-mannosyltransferase, necessary for growth over 37°C, but is not involved in virulence.311 A putative α-1,2-mannosyltransferase coded by afmnt1 was also shown to be necessary for growth at 48°C.289 These authors showed that the Δafmnt1 mutant grows normally at 37°C, and that the observed growth defect of the mutant at 48°C can be attributed to cell wall instability resulting in leakage at the hyphal tips. This Δafmnt1 mutant was attenuated in a mouse model of infection, and showed an increased sensitivity to azoles.289 Likewise the deletion of the cgrA gene, which is involved in ribosome biogenesis, produced a hypovirulent strain in a murine model of invasive aspergillosis but not in a fruit fly model, being so related to the growth at 37°C.32
Aspergillus fumigatus thermotolerance genes and their relationship with virulence
| Genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| thtA | Unknown | Function unknown; essential for growth at 48°C | Normal virulence | 54 | ||
| afpmt1 | Afpmt1 (o-mannosyl-transferase) | Necessary for growth > 37°C | Cell-wall assembly and morphogenesis | Normal virulence | 311 | |
| kre2 afmnt1 | Kre2/Afmnt1 (α-1,2-mannosyltransferase) | Necessary to growth at 48°C | Cell-wall assembly and morphogenesis | Hypovirulent | New antifungal target | 289 |
| Cell wall integrity | ||||||
| cgrA | CgrA (nucleolar protein) | Ribosome biogenesis at 37°C | Growth at 37°C | Hypovirulent | 32 | |
| hsp1/asp f 12 | Hsp1/Asp f 12 (heat shock protein, Hsp90 family) | Chaperone | Chaperone activity and protein transport in growth at 37°C | 136 | ||
| Stress response during inflammation | ||||||
| Autoimmunity | ||||||
| Type I hypersensitivity |
Cells exposed to non-lethal high temperatures become transiently resistant to subsequent heat shock, producing proteins named heat shock proteins (HSPs). Thermotolerance development is paralleled by expression of these HSPs.203 HSPs have been identified as molecular chaperones conserved between organisms.46 It has also been reported that a protein, Hsp1/Asp f 12,136 classified as a member of the family of Hsp90 could be related to thermotolerance. In addition, the protein Hsp1/Asp f 12 may also play a role in protective immunity and autoimmunity, as it is one of the immunodominant antigens in allergic aspergillosis.136
Nierman et al.201 studied the differences in gene expression between 30 and 37°C and between 30 and 48°C, and detected some upregulated genes at 37°C, but to date none of the genes related to pathogenicity have been found to be more highly expressed at 37°C than that at 48°C. They concluded that host temperature alone is not sufficient to turn on many virulence-related genes. On the other hand, Do et al.76 proposed that the thermal tolerance of A. fumigatus might be due to the efficient regulation of metabolic genes by HSPs. These authors used a state space model to examine transcriptional regulation and found a negative association between many HSPs and the metabolic genes they regulate. Little is known about A. fumigatus proteome changes at different temperatures, but a recent study has described 64 proteins to be up or downregulated from 30 to 48°C.4 Of them, Hsp 30/Hsp 42 and Hsp 90 showed the highest increase in expression during the heat shock response of A. fumigatus. More studies of changes in the proteome and their relationship with transcriptome changes could enhance our understanding of the thermoregulation of this fungus, and would help identify new possible targets for IA treatment.
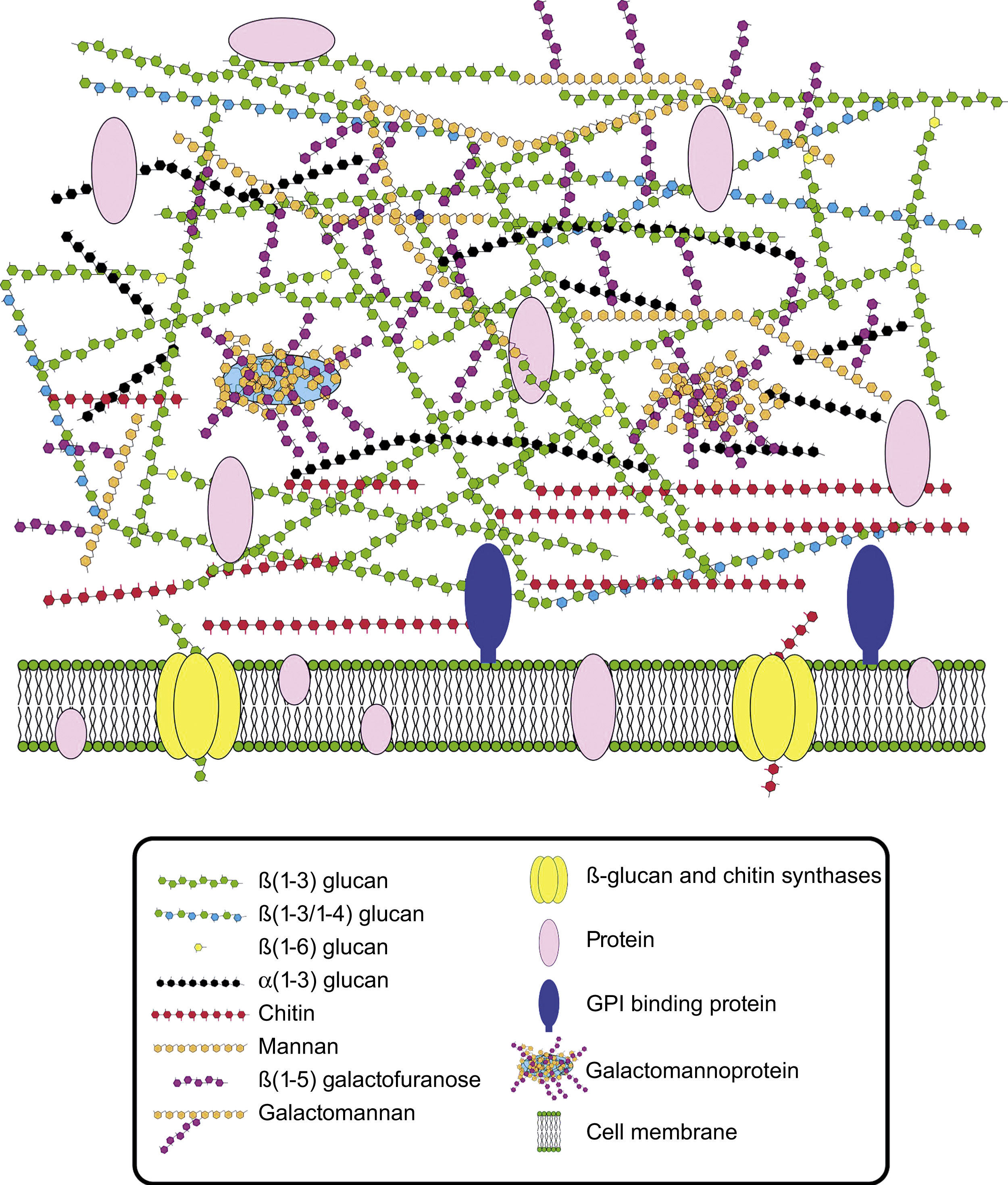
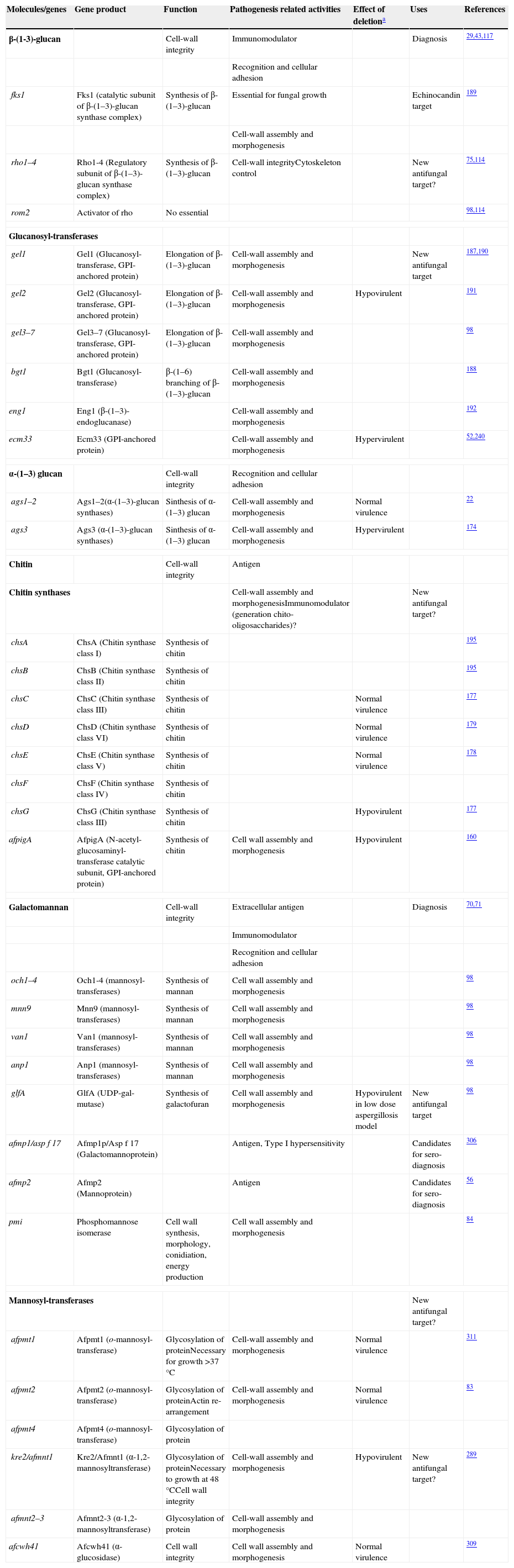
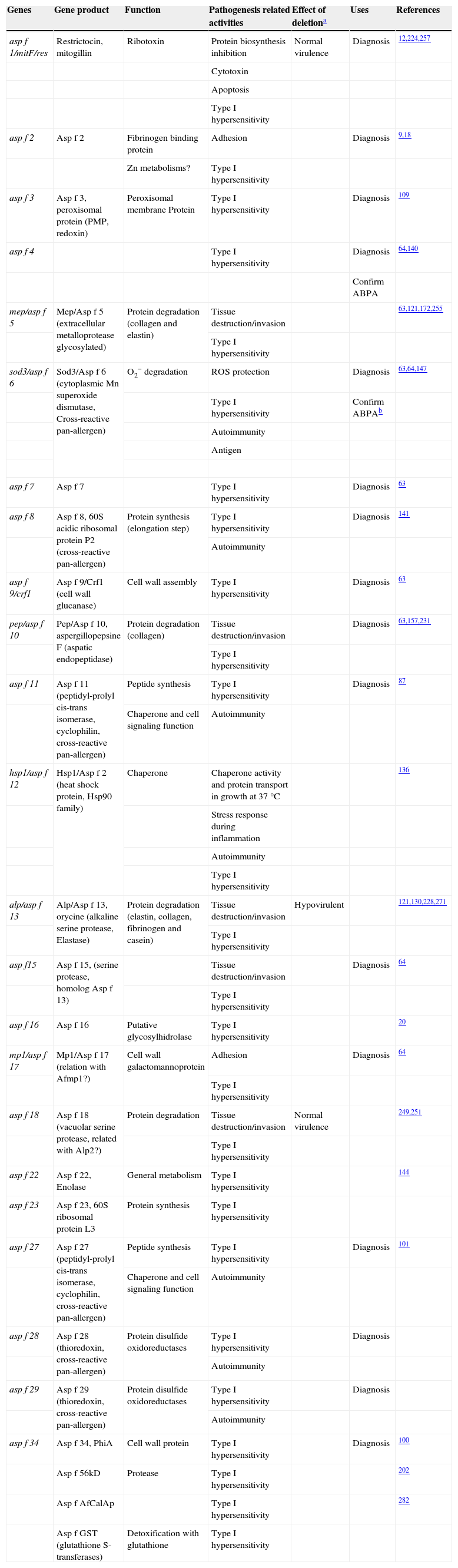
Cell wall composition and maintenanceThe cell wall is the main line of defense of the fungus against a hostile environment providing structural integrity and physical protection to the cell. The fungal cell wall is also the structure responsible for the interaction with the host and their components are often the targets of the host immune system during fungal infections. In A. fumigatus, the cell wall is mainly composed of polysaccharides (at least 90%) and proteins.98 Among the polysaccharides there are linear β(1–3)-glucans (20–35%) branched with β(1–6) links (4%); linear β(1–3/1–4)-glucans (10%); α(1–3)-glucans (35–46%); chitins; and galactomannans (20–25%).98,152,156Fig. 2 shows a schematic drawing of the cell wall structure. The genes and molecules related to the cell wall and virulence included in this review are listed in Table 2. Additional layers in the outer part of the cell wall may be also present. A layer of hydrophobic components is detected on both hypha and conidia, and a melanin layer only on the conidia. The effect of melanin and hydrophobic components on the immune response is addressed in the next section. Further, the presence of sialic acids has been detected on the surface of conidia. These sialic acids are unsubstituted N-acetyl-neuraminic acids linked to galactose by α-2,6 bonds29 and could play an important role in their adhesion to the extracellular matrix.292
Genes and proteins related with cell wall structure and virulence
| Molecules/genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| β-(1-3)-glucan | Cell-wall integrity | Immunomodulator | Diagnosis | 29,43,117 | ||
| Recognition and cellular adhesion | ||||||
| fks1 | Fks1 (catalytic subunit of β-(1–3)-glucan synthase complex) | Synthesis of β-(1–3)-glucan | Essential for fungal growth | Echinocandin target | 189 | |
| Cell-wall assembly and morphogenesis | ||||||
| rho1–4 | Rho1-4 (Regulatory subunit of β-(1–3)-glucan synthase complex) | Synthesis of β-(1–3)-glucan | Cell-wall integrityCytoskeleton control | New antifungal target? | 75,114 | |
| rom2 | Activator of rho | No essential | 98,114 | |||
| Glucanosyl-transferases | ||||||
| gel1 | Gel1 (Glucanosyl-transferase, GPI-anchored protein) | Elongation of β-(1–3)-glucan | Cell-wall assembly and morphogenesis | New antifungal target | 187,190 | |
| gel2 | Gel2 (Glucanosyl-transferase, GPI-anchored protein) | Elongation of β-(1–3)-glucan | Cell-wall assembly and morphogenesis | Hypovirulent | 191 | |
| gel3–7 | Gel3–7 (Glucanosyl-transferase, GPI-anchored protein) | Elongation of β-(1–3)-glucan | Cell-wall assembly and morphogenesis | 98 | ||
| bgt1 | Bgt1 (Glucanosyl-transferase) | β-(1–6) branching of β-(1–3)-glucan | Cell-wall assembly and morphogenesis | 188 | ||
| eng1 | Eng1 (β-(1–3)-endoglucanase) | Cell-wall assembly and morphogenesis | 192 | |||
| ecm33 | Ecm33 (GPI-anchored protein) | Cell-wall assembly and morphogenesis | Hypervirulent | 52,240 | ||
| α-(1–3) glucan | Cell-wall integrity | Recognition and cellular adhesion | ||||
| ags1–2 | Ags1–2(α-(1–3)-glucan synthases) | Sinthesis of α-(1–3) glucan | Cell-wall assembly and morphogenesis | Normal virulence | 22 | |
| ags3 | Ags3 (α-(1–3)-glucan synthases) | Sinthesis of α-(1–3) glucan | Cell-wall assembly and morphogenesis | Hypervirulent | 174 | |
| Chitin | Cell-wall integrity | Antigen | ||||
| Chitin synthases | Cell-wall assembly and morphogenesisImmunomodulator (generation chito-oligosaccharides)? | New antifungal target? | ||||
| chsA | ChsA (Chitin synthase class I) | Synthesis of chitin | 195 | |||
| chsB | ChsB (Chitin synthase class II) | Synthesis of chitin | 195 | |||
| chsC | ChsC (Chitin synthase class III) | Synthesis of chitin | Normal virulence | 177 | ||
| chsD | ChsD (Chitin synthase class VI) | Synthesis of chitin | Normal virulence | 179 | ||
| chsE | ChsE (Chitin synthase class V) | Synthesis of chitin | Normal virulence | 178 | ||
| chsF | ChsF (Chitin synthase class IV) | Synthesis of chitin | ||||
| chsG | ChsG (Chitin synthase class III) | Synthesis of chitin | Hypovirulent | 177 | ||
| afpigA | AfpigA (N-acetyl-glucosaminyl-transferase catalytic subunit, GPI-anchored protein) | Synthesis of chitin | Cell wall assembly and morphogenesis | Hypovirulent | 160 | |
| Galactomannan | Cell-wall integrity | Extracellular antigen | Diagnosis | 70,71 | ||
| Immunomodulator | ||||||
| Recognition and cellular adhesion | ||||||
| och1–4 | Och1-4 (mannosyl-transferases) | Synthesis of mannan | Cell wall assembly and morphogenesis | 98 | ||
| mnn9 | Mnn9 (mannosyl-transferases) | Synthesis of mannan | Cell wall assembly and morphogenesis | 98 | ||
| van1 | Van1 (mannosyl-transferases) | Synthesis of mannan | Cell wall assembly and morphogenesis | 98 | ||
| anp1 | Anp1 (mannosyl-transferases) | Synthesis of mannan | Cell wall assembly and morphogenesis | 98 | ||
| glfA | GlfA (UDP-gal-mutase) | Synthesis of galactofuran | Cell wall assembly and morphogenesis | Hypovirulent in low dose aspergillosis model | New antifungal target | 98 |
| afmp1/asp f 17 | Afmp1p/Asp f 17 (Galactomannoprotein) | Antigen, Type I hypersensitivity | Candidates for sero-diagnosis | 306 | ||
| afmp2 | Afmp2 (Mannoprotein) | Antigen | Candidates for sero-diagnosis | 56 | ||
| pmi | Phosphomannose isomerase | Cell wall synthesis, morphology, conidiation, energy production | Cell wall assembly and morphogenesis | 84 | ||
| Mannosyl-transferases | New antifungal target? | |||||
| afpmt1 | Afpmt1 (o-mannosyl-transferase) | Glycosylation of proteinNecessary for growth >37°C | Cell-wall assembly and morphogenesis | Normal virulence | 311 | |
| afpmt2 | Afpmt2 (o-mannosyl-transferase) | Glycosylation of proteinActin re-arrangement | Cell-wall assembly and morphogenesis | Normal virulence | 83 | |
| afpmt4 | Afpmt4 (o-mannosyl-transferase) | Glycosylation of protein | ||||
| kre2/afmnt1 | Kre2/Afmnt1 (α-1,2-mannosyltransferase) | Glycosylation of proteinNecessary to growth at 48°CCell wall integrity | Cell-wall assembly and morphogenesis | Hypovirulent | New antifungal target? | 289 |
| afmnt2–3 | Afmnt2-3 (α-1,2-mannosyltransferase) | Glycosylation of protein | Cell-wall assembly and morphogenesis | |||
| afcwh41 | Afcwh41 (α-glucosidase) | Cell wall integrity | Cell wall assembly and morphogenesis | Normal virulence | 309 | |
The cell wall consists in a polysaccharide-based three-dimensional network and is now seen as a dynamic structure that is continuously changing as a result of the modification of culture conditions and environmental stress.152 The maintenance of cell wall integrity and functionality as well as changes in cell wall composition to adapt to the environment of the host could be involved in pathogenicity. Genes participating in the biosynthesis of most of A. fumigatus cell wall components have been identified.98 The study of these genes has revealed that mutant strains for enzymes required to synthesize cell wall polysaccharides were at least as virulent as the reference strain on almost all occasions.
The major polysaccharides in the A. fumigatus cell wall are the α(1–3)-glucans, and these have been shown to contribute to the virulence of diverse fungal pathogens. In particular, three α(1–3)-glucan synthase genes, ags1, ags2, and ags3, have been identified and were found to be responsible for cell wall α(1–3)-glucan biosynthesis. The Δags1 and Δags2 strains were not defective in virulence,22 while the Δags3 mutant was hypervirulent in an experimental mouse model of aspergillosis.174 Hypervirulence was correlated with an increased melanin content of the conidial cell wall, which could protect the cells from oxidative stress, and a quicker germination rate, that could evade macrophage killing. These authors did not observe significant changes in cell wall composition of the mutants, probably because of the redundancy between ags1 and ags3.13
β(1–3)-glucan branched with β(1–6)-glucan form the skeleton of the wall, and these are covalently bound to chitin and β(1–3/1–4)-glucan. This component is an important fungal pathogen-associated molecular pattern (PAMP) being recognized by receptor dectin1 on immune cells,29,43 and has different types of biological activity, triggering the activation of complement and inflammatory responses through mediators such as leukotrienes and TNFα117. β-glucan is a compound that is present in almost all fungi and has been used for the diagnosis of invasive mycosis,117 its kinetics correlating very well with that of galactomannan in patients with IA.218 Several authors have reviewed the synthesis of this component.78,98 Briefly, β(1–3)-glucan synthase is a transmembrane complex formed by several different proteins.78,98 The fks1 gene encodes the catalytic subunit and some of the four rho genes (rho1–4) detected in A. fumigatus and may be the regulatory subunit of glucan synthase.78,189 Although it is not a real virulence factor, Fks1 is essential for the fungus and its interest lies in being the target for the antifungal echinocandins. Research has also indicated that Rho1 and Rho3 are involved in controlling cell wall integrity and the cytoskeleton, and these are localized in the hyphal tip.75 Therefore, in the future, Rho molecules could also be potential targets for developing new antifungal agents.
A. fumigatus has at least seven chitin synthase encoding genes, but just four of them have been assayed for virulence: chsC, chsD, chsE, and chsG.14,177–179 Only chsG seems to have an influence on virulence, with a ΔchsG mutant strain having been shown to produce lower mortality rates than the reference strain in a mice infection model.177 However, these results could also be explained by redundancy of this type of enzymes.
The galactomannans in the cell wall are composed of mannose chains (α-mannan), shorter than those of yeasts, with branches formed by small side chains of five molecules of β(1–5)-galactose linked to mannan.98 Galactomannan synthesis requires mannosyl- and galactosyl-transferases. In the A. fumigatus genome there are orthologs of the S. cerevisiae genes related to mannan synthesis, four of the OCH genes, that initiate the synthesis of mannan chains, and orthologs of MNN9, VAN1, and ANP1 genes, which encode for mannosyltransferases.98 The functional role of each gene remains unknown. Galactofuranose biosynthesis starts with the isomerization of UDP-galactopyranose to UDP galactofuranose by UDP galactomutase encoded by the glfA gene. ΔglfA strains displayed attenuated virulence in a low-dose mouse model of IA and showed an increased susceptibility to various antifungal agents.244 UDP-galactomutase thus appears to be an appealing target for adjuvant therapy due to its absence from mammalian cells.244 This galactomannan could be a PAMP of the fungus, and useful for adhesion to host components such as fibronectin and laminin, or to interact with pentraxin 3 and other surface receptors of macrophages, dendritic cells, and Langerhans cells.29,112 Galactomannan is the principal exoantigen released during tissue invasion154 and may activate the innate immune response away from the focus of the infection. At present, the galactomannan produced and released by A. fumigatus is used in a commercial test for the diagnosis of IA (Platelia®Aspergillus).70,71
Several proteins of the cell wall are also mannosylated. For example, afmp1 and afmp2 genes encode for a galactomannoprotein and a mannoprotein, respectively. Their role in virulence has not been investigated, but it is worth mentioning them as they are antigenic determinants and therefore possible candidates for serodiagnosis.56,232,306 The addition of N-linked and/or O-linked oligosaccharides is a common modification of cell wall proteins. Mannosyltransferases play a crucial role in this process and most likely are also engaged in the generation of other glycoconjugates. Mannosyltransferases are localized in intracellular compartments of the secretory pathway, e.g., the Golgi apparatus or the endoplasmic reticulum (ER)289 and initiate mannosylation of secretory proteins. In A. fumigatus three members of O-mannosyltransferases, orthologs of PMT family of S. cerevisiae, afpmt1, afpmt2 and afpmt4 have been detected. Two of these have been studied but were found not to be necessary for virulence. The Δafpmt1 mutant showed sensitivity to high temperatures, as mentioned above in the thermotolerance section, and also defects in growth and cell wall integrity, thereby affecting cell morphology, conidium formation, and germination in A. fumigatus.311 Reduced expression of the afpmt2 gene also led to delayed germination, retarded hyphal growth, reduced conidiation, and defects in cell wall integrity; but growth was not found to be temperature-sensitive.83 The reduced production of Afpmt2 also caused actin rearrangement to fail.83 The afpmt4 gene has not yet been studied. The genome of A. fumigatus also harbors three putative α-1,2-mannosyltransferases genes with homology with members of KTR family of S. cerevisiae. One of these, kre2/afmnt1, was studied by Wagener et al.289 and given the findings this has been discussed above in the thermotolerance section. The function of the other two genes still remains unknown. The importance of α-1,2-mannosyltransferases for the synthesis of O- and N-linked carbohydrates and their possible role in the generation of other glycoconjugates, as well as the fact that humans do not possess any homologous enzymes, make α-1,2-mannosyltransferases promising targets for novel antifungal therapies.289
Other proteins present in the cell wall and related to virulence are linked to glycosyl-phosphatidyl-inositol (GPI) motifs. The glucanosyltransferases are enzymes linked to the cell membrane and the cell wall by GPI motifs. Some of these enzymes are thought to participate in the elongation of β(1–3)-glucan side chains.13 For example, the Gel family which is composed of seven proteins coded for by gel1–7. One of these enzymes, encoded by the gel2 gene, was observed to be related to virulence in a coinfection study. Specifically, the presence of DNA from a Δgel2 mutant strain was lower than the DNA of the reference strain in the lungs of coinfected mice.191 Another gene, afpigA, encodes the catalytic subunit of a complex that catalyzes GPI anchor biosynthesis. The GPI anchor is not essential for viability, but does seem to be required for cell wall integrity, morphogenesis, and virulence in A. fumigatus, and accordingly disruption of this gene caused a hypovirulent strain in a model of infection.160 However, the deletion of the ecm33 gene, that codes for a GPI-linked protein, enhanced virulence and resulted in a higher rate of germination, with more resistant conidia but more susceptible hypha.52,240
The phosphomannose isomerase enzyme, Pmi1, is essential for viability and plays a central regulatory role in both cell wall synthesis and energy production in A. fumigatus. The deletion of this gene led to phenotypes showing defects in cell wall integrity, abnormal morphology, and reduced conidiation,84 but their effect on virulence was not tested.
Zhang et al.309 identified a gene in A. fumigatus encoding an α-glucosidase, afcwh41, involved in cell wall integrity, polarity, septation and conidiation, probably by affecting the proper function of the proteins required for cell wall synthesis. However, this gene was not essential for hyphal growth and virulence.
In addition, the polysaccharide matrix of the cell wall, mainly composed of α-glucans and galactomannans, can bind the hyphae of a colony to generate a biofilm. Such biofilms may have an impact on virulence increasing the resistance to antifungals, and concentrating the extracellular enzymes produced during growth, which are also necessary for tissue colonization and infection.25 They might also help fungi to resist the immune response, although more studies are needed.
Genes and molecules associated with resistance to immune responseAs mentioned before, the small size of resting Aspergillus conidia means that some of the inhaled conidia are able to reach the respiratory zone of the lungs, beyond the ciliated epithelium. Various genes and molecules on surface structures of A. fumigatus form a set called PAMPs that interact with and activate the immune system. Host defense relies on soluble and cellular pattern recognition receptors; activation of the effector mechanisms of innate immunity, including the antimicrobial mechanisms of resident leukocytes in the lung, such as alveolar macrophages and dendritic cells; recruitment of other leukocytes; and activation of recruited leukocytes after their arrival at the site of infection. Several reviews focusing on immune response to A. fumigatus infections have been published in the recent years.17,55,69,112,185 With these defenses weakened, conidia are able to germinate and form hypha within 12–15h of arrival.217
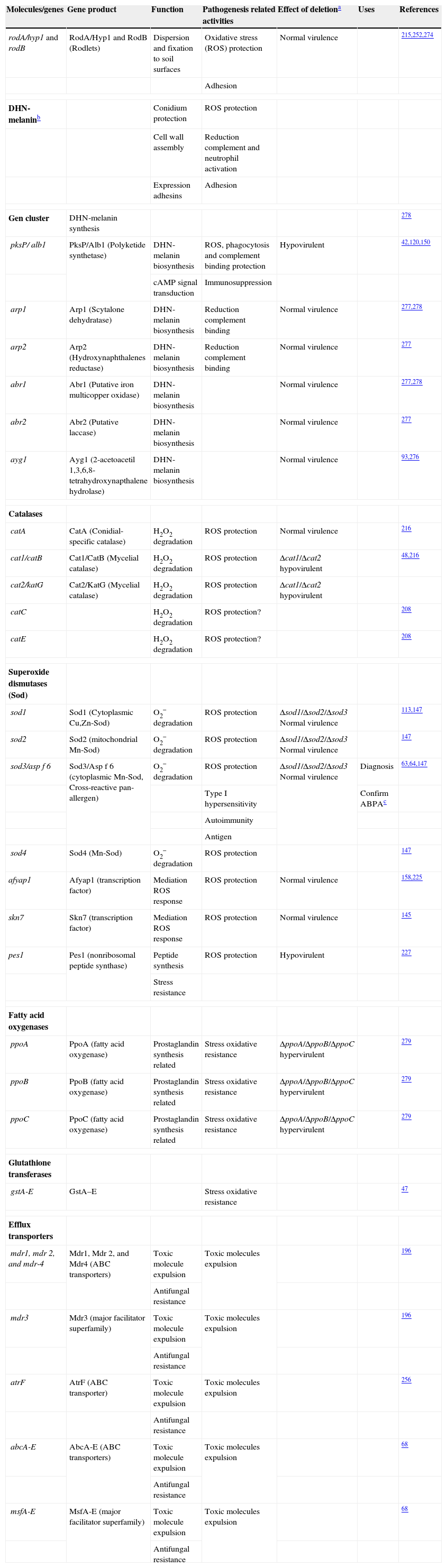
In addition to the weakening of host immune response, A. fumigatus has a combination of characteristics that helps the fungus to evade or resist to immune response (Table 3). Pigmentation on A. fumigatus conidial surface has been shown to affect virulence by limiting C3 complement deposition and neutrophil activation.275 Further, A. fumigatus has demonstrated an ability to bind Factor H, FHL-1, and C4BP on their surface to down-regulate the complement cascade,27,180,288 and to produce a soluble complement-inhibitory factor, which may be lipid derived, that prevented the activation of the alternative pathways.293,294 Moreover the thick fungal cell wall is largely resistant to direct lysis by the terminal membrane attack complex of the complement system.132
Genes and molecules associated with resistance to host immune response
| Molecules/genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| rodA/hyp1 and rodB | RodA/Hyp1 and RodB (Rodlets) | Dispersion and fixation to soil surfaces | Oxidative stress (ROS) protection | Normal virulence | 215,252,274 | |
| Adhesion | ||||||
| DHN-melaninb | Conidium protection | ROS protection | ||||
| Cell wall assembly | Reduction complement and neutrophil activation | |||||
| Expression adhesins | Adhesion | |||||
| Gen cluster | DHN-melanin synthesis | 278 | ||||
| pksP/ alb1 | PksP/Alb1 (Polyketide synthetase) | DHN-melanin biosynthesis | ROS, phagocytosis and complement binding protection | Hypovirulent | 42,120,150 | |
| cAMP signal transduction | Immunosuppression | |||||
| arp1 | Arp1 (Scytalone dehydratase) | DHN-melanin biosynthesis | Reduction complement binding | Normal virulence | 277,278 | |
| arp2 | Arp2 (Hydroxynaphthalenes reductase) | DHN-melanin biosynthesis | Reduction complement binding | Normal virulence | 277 | |
| abr1 | Abr1 (Putative iron multicopper oxidase) | DHN-melanin biosynthesis | Normal virulence | 277,278 | ||
| abr2 | Abr2 (Putative laccase) | DHN-melanin biosynthesis | Normal virulence | 277 | ||
| ayg1 | Ayg1 (2-acetoacetil 1,3,6,8-tetrahydroxynapthalene hydrolase) | DHN-melanin biosynthesis | Normal virulence | 93,276 | ||
| Catalases | ||||||
| catA | CatA (Conidial-specific catalase) | H2O2 degradation | ROS protection | Normal virulence | 216 | |
| cat1/catB | Cat1/CatB (Mycelial catalase) | H2O2 degradation | ROS protection | Δcat1/Δcat2 hypovirulent | 48,216 | |
| cat2/katG | Cat2/KatG (Mycelial catalase) | H2O2 degradation | ROS protection | Δcat1/Δcat2 hypovirulent | ||
| catC | H2O2 degradation | ROS protection? | 208 | |||
| catE | H2O2 degradation | ROS protection? | 208 | |||
| Superoxide dismutases (Sod) | ||||||
| sod1 | Sod1 (Cytoplasmic Cu,Zn-Sod) | O2− degradation | ROS protection | Δsod1/Δsod2/Δsod3 Normal virulence | 113,147 | |
| sod2 | Sod2 (mitochondrial Mn-Sod) | O2− degradation | ROS protection | Δsod1/Δsod2/Δsod3 Normal virulence | 147 | |
| sod3/asp f 6 | Sod3/Asp f 6 (cytoplasmic Mn-Sod, Cross-reactive pan-allergen) | O2− degradation | ROS protection | Δsod1/Δsod2/Δsod3 Normal virulence | Diagnosis | 63,64,147 |
| Type I hypersensitivity | Confirm ABPAc | |||||
| Autoimmunity | ||||||
| Antigen | ||||||
| sod4 | Sod4 (Mn-Sod) | O2− degradation | ROS protection | 147 | ||
| afyap1 | Afyap1 (transcription factor) | Mediation ROS response | ROS protection | Normal virulence | 158,225 | |
| skn7 | Skn7 (transcription factor) | Mediation ROS response | ROS protection | Normal virulence | 145 | |
| pes1 | Pes1 (nonribosomal peptide synthase) | Peptide synthesis | ROS protection | Hypovirulent | 227 | |
| Stress resistance | ||||||
| Fatty acid oxygenases | ||||||
| ppoA | PpoA (fatty acid oxygenase) | Prostaglandin synthesis related | Stress oxidative resistance | ΔppoA/ΔppoB/ΔppoC hypervirulent | 279 | |
| ppoB | PpoB (fatty acid oxygenase) | Prostaglandin synthesis related | Stress oxidative resistance | ΔppoA/ΔppoB/ΔppoC hypervirulent | 279 | |
| ppoC | PpoC (fatty acid oxygenase) | Prostaglandin synthesis related | Stress oxidative resistance | ΔppoA/ΔppoB/ΔppoC hypervirulent | 279 | |
| Glutathione transferases | ||||||
| gstA-E | GstA–E | Stress oxidative resistance | 47 | |||
| Efflux transporters | ||||||
| mdr1, mdr 2, and mdr-4 | Mdr1, Mdr 2, and Mdr4 (ABC transporters) | Toxic molecule expulsion | Toxic molecules expulsion | 196 | ||
| Antifungal resistance | ||||||
| mdr3 | Mdr3 (major facilitator superfamily) | Toxic molecule expulsion | Toxic molecules expulsion | 196 | ||
| Antifungal resistance | ||||||
| atrF | AtrF (ABC transporter) | Toxic molecule expulsion | Toxic molecules expulsion | 256 | ||
| Antifungal resistance | ||||||
| abcA-E | AbcA-E (ABC transporters) | Toxic molecule expulsion | Toxic molecules expulsion | 68 | ||
| Antifungal resistance | ||||||
| msfA-E | MsfA-E (major facilitator superfamily) | Toxic molecule expulsion | Toxic molecules expulsion | 68 | ||
| Antifungal resistance | ||||||
Various different types of behaviour have been detected on activation of immune cells through Toll-like receptors (TLR) by conidia and hyphae of this fungus. A. fumigatus conidia induce signal transduction after their recognition by TLR2 and TLR4; during tissue invasion, the conidia germinate into hyphae with loss of TLR4 stimulation, leading to a less pronounced stimulation of proinflammatory cytokines.53,199 TLR4-mediated proinflammatory effects have been demonstrated to be important in the protection against IA.28 Hence the tissue-invasive hypha of A. fumigatus is able to tilt the balance towards a non-protective Th2 response by a predominant TLR2 activation.53 On the other hand, it has been demonstrated that A. fumigatus conidia can bind and become internalized by human epithelial cell lines,214 which also may limit the induced levels of protective proinflammatory cytokines. These endocytosed conidia remained viable for relatively longer periods of time compared to conidia within macrophages,296 and may eventually germinate and disseminate.295
Genes and molecules involved in resistance to immune response could be considered defensive virulence factors as proposed by Osherov.208 It is well known that A. fumigatus has certain hydrophobic proteins on the surface of its conidial and aerial hyphae which help conidial dispersion, fixation to soil surfaces,154,164 and conidial adherence to the respiratory epithelium,274 and are related to the protection against the oxidative stress produced by alveolar macrophages.215 These proteins are clustered in microfibrils called rodlets. A. fumigatus has at least six genes that code for hydrophobins, but only rodA/hyp1 and rodB have been studied for virulence implication. The rodA gene encodes a small hydrophobic cysteine-rich polypeptide and the mutant strains for this gene showed high sensitivity to destruction by alveolar macrophages but were as virulent as the wild strain.215,274 However, the ΔrodA strain produced smaller lung lesions and weaker inflammatory response than the reference strain.252 On the other hand, the ΔrodB mutant did not show high sensitivity to killing by alveolar macrophages and did not lose their virulence.215
Another surface component of the fungi that has been associated with virulence is melanin, a pigment that protects the integrity of the genome in conidia from ultraviolet light, enzymatic lysis, and oxidation. The conidia of A. fumigatus possess a greyish-green melanin layer, absent in hyphae,305 which contributes to their survival and longevity in the environment.297 Some reviews have focused on the synthesis of melanin in pathogenic fungus and its importance.15,151,221 This pigment appears adhered to the cell wall of the A. fumigatus conidia, coming into direct contact with the host immune system.119,150 The presence of melanin on the surface of the conidium appears to protect the fungus in three ways. Firstly, as described above, the pigmentation on A. fumigatus conidial surface has been shown to affect virulence by limiting the activation of the complement cascade and neutrophils, and through interference with intracellular trafficking of phagocytised conidia.29,275 Secondly, the wild pigmented strains have a 10- to 20-fold greater resistance against reactive oxygen species (ROS) than the white mutant strains, presumably due to their capacity to quench and detoxify these ROS.154 Finally, the melanin could be masking β-glucan. In fact, the absence of pigment produces white conidia, decreases their virulence and makes them more sensitive to the action of H2O2 and sodium hypochlorite, and more susceptible to phagocytosis and to damage by macrophages in vitro.118,150,275 Melanin synthesis seems to be produced in the synthesis route of melanin-1,8 dihydroxynaphthalene (DHN-melanin) and is regulated by a cluster of six genes, pksP/alb1, ayg1, arp1, arp2, abr1, and abr2.41,93,150,275,276,278 Of all these, the most interesting, from the point of view of virulence, is the pksP/alb1 gene which encodes a polyketide synthase and catalyses the first step of this pathway. The deletion in other genes of this pathway produces conidia with different coloration, and in some cases with less deposition of complement (arp1 and arp2), but they do not have any obvious effects on the virulence.277,278 However, the ΔpksP/alb1 mutant has been shown to produce a smooth white conidium, increased C3 deposition on the surface and increased phagocytosis and killing of conidia.42,120 Resting conidia of this mutant strain express β-glucan abundantly on their surface encouraging its recognition through dectin1 receptors. Moreover, a product of the pksP gene could act as an immunosuppressant due to the presence of a functioning pksP gene, which is associated with inhibition of phagosome–lysosome fusion following conidial phagocytosis,120 and may have a direct role in the virulence of the fungus in a murine infection model.150 Melanin is also a structural component of the conidial wall that is required for correct assembly of the cell wall layers and the expression at the conidial surface of adhesins and other virulence factors.221
A. fumigatus also has specific enzymes for detoxification of ROS produced by macrophages and neutrophils, such as five catalases (catA, cat1/catB, catC, catE, and cat2)48,208,215 and four superoxide dismutases (SODs): a cytoplasmic Cu/ZnSOD (Sod1), a mitochondrial MnSOD (Sod2), a cytoplasmic MnSOD (Sod3), and Sod4 displaying a MnSOD C-terminal domain.87,113,147 Deletion of catA, a conidial catalase, resulted in increased susceptibility of conidia to H2O2in vitro, but the virulence of the mutant strain did not change in a murine model.215. Disruptions of either cat1 or cat 2 genes, encoding the hyphal catalases, did not affect sensitivity to H2O2in vitro or the virulence of mutants in animal infection models.48,215 However, double mutant Δcat1/Δcat2 exhibited reduced virulence in immunosuppressed rats.48,215 In any case, as noted above, the redundancy of these genes for detoxification of ROS makes it difficult to verify their relationship with the virulence of the fungus. Fungal SODs that detoxify superoxide anions could be putative virulence factors for this opportunistic pathogen. During growth, Sod1 and Sod2 were highly expressed in conidia whereas Sod3 was only strongly expressed in mycelium and Sod4 was weakly expressed compared to other SODs.147 The deletion of Sod4 was lethal. The Δsod1 and Δsod2 mutants showed an inhibition of growth at high temperatures and hypersensitivity to menadione, whereas the sod3 mutant had only slightly delayed growth at high temperatures. The triple sod1/sod2/sod3 mutant was characterized by a delay in conidial germination, lower rates of conidial survival over time during storage, the highest sensitivity to menadione and an increased sensitivity to killing by alveolar macrophages of immunocompetent mice. In spite of these phenotypes, no significant virulence difference was observed between the triple mutant and the parental strain in experimental murine aspergillosis models with immunocompromised animals.147 Recently, Lessing et al.158 investigated the enzymatic ROS detoxifying system by proteome analysis of A. fumigatus challenged by H2O2. These researchers discovered that many of the identified proteins and genes were apparently regulated by a putative S. cerevisiae YAP1 homologous gene. This gene codes for a bZip-type transcription factor that contributes to the response against oxidative stress. Deletion of this afyap1 homologous gene in A. fumigatus led to drastically increased sensitivity to H2O2, but this mutant strain did not show attenuated virulence in a murine model of Aspergillus infection.158 These data have been corroborated in another study by Qiao et al.225 Other researchers have suggested that catalase activity in the Δafyap1 strain could be sufficient or more than sufficient to provide protection during incubation with neutrophils or in vivo, than after exposure to H2O2in vitro.69 These authors also argued that this similarity in virulence could be due to the use of a severe immunosuppression model, which made it difficult to detect small variations in virulence between mutants and their reference strains. Another transcription factor that contributes to the response against oxidative stress in yeast is SKN7. The homolog of this gene in A. fumigatus showed a similar role to the YAP1 gene we have just discussed. The Δskn7 strain of A. fumigatus had an increased sensitivity to peroxides in vitro but this was not correlated with a modification of fungal virulence.145 These results suggest that reactive oxygen intermediates have a relatively low importance in the destruction of the hyphae and conidia of A. fumigatus. Other mechanisms, such as the production of nitric oxide by macrophages or lactoferrin by neutrophils, a molecule with an ability to sequester iron, could be more relevant in the immune response against this fungus.104,197,307 Therefore, the role of other genes and molecules of the fungus in combating stress should be studied.
Three glutathione transferases (GST) genes, termed gstA–C in A. fumigatus, have also been described.47 The results from studying these genes suggested a role for these enzymes in the response of the organism to both oxidative stress and presence of xenobiotic compounds,47 but they have not been tested for virulence. It has also been suggested that the nonribosomal peptide synthetase gene, pes1, contribute to the resistance of A. fumigatus to oxidative stress. Disruption of this gene led to decreased fungal virulence in a moth model system, as well as an increased susceptibility to oxidative stress and neutrophil-mediated killing, in addition to altered conidial morphology and hydrophobicity.227
Three fatty acid oxygenases encoding genes (ppoA, ppoB, and ppoC) have also been tested for their role in the virulence of A. fumigatus. The triple mutant strain was found to be hypervirulent in an invasive murine model and showed increased tolerance to H2O2 stress relative to that of the wild type.279 These authors suggested that part of the increased virulence of the triple mutant strain might be due to the Ppo-generated prostaglandins, which could enhance host defense mechanisms, perhaps through initiation of inflammation responses involved in recruiting phagocytic cells.
Four genes that encode ATP-binding cassette (ABC)-type transporters (mdr1, mdr2, atrF, and mdr4), and one gene that codes for a protein of the major facilitator superfamily (MFS)(mdr3) related to azole resistance149,196,256 have been described in A. fumigatus. Other genes (abcA–E and mfsA–E) that encode for these types of transporter could be related to voriconazole resistance.68 These two classes of transporters or efflux pumps are associated with the membrane and could detoxify immune system components in a similar way to their involvement in resistance to antifungals.208 Today, thanks to genome sequencing of A. fumigatus, at least 327 genes that encode putative multidrug resistance efflux pumps have been reported, including 49 ABC-type genes, and 278 genes that encode MFS proteins.86,201
However, despite the varied capabilities possessed by the fungal pathogen to evade host detection, it should be emphasized that the normal host defense is generally effective against most fungal infections and the host has first to be in an immune suppressed state before it becomes susceptible to opportunistic pathogens.53
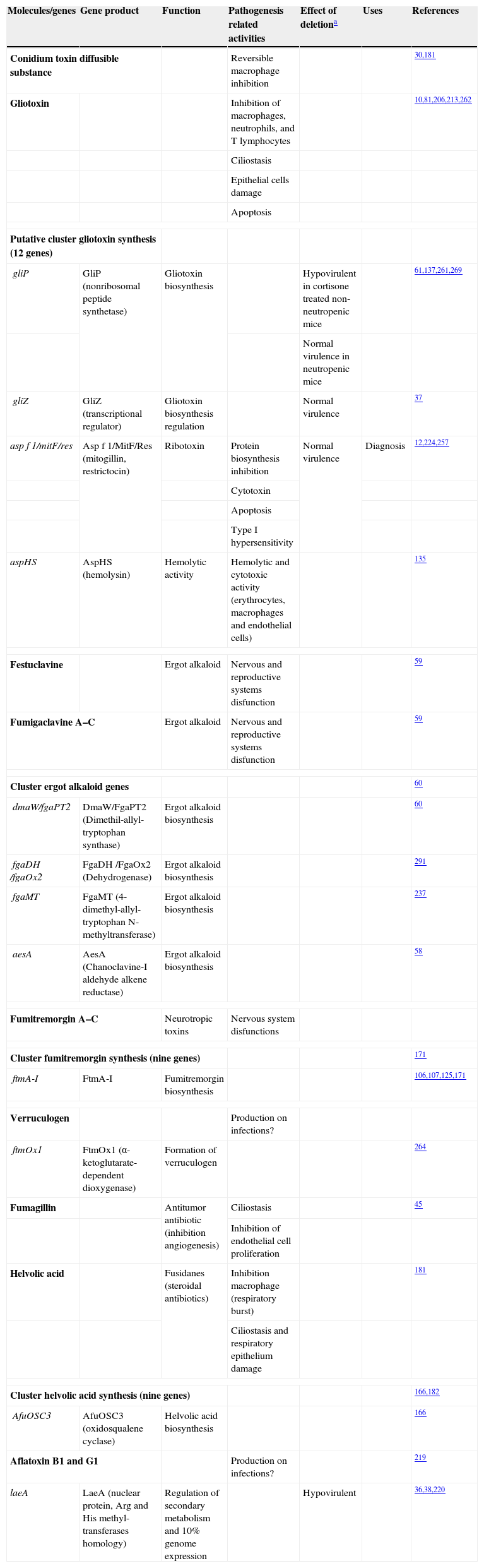
ToxinsMycotoxins can be described as a chemically diverse group of low molecular weight organic substances produced by fungi. These substances are formed in the hyphae during growth, and may be actively expelled into the environment, or released after the death of the hyphae. The presence of preformed mycotoxins in conidia means that the toxins must be incorporated during conidiogenesis. However, these substances might be also produced during germination. Toxins are apparently produced by the fungus to protect itself from predators and competitors in its ecological niche,208 but they could also contribute to A. fumigatus pathogenesis, since they can directly attack the host (Table 4). Many of these toxins are secondary metabolites of these fungi. Depending on the mycotoxin, they can affect the synthesis of proteins, DNA and RNA, or alter the cell membrane, the consequences of which may be death or impairment of cellular functions.
Toxins related to the direct attack to the host organism
| Molecules/genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| Conidium toxin diffusible substance | Reversible macrophage inhibition | 30,181 | ||||
| Gliotoxin | Inhibition of macrophages, neutrophils, and T lymphocytes | 10,81,206,213,262 | ||||
| Ciliostasis | ||||||
| Epithelial cells damage | ||||||
| Apoptosis | ||||||
| Putative cluster gliotoxin synthesis (12 genes) | ||||||
| gliP | GliP (nonribosomal peptide synthetase) | Gliotoxin biosynthesis | Hypovirulent in cortisone treated non-neutropenic mice | 61,137,261,269 | ||
| Normal virulence in neutropenic mice | ||||||
| gliZ | GliZ (transcriptional regulator) | Gliotoxin biosynthesis regulation | Normal virulence | 37 | ||
| asp f 1/mitF/res | Asp f 1/MitF/Res (mitogillin, restrictocin) | Ribotoxin | Protein biosynthesis inhibition | Normal virulence | Diagnosis | 12,224,257 |
| Cytotoxin | ||||||
| Apoptosis | ||||||
| Type I hypersensitivity | ||||||
| aspHS | AspHS (hemolysin) | Hemolytic activity | Hemolytic and cytotoxic activity (erythrocytes, macrophages and endothelial cells) | 135 | ||
| Festuclavine | Ergot alkaloid | Nervous and reproductive systems disfunction | 59 | |||
| Fumigaclavine A–C | Ergot alkaloid | Nervous and reproductive systems disfunction | 59 | |||
| Cluster ergot alkaloid genes | 60 | |||||
| dmaW/fgaPT2 | DmaW/FgaPT2 (Dimethil-allyl-tryptophan synthase) | Ergot alkaloid biosynthesis | 60 | |||
| fgaDH /fgaOx2 | FgaDH /FgaOx2 (Dehydrogenase) | Ergot alkaloid biosynthesis | 291 | |||
| fgaMT | FgaMT (4-dimethyl-allyl-tryptophan N-methyltransferase) | Ergot alkaloid biosynthesis | 237 | |||
| aesA | AesA (Chanoclavine-I aldehyde alkene reductase) | Ergot alkaloid biosynthesis | 58 | |||
| Fumitremorgin A–C | Neurotropic toxins | Nervous system disfunctions | ||||
| Cluster fumitremorgin synthesis (nine genes) | 171 | |||||
| ftmA-I | FtmA-I | Fumitremorgin biosynthesis | 106,107,125,171 | |||
| Verruculogen | Production on infections? | |||||
| ftmOx1 | FtmOx1 (α-ketoglutarate-dependent dioxygenase) | Formation of verruculogen | 264 | |||
| Fumagillin | Antitumor antibiotic (inhibition angiogenesis) | Ciliostasis | 45 | |||
| Inhibition of endothelial cell proliferation | ||||||
| Helvolic acid | Fusidanes (steroidal antibiotics) | Inhibition macrophage (respiratory burst) | 181 | |||
| Ciliostasis and respiratory epithelium damage | ||||||
| Cluster helvolic acid synthesis (nine genes) | 166,182 | |||||
| AfuOSC3 | AfuOSC3 (oxidosqualene cyclase) | Helvolic acid biosynthesis | 166 | |||
| Aflatoxin B1 and G1 | Production on infections? | 219 | ||||
| laeA | LaeA (nuclear protein, Arg and His methyl-transferases homology) | Regulation of secondary metabolism and 10% genome expression | Hypovirulent | 36,38,220 | ||
A diffusible, heat-stable substance, with a mass of less than 14kDa, can be rapidly extracted from the surface of the conidium. This diffusible substance has been shown to affect competent macrophages, inhibiting the respiratory burst, phagocytosis and the release of cytokines by macrophages,30,181 and its effect is reversible. This component has still not been identified, but may allow the fungus to remain in the lungs and express its pathogenic effects. In particular, it has been associated with the pathogenicity level of A. fumigatus strains, but not all strains produce it.30
Ergot alkaloids are a complex family of indole-derived mycotoxins that affect the nervous and reproductive systems of exposed individuals through interactions with monoamine receptors.59 The ergot alkaloids festuclavine and fumigaclavines A–C are present in or on conidia of A. fumigatus.59 An ergot alkaloid gene cluster in A. fumigatus genome has been described,60 of which the dmaW gene has been studied. This gene encodes a dimethylallyl tryptophan synthase that appears to control a determinant step in ergot alkaloid biosynthesis, as when dmaW was knocked out all known ergot alkaloids were eliminated from A. fumigatus.60 Another recently studied gene, easA encodes an enzyme which catalyzes the reduction of the chanoclavine-I aldehyde alkene to dihydrochanoclavine aldehyde, and facilitates an intramolecular reaction to generate the immediate precursor to festuclavine.58 Some other genes, like the 4-dimethylallyltryptophan N-methyltransferase encoded gene, fgaMT237 and a dehydrogenase gene, fgaDH/fgaOx2, that catalyzed the oxidation of chanoclavine-I to chanoclavine-I aldehyde,291 have also been reported. However, none of these genes have yet been tested for virulence.
Gliotoxin is the major and the most potent toxin produced by A. fumigatus.143 It belongs to the family of epipolythiodioxopiperazines, which are characterized by a disulfide bridge across a piperazine ring which is essential for their toxicity.97 Gliotoxin has several immunosuppressive roles including inhibition of macrophage phagocytosis, mitogen-activated T cell proliferation, mast cell activation, cytotoxic T-cell response, and monocyte apoptosis.81,194,262,301 It also inhibits the NADPH of neutrophils,280 suppresses ROS production and impairs neutrophil phagocytic capacity,206 reduces the ciliary movement of epithelial cells and leads to epithelial cells damage.10 It has also been reported that gliotoxin induces ROS-facilitated apoptotic cell death by activating the Bak gene of mice, a member of proapoptotic Bcl-2 family.213 It has been proven that this toxin is produced in experimental animal aspergillosis159,234 as well as in human IA, with serum concentrations of 166–785ng/ml in 80% of patients with IA.159 Although some studies have reported that a low proportion of strains produce this toxin,77,92 a recent study reported that gliotoxin is produced by more than 95% of A. fumigatus isolates from both clinical and environmental origins, while it is less often produced by other Aspergillus species.138 A putative cluster of 12 genes involved in gliotoxin biosynthesis was discovered.96 The gliZ gene controls expression of the remaining 11 genes in this cluster,37 while gliP encodes a multimodular nonribosomal peptide synthase that catalyzes the condensation of serine and phenylalanine, the first step of the pathway.16 In neutropenic models of IA, the mutant strains for these two genes were as virulent as the reference strain.37,61,137 Nevertheless, in non-neutropenic mice treated with cortisone, the virulence of gliP mutant strains was lower than the reference strains.261,269 These results suggest that gliotoxin induces neutrophil apoptosis261 and a direct role of gliotoxin in aspergillosis virulence in non-neutropenic immunocompromised individuals.
A. fumigatus is able to produce ribotoxins, proteins that have a highly specific activity against the sarcin/ricin domain universally preserved in 28S ribosomal RNA, inhibiting protein biosynthesis.123,124 One of these proteins is restrictocin, also known as mitogillin, encoded by the asp f 1/mitF/res gene. This toxin is related to the allergic process, since it is one of the immunodominant antigens of allergic aspergillosis.12 Mitogillin is secreted in vivo by A. fumigatus148 and has strong toxic effects that can cause cell death at low concentrations.224 Ok et al 205 showed in vitro that Asp f 1 is also able to induce cytokine release and apoptosis in human immature dendritic cells. This immunomodulator effect could be helping the immune evasion of A. fumigatus. However, the deletion of asp f 1/mitF/res did not affect fungal virulence in a neutropenic model of IA.257 The fungus also produces a hemolysin encoded by the aspHS gene. This molecule has hemolytic activity on rabbit and sheep erythrocytes, cytotoxic effects on macrophages and endothelial cells in vitro,135 and can be detected during infection in vivo304.
It is worth mentioning that in a recent study the levels of expression of certain of the genes discussed above (gliP, aspHS, asp f 1, and dmaW) were determined by real-time RT-PCR analysis, and higher expression was observed in vivo than in vitro.102 These results suggest an overexpression of these toxins during infection.
Other toxins produced by A. fumigatus are helvolic acid and fumagillin. Helvolic acid is part of a small family of steroidal antibiotics known as fusidanes. At high concentrations it can affect the oxidative burst of macrophages,181 the metabolism of low density lipoproteins254 and in vivo it induces ciliostasis and rupture of epithelial cells.10 On the other hand, fumagillin is an antitumor antibiotic that inhibits angiogenesis and in vitro directly inhibits endothelial cell proliferation and cilial movement in respiratory epithelium.45 The active concentrations of these toxins are considerably higher than those of gliotoxin, but it is still unknown in what concentrations are produced in vivo232. It has also been reported that fumitremorgin A302 fumitremorgin B165, and fumitremorgin C77, neurotropic toxins that cause tremors, seizures, and abnormal behavior in mice, are produced in a dose-dependent manner. Another toxin described to be produced by A. fumigatus that causes tremors is tryptoquivaline A.303 Further toxins, such as aflatoxin B1 and G1, and verruculogen, have been detected in culture filtrates of A. fumigatus, but their presence during infection has not yet been demonstrated.219 Other genes involved in the biosynthesis of these toxins have also been identified, such as ftmOx1, that encodes a non-heme Fe(II) α-ketoglutarate-dependent dioxygenase, which catalyses the endoperoxide formation of verruculogen in A. fumigatus.264 Fumitremorgin biosynthesis seems to be encoded by a cluster of nine genes, ftmA–I,171 and most of which have been described recently.106,107,125,171 A cluster of nine genes involved in helvolic acid biosynthesis has also been described.166,182 However, none of these genes have yet been tested for virulence.
The transcription factor leaA is a global regulator of secondary metabolite biosynthesis38 that modulates the expression of approximately a 10% of the genome of this fungus.220 The deletion of this gene in A. fumigatus blocked the production of almost all secondary metabolites, including gliotoxin,268 and a leaA mutant strain was hypovirulent after intranasal inoculation of neutropenic mice.36 These authors also showed that ΔlaeA mutants lost pigment production and their conidia were more susceptible than wild type A. fumigatus conidia to phagocytosis by macrophages.
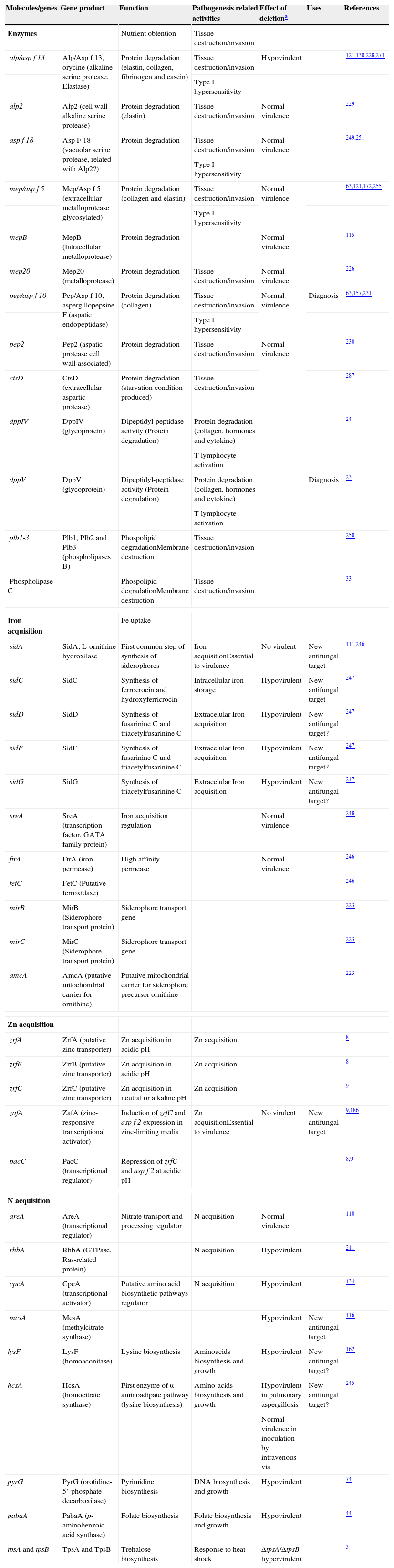
Nutrient uptake in invasive growthMammalian organisms present a broad variety of microenvironments in which A. fumigatus must survive to cause disease, and these environmental conditions can rapidly change depending on the current stage of infection.298 Normal nutrient uptake systems, used in their ecological niche, might serve the fungus during infection, but it is possible that other systems could be activated by environmental conditions. Table 5 shows the major molecules and genes related to virulence covered in this section.
Genes and molecules related with nutrient uptake in invasive growth
| Molecules/genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| Enzymes | Nutrient obtention | Tissue destruction/invasion | ||||
| alp/asp f 13 | Alp/Asp f 13, orycine (alkaline serine protease, Elastase) | Protein degradation (elastin, collagen, fibrinogen and casein) | Tissue destruction/invasion | Hypovirulent | 121,130,228,271 | |
| Type I hypersensitivity | ||||||
| alp2 | Alp2 (cell wall alkaline serine protease) | Protein degradation (elastin) | Tissue destruction/invasion | Normal virulence | 229 | |
| asp f 18 | Asp F 18 (vacuolar serine protease, related with Alp2?) | Protein degradation | Tissue destruction/invasion | Normal virulence | 249,251 | |
| Type I hypersensitivity | ||||||
| mep/asp f 5 | Mep/Asp f 5 (extracellular metalloprotease glycosylated) | Protein degradation (collagen and elastin) | Tissue destruction/invasion | Normal virulence | 63,121,172,255 | |
| Type I hypersensitivity | ||||||
| mepB | MepB (Intracellular metalloprotease) | Protein degradation | Normal virulence | 115 | ||
| mep20 | Mep20 (metalloprotease) | Protein degradation | Tissue destruction/invasion | Normal virulence | 226 | |
| pep/asp f 10 | Pep/Asp f 10, aspergillopepsine F (aspatic endopeptidase) | Protein degradation (collagen) | Tissue destruction/invasion | Normal virulence | Diagnosis | 63,157,231 |
| Type I hypersensitivity | ||||||
| pep2 | Pep2 (aspatic protease cell wall-associated) | Protein degradation | Tissue destruction/invasion | Normal virulence | 230 | |
| ctsD | CtsD (extracellular aspartic protease) | Protein degradation (starvation condition produced) | Tissue destruction/invasion | 287 | ||
| dppIV | DppIV (glycoprotein) | Dipeptidyl-peptidase activity (Protein degradation) | Protein degradation (collagen, hormones and cytokine) | 24 | ||
| T lymphocyte activation | ||||||
| dppV | DppV (glycoprotein) | Dipeptidyl-peptidase activity (Protein degradation) | Protein degradation (collagen, hormones and cytokine) | Diagnosis | 23 | |
| T lymphocyte activation | ||||||
| plb1-3 | Plb1, Plb2 and Plb3 (phospholipases B) | Phospolipid degradationMembrane destruction | Tissue destruction/invasion | 250 | ||
| Phospholipase C | Phospolipid degradationMembrane destruction | Tissue destruction/invasion | 33 | |||
| Iron acquisition | Fe uptake | |||||
| sidA | SidA, L-ornithine hydroxilase | First common step of synthesis of siderophores | Iron acquisitionEssential to virulence | No virulent | New antifungal target | 111,246 |
| sidC | SidC | Synthesis of ferrocrocin and hydroxyferricrocin | Intracellular iron storage | Hypovirulent | New antifungal target | 247 |
| sidD | SidD | Synthesis of fusarinine C and triacetylfusarinine C | Extracelular Iron acquisition | Hypovirulent | New antifungal target? | 247 |
| sidF | SidF | Synthesis of fusarinine C and triacetylfusarinine C | Extracelular Iron acquisition | Hypovirulent | New antifungal target? | 247 |
| sidG | SidG | Synthesis of triacetylfusarinine C | Extracelular Iron acquisition | Hypovirulent | New antifungal target? | 247 |
| sreA | SreA (transcription factor, GATA family protein) | Iron acquisition regulation | Normal virulence | 248 | ||
| ftrA | FtrA (iron permease) | High affinity permease | Normal virulence | 246 | ||
| fetC | FetC (Putative ferroxidase) | 246 | ||||
| mirB | MirB (Siderophore transport protein) | Siderophore transport gene | 223 | |||
| mirC | MirC (Siderophore transport protein) | Siderophore transport gene | 223 | |||
| amcA | AmcA (putative mitochondrial carrier for ornithine) | Putative mitochondrial carrier for siderophore precursor ornithine | 223 | |||
| Zn acquisition | ||||||
| zrfA | ZrfA (putative zinc transporter) | Zn acquisition in acidic pH | Zn acquisition | 8 | ||
| zrfB | ZrfB (putative zinc transporter) | Zn acquisition in acidic pH | Zn acquisition | 8 | ||
| zrfC | ZrfC (putative zinc transporter) | Zn acquisition in neutral or alkaline pH | Zn acquisition | 9 | ||
| zafA | ZafA (zinc-responsive transcriptional activator) | Induction of zrfC and asp f 2 expression in zinc-limiting media | Zn acquisitionEssential to virulence | No virulent | New antifungal target | 9,186 |
| pacC | PacC (transcriptional regulator) | Repression of zrfC and asp f 2 at acidic pH | 8,9 | |||
| N acquisition | ||||||
| areA | AreA (transcriptional regulator) | Nitrate transport and processing regulator | N acquisition | Normal virulence | 110 | |
| rhbA | RhbA (GTPase, Ras-related protein) | N acquisition | Hypovirulent | 211 | ||
| cpcA | CpcA (transcriptional activator) | Putative amino acid biosynthetic pathways regulator | N acquisition | Hypovirulent | 134 | |
| mcsA | McsA (methylcitrate synthase) | Hypovirulent | New antifungal target | 116 | ||
| lysF | LysF (homoaconitase) | Lysine biosynthesis | Aminoacids biosynthesis and growth | Hypovirulent | New antifungal target? | 162 |
| hcsA | HcsA (homocitrate synthase) | First enzyme of α-aminoadipate pathway (lysine biosynthesis) | Amino-acids biosynthesis and growth | Hypovirulent in pulmonary aspergillosis | New antifungal target? | 245 |
| Normal virulence in inoculation by intravenous via | ||||||
| pyrG | PyrG (orotidine-5’-phosphate decarboxilase) | Pyrimidine biosynthesis | DNA biosynthesis and growth | Hypovirulent | 74 | |
| pabaA | PabaA (p-aminobenzoic acid synthase) | Folate biosynthesis | Folate biosynthesis and growth | Hypovirulent | 44 | |
| tpsA and tpsB | TpsA and TpsB | Trehalose biosynthesis | Response to heat shock | ΔtpsA/ΔtpsB hypervirulent | 3 | |
A. fumigatus can obtain important nutrients from destruction of host tissue. A. fumigatus secretes extracellular enzymes, most of them proteases, that degrade and recycle organic matter in the environment, but during infection they could serve to break down the structural barriers of the host and to obtain nutrients. As indicated above, one of the host antimicrobial mechanisms is nutrient deprivation, and the amount of secreted hydrolases encoded on the genome201,239 may allow A. fumigatus to obtain nutrients from mammalian tissues without the need to activate the autophagic network.209 Several articles have reviewed these proteases and their relationship with pathogenicity.112,133,208,232 Some of these proteases can degrade collagen and elastin, which are the main components of the lung matrix. Various researchers have demonstrated a clear link between elastase activity of A. fumigatus strains and their invasiveness,35,130 so the fungus seems to be able to adapt to the host environment increasing elastase activity.95 However, other authors found no statistical correlation between the existence of elastase or acid proteinase activity and the development of invasive disease.5 These enzymes include serine alkaline protease (Alp) from the family of subtilisins, which can degrade elastin, collagen, fibrinogen, and casein,130,228 and corresponds to the allergen Asp f 13; Alp2, a serine protease that is associated with the cell wall;229 and a vacuolar serine protease, the allergen Asp f 18.251 The extracellular metalloprotease Mep can degrade collagen and elastin,172,255 and is also known as Asp f 5 allergen. Other metalloproteases have been identified in A. fumigatus such as that encoded by the mep20 gene226 or the intracellular metalloproteinase encoded by the mepB gene, which appears to be associated with the cytoplasmic degradation of small peptides.115 Another group of extracellular enzymes produced by A. fumigatus are aspartic proteases, also called aspergillopepsins. Two aspergillopepsins have been identified, a secreted aspergillopepsin (Pep)157 which matches the known Asp f 10 allergen, and another one associated to the cell wall (Pep2).230,231 A novel aspartic protease, CtsD, has been described in culture supernants.287 The expression of the ctsD gene was absent under nutrient-rich conditions, but it was detected, in vivo, in a Galleria mellonella infection model.287 In culture supernatants of A. fumigatus two members of dipeptidylpeptidases family (Dpp) have also been detected, DppIV and DppV, which cut at the amino-terminal end of peptides and proteins. These enzymes can bind to collagen, and even to hormones and cytokines, and degrade them. Their role in T cell activation has also been described.23,24
Finally A. fumigatus also secretes phospholipases, which break the ester bond of phosphoglycerides and thus may destabilize the host cell membranes causing cell lysis.232 Activity of phospholipases A–D has also been detected in culture filtrates of A. fumigatus.34 The genes plb1, plb2, and plb3 of A. fumigatus encode for B phospholipases, which are characterized by their phospholipase, lysophospholipase, and lysophospholipase transacylase activity.250 Two of them, Plb1 and Plb3, are known to be secreted.250 The genome of A. fumigatus codes for at least another three putative secreted phospholipases.208 Although these enzymes have been considered virulence factors for other species such as C. albicans or C. neoformans, in clinical isolates of A. fumigatus the production of B phospholipases is lower than in environmental isolates, making unlikely, if not excluding, their involvement in the virulence of the fungus. This could be explained by the secretion of other phospholipases by A. fumigatus, such as phospholipase C which has not been detected in other species and is produced in a higher proportion in clinical than environmental isolates.33 It should however be noted that while high phospholipase production was found to be associated with development of invasive aspergillosis, not all isolates that caused invasive diseases have displayed high phospholipase activity.5
Different proteases may play unique or overlapping roles during pathogenesis, and is difficult to obtain evidence of them as individual virulence factors.298 Only one mutant strain of A. fumigatus in a 33-kDa protein, coded for by the alp/asp f 13 gene and which has elastase activity, has produces lower rates of mortality when neutropenic mice were infected by intranasal inoculation.130 However none of these extracellular enzymes, metalloproteases,115,121 alkaline proteases121,184,271 or aspartic proteases231 have demonstrated a direct role in virulence, probably due to their redundancy. It is worth noting that there are at least 99 putative secreted proteases for the A. fumigatus genome.168,201
Recently, the biosynthesis of trehalose has been linked to virulence in pathogenic fungi. Trehalose is a non-reducing disaccharide the expression of which increases during the life cycle of A. fumigatus. Its concentration also increases after heat shock but not in response to other types of stress and in this fungus it is related with reduction in pathogenicity.3 In A. fumigatus the proteins involved in threhalose biosynthesis are encoded by two genes, tpsA and tpsB. The deletion of both genes showed conidia with delayed germination at 37°C and susceptibility to oxidative stress. The double mutation was required to block the trehalose accumulation, and this double mutant was hypervirulent in murine model of IA and was also associated with alterations in the cell wall and resistance to macrophage phagocytosis.3
The uptake of certain components is essential for most organisms and the ability to acquire these components in limiting environments, such as in the human host, is a necessary requirement for virulence of human pathogens. One of these limiting components in the human host is iron. A. fumigatus can acquire iron in two different ways, by reductive iron assimilation and by siderophore-assisted iron uptake, both of which are induced upon iron starvation246. The reductive mechanism for iron assimilation consists in the reduction of ferric to ferrous iron and the subsequent uptake of ferrous iron by the FtrA/FetC complex.246 Inactivation of the high affinity iron permease FtrA did not produce a reduction in virulence in a murine infection model, suggesting that virulence of A. fumigatus does not depend on reductive iron assimilation.246 By contrast, the inactivation of the sidA gene, which catalyses the first step of the biosynthesis of all known siderophores, namely the hydroxylation of L-ornithine,111 was found to be absolutely essential for virulence.111,246 Siderophores are low-molecular weight proteins (Mr<1500), that act as ferric iron-specific, high-affinity chelators.198A. fumigatus possesses at least four siderophores: fusaricine C and triacetylfusaricine C are excreted for iron acquisition; and ferricrocin and hydroxyferricrocin are used for intracellular iron storage.247 The study and deletion of the four genes needed for the biosynthesis of these two types of siderophores, sidC, sidD, sidF, and sidG revealed that the nonribosomal peptide synthetase, sidC, is involved in intracellular siderophore biosynthesis and that this type of siderophore is required for germ tube formation, asexual sporulation, resistance to oxidative stress, catalase A activity, and virulence.247 The strains with deletion of sidD and sidF genes, which are involved in biosynthesis of extracellular siderophores, were found to have attenuated virulence in animal infections and partial sensitivity to oxidative stress.247 The acquisition of iron is also regulated by the protein SreA, of the GATA family, but as this gene acts as a repressor under high iron conditions, its genetic inactivation results in overaccumulation of iron. Although the ΔsreA strain showed increased sensitivity to iron and oxidative stress, it did not demonstrate a role in virulence in a murine infection model.248 Certain other genes, including amcA, a putative mitochondrial carrier for the siderophore precursor ornithine, and the siderophore transport gene mirB, have shown to be upregulated during iron starvation conditions,223 but have not yet been studied for their role in virulence. As humans do not produce siderophores, most of these genes, and particulary sidA and sidC, could be good targets for new antifungal therapies.
Zinc is another essential element for fungal growth. The genome of A. fumigatus contains three putative zinc transporter-encoding genes (zrfA–C) whose expression is regulated by both pH and the environmental concentration of zinc.8,9 Two of these transporters, coded by genes zrfA and zrfB, are transcribed at higher levels and are required for fungal growth under acidic zinc-limiting conditions, while they are not required for growth in neutral or alkaline zinc-limiting media,286 the conditions found in lung tissues. It has recently been described that the zrfC gene encodes a transporter devoted to obtaining zinc from alkaline zinc-limiting media.9 This gene is adjacent to the asp f 2 gene, which encodes an allergen secreted by A. fumigatus. In alkaline and extreme zinc-limiting conditions, the transcriptional regulators ZafA and PacC induce the simultaneous transcription of zrfC and asp f 2 genes. Specifically, ZafA upregulates the expression of zrfC and Asp f 2 under zinc-limiting conditions regardless of the environmental pH, whereas PacC represses the expression of these genes under acidic growth conditions.9 The role in virulence of these transporters has not yet been studied. However, the deletion of the transcriptional regulator zafA gene impairs the germination and growth capacity of A. fumigatus in zinc-limiting media and the ΔzafA strain abrogated A. fumigatus virulence in a murine model of IA.186 The zafA gene may constitute a new target for the development of chemotherapeutic agents against Aspergillus, especially since no zafA orthologues have been found in mammals.186
Nitrogen metabolism has also been related to A. fumigatus virulence. Several sources of nitrogen may be used by A. fumigatus, such as nitrate or amino acids released during host tissue destruction or biosynthesized in their metabolism. The proteins that are involved in nitrate transport and processing are transcriptionally regulated by the areA gene.69 The study of an ΔareA mutant strain in a neutropenic model of IA showed similar virulence to the reference strain. However, this mutant strain presented a delayed-growth phenotype in the lung tissue.110 The expression of another gene, rhbA, was induced under nitrogen starvation conditions.210 This gene codes for a Ras-related protein and has been considered a virulence factor because ΔrhbA mutant strains displayed a significantly lower virulence in a murine infection model.211 Amino acids can be another source of nitrogen for microorganisms but not all amino acids are readily available in mammalian hosts during infections.298 The cpcA gene of the Cross-Pathway Control (CPC) system (also known as General Control of amino acid biosynthesis) is activated in amino acid-limiting conditions. It has been proposed that this system regulates the A. fumigatus amino acid biosynthetic pathways, and the deletion of this gene produced mutants with decreased virulence.134 The deletion of essential functional genes, such as lysF, which encodes a homoaconitase of lysine biosynthesis, produces mutants with decreased virulence in murine models of IA.162 The fungal α-aminoadipate pathway is also essential for lysine biosynthesis, and the first pathway specific enzyme, homocitrate synthase (HcsA), has recently been described.245 The hcsA deletion mutant was lysine auxotrophic, but although virulence of the mutant was strongly attenuated in murine models of bronchopulmonary aspergillosis, the mutant retained full virulence when injected intravenously.245 Therefore, inhibition of fungal lysine biosynthesis does not appear to provide a suitable target for new antifungals, at least not for disseminating invasive aspergillosis. The degradation of amino acids could be important in A. fumigatus pathogenesis, and during invasive growth the amino acid metabolism can produce propionyl-CoA accumulation, which is a toxic metabolite. The fungus metabolizes propionyl-CoA via the methylcitrate cycle.169 Recently the deletion of mcsA gene, which codes for the first enzyme of the methylcitrate cycle, a methylcitrate synthase, has been studied. This mutant strain displayed attenuated virulence in a murine model of IA, so that this activity does provide a suitable target for new antifungals.116
The genome of A. fumigatus contains some putative genes for the uptake of other essential elements such as magnesium or phosphate, but none of them have yet been studied for their role in virulence. That is the case, for example, of four putative inorganic phosphate transporters and six secreted acid phosphatases.208
Like other essential genes, strains with deletion of the pyrG gene that encodes an orotidine-5′-phosphate decarboxylase and catalyzes the last step of pyrimidine biosynthesis, has a reduced virulence and produced a low germination rate in murine models of IA.74 In the same way, mutant strains lacking the pabaA gene, that encodes for p-aminobenzoic acid synthase and is involved in folate biosynthesis, showed a severe reduction of virulence.44 In the case of these two latter genes, their involvement in virulence is attributed to a supposed low concentration of pyrimidine and p-amino benzoic acid in vivo.
Signaling, metabolic regulation and response to stress conditionsThe environmental conditions found by pathogenic fungi in the colonization and infection of the host are different to those found in their normal environmental niche. The signals must be detected and transmitted through mechanisms of gene regulation and metabolism, enabling the fungus to adapt to them. Several regulatory mechanisms have been studied in A. fumigatus including mitogen-activated protein kinase (MAPK) pathways, signal transduction pathways activated by G-proteins, Ras proteins, histidine kinases, calcium signaling, and a CPC system, among others (Table 6).
Molecules and genes involved in signaling, metabolic regulation and response to stress conditions
| Molecules/genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| MAP kinasebpathways | ||||||
| sakA/hogA | SakA/HogA (MAP kinases) | Stress regulation (osmotic, C and N starvation) | Response to stress | 300 | ||
| Regulation of conidium germination | ||||||
| mpkA | MpkA (MAP kinases) | Regulation of cell wall integrity signaling | Response to stress | |||
| Regulation of pyomelanin formation | Normal virulence | 283,284 | ||||
| mpkB | MpkB (MAP kinases) | Mating (putative pheromone) | Response to stress | 175 | ||
| mpkC | MpkC (MAP kinases) | Regulation of conidium germination | Response to stress | 233 | ||
| ste7 | Ste7 (MAPK kinases) | Mating | 175 | |||
| pbs2 | Pbs2 (MAPK kinases) | Osmotic regulation | 175 | |||
| mkk2 | Mkk2 (MAPK kinases) | Cell wall integrity | 175 | |||
| steC/ste11 | SteC/Ste11 (MAPKK kinases) | Mating | 175 | |||
| bck1 | Bck1 (MAPKK kinases) | Cell-wall integrity | 175 | |||
| ssk2 | Ssk2 (MAPKK kinases) | Osmotic regulation | 175 | |||
| sho1 | Sho1(adaptor protein) | HOG–MAPK pathwayc | Response to stress | Normal virulence | 167 | |
| G-proteins | ||||||
| gpaA | GpaA (G protein α subunit) | Regulation vegetative growth and conidium germination | 170 | |||
| sfaD | SfaD (G protein β subunit) | Regulation vegetative growth and conidium germination | 253 | |||
| Regulation metabolite production (gliotoxin, etc.) | ||||||
| gpgA | GpgA (G protein γ subunit) | Regulation vegetative growth and conidium germination | 253 | |||
| Regulation metabolite production (gliotoxin, etc.) | ||||||
| cAMP-PKA signaling | ||||||
| acyA | AcyA (adenylate cyclase) | cAMP signal transduction | 161 | |||
| gpaB | GpaB (G protein α subunit) | cAMP signal transduction, Stimulator of adenylate cyclase | Almost avirulent | 163 | ||
| pkaC1 | PkaC1 (cAMP-dependent PKA catalytic subunit) | cAMP signal transduction | Almost avirulent | 163 | ||
| pkaR | PkaR (PKA regulatory subunit ) | cAMP signal transduction | Hypovirulent | 310 | ||
| His kinases | Osmolarity stress response | 222 | ||||
| Dicarboximide fungicides resistance | ||||||
| Cell-wall assembly | ||||||
| fos1 | Fos1 (histidine kinase) | Stress response | Hypovirulent | 57,222 | ||
| tcsB | TcsB (histidine kinase) | |||||
| Other kinases (Cross-Pathways Control) | 79 | |||||
| cpcA/gcn4p | CpcA/Gcn4p (Transcriptional activator) | Putative amino acid biosynthetic pathways regulator | N acquisition | Hypovirulent | 134 | |
| cpcC/gcn2pC | CpcC/Gcn2p (eIF2a kinase) | Sensor kinase, in amino acid starvation, down-regulation of general translations | N adquisition | Normal virulence | 243 | |
| Derepress cpcA in nutritional stress conditions | Adaptation amino acid starvation | |||||
| Ca2+ signaling | ||||||
| calA/cnaA | CalA/CnaA (calcineurine catalytic subunit A) | Septum formation Conidiophore development | Stress response | Hypovirulent | Adjunct therapeutic target | 265,266 |
| crzA | CrzA (zinc finger transcription factor) | Ca2+–Mn2+-tolerance | Stress response | Hypovirulent | New antifungal target | 62,258 |
| gprC and gprD | GprC and GprD (putative G protein-coupled receptors) | Stress signals via modulation of the calcineurin pathway | Adaptation stress signaling | Hypovirulent | 99 | |
| Ras family | ||||||
| rasA | RasA (GTPase) | Hyphal growth and asexual development | 88 | |||
| Cell wall integrity | ||||||
| rasB | RasB (GTPase) | Germination and growth rates | Hypovirulent | 90 | ||
| rhbA | RhbA (GTPase, Ras-related protein) | N acquisition | Hypovirulent | 211 | ||
| ace2 | Ace2 (transcription factor) | Pigment production and conidiation | Hypervirulent | 82 | ||
| medA | MedA | Adherence | Host interactions (adherence to pulmonary epithelial cells, endothelial cells and fibronectin) | Hypovirulent | New antifungal target | 103 |
| Biofilm formation | ||||||
| srbA | SrbA (related with SREBPd, homolog to Sre1) | Ergosterol biosynthesis | Hypoxia adaptation | Hypovirulent | 299 | |
| Maintenance of cell polarity | Azole resistance | |||||
Fungi, like other eukaryotes, can regulate their cellular physiology in response to environmental changes via MAPK pathways. These environmental changes include conditions of stress (increased osmolarity, heat shock, high concentrations of heavy metals, and reactive oxygen species), nutrient limitation, disruption of cell wall integrity, and mating pheromones.176 For a better understanding of MAPK pathways in Aspergillus see the review of May.175 The MAPK pathways consist in three protein kinases that act subsequently by phosphorylation. The genome of A. fumigatus has four MAPK described genes, sakA/hogA, mpkA, mpkB, and mpkC, three putative MAPK kinases (MAPKK) and three MAPKK kinases (MAPKKK). The three MAPKKs are Ste7 like, Pbs2 like, and Mkk2 like, suggesting their possible roles in mating, osmotic regulation, and cell wall integrity, respectively. Similarly, the MAPKKKs are SteC/Ste11, Bck1, and Ssk2, with possible relations in mating, cell-wall integrity, and osmotic regulation, respectively.175 Of all of these genes, the sakA is the most intensively studied. This gene is necessary for the osmotic stress response, it negatively regulates conidial germination in response to less-preferred nitrogen sources; and is activated upon either carbon or nitrogen starvation during vegetative growth.300 On the other hand, the mpkA regulates cell wall integrity signaling and pyomelanin formation,284 and mpkC regulates conidial germination in response to the carbon source in the medium.233 The mpkA deletion has been carried out but no influence was observed on virulence of the mutant strain in a murine infection model,283 while the other genes have not been yet tested for virulence.
The high osmolarity–glycerol (HOG) MAPK (HOG–MAPK) signaling pathway plays an important role in regulating morphology, growth, and adaptation to stress and virulence in a number of fungal pathogens. The Sho1 adaptor protein is an important element of the two upstream branches of the HOG–MAPK pathway in S. cerevisiae. However, although the deletion of this gene in A. fumigatus produces a mutant sensitive to oxidative stress, it was still as virulent as the wild-type strain in an immunosuppressed mouse infection model.167
Many signal transduction pathways are activated by heterotrimeric G-proteins whose activation is frequently coupled to cell surface receptors. In fungi, G-proteins play integral roles in germination, vegetative growth, cell cycle control, mating, cell–cell fusion, morphogenesis, chemotaxis, pathogenicity, and secondary metabolism.253 The system consists of a membrane bound G-protein coupled receptor (GPCR), heterotrimeric G-protein α, β, γ subunits, and a diverse group of effectors. The G protein α subunit, GpaA, mediates signaling for vegetative growth and negative-regulation of conidiation in A. fumigatus,170 while the β subunit, SfaD, and γ subunit, GpgA, play crucial roles in proper control of vegetative growth, spore germination, asexual development and production of certain metabolites.253 The deletion of the sfaD and gpgA genes resulted in no or very low gliotoxin detection,253 suggesting a possible role of these proteins in gliotoxin biosynthesis.
The gpaB gene encodes a G protein α subunit involved in cAMP signal transduction that was found to be an upstream stimulator of adenylate cyclase, acyA. Deletion of these genes was studied and the mutant strains showed reduced conidiation, and also a slower growth rate in the ΔacyA mutant strain.161 The same effect was observed with the deletion of pkaC1 gene, which encodes the cAMP-dependent protein kinase A (PKA) catalytic subunit.163 The ΔgpaB and ΔpkaC1 strains were almost avirulent in an animal infection model of IA.163 The regulatory subunit of PKA is encoded by the pkaR gene. A ΔpkaR mutant had reduced growth and germination rates, increased susceptibility to oxidative stress, and reduced virulence in an immunosuppressed mouse model of IA.310 However, the reduced virulence of ΔpkaC1 and ΔpkaR observed in mice could be a general outcome of impaired growth.208 Recent studies have also related the cAMP–PKA signal transduction pathway with pigment formation105 and the nuclear duplication cycle.94 In fact, the sporulation and expression of the pksP/alb1 gene, which codes for the first enzyme of melanin production, is controlled by the cAMP signal transduction pathway, which includes a G protein α subunit, acenylate cyclase, and protein kinase A.42,161 Recently, two putative G protein-coupled receptors, GprC and GprD, have been characterized.99 Deletion of the corresponding genes resulted in drastic growth defects, including reduced hyphal extension, retarded germination and elevated levels of hyphae branching. Furthermore, compared with the wild type, the sensitivity of the mutant strains towards reactive oxygen intermediates was greater, and the mutants displayed attenuated virulence in a murine infection model. These authors concluded that the receptors are involved in integrating and processing stress signals via modulation of the calcineurin pathway.
Ras proteins are monomeric GTPases which act as molecular switches that transduce signals from the outside of the cell to signaling cascades inside the cell. In A. fumigatus, three of these proteins have been studied: RasA, RasB, and RhbA. The first, RasA, appears to have a crucial role in hyphal growth and asexual development, and its function is linked to cell wall integrity,88 while deletion of the A. fumigatus rasB gene caused decreased germination and growth rates as well as a diminished virulence in a mice infection model.90 The role of rhbA gene was discussed above in the nutrient uptake section.
In fungi, two-component histidine kinases are involved in response mechanisms to extracellular changes in osmolarity, resistance to dicarboximide fungicides, and cell-wall assembly.222 The A. fumigatus genome has at least 15 putative histidine kinase genes, of which only two have been studied, fos1 and tcsB.208 The Δfos1 mutant strain did not exhibit any detectable defects in either hyphal growth or morphology when grown on solid or liquid media222 but it had significantly lower virulence than the wild-type strain.57 The ΔtcsB mutant was similar to the wild type strain with regard to growth and morphology79 but its role in virulence has not been established.
Calcium signalling through the Ca2+-binding protein, calmodulin, and the Ca2+–calmodulin-dependent phosphatase, calcineurin, has been associated with a multitude of processes, including stress response, mating, budding, and actin-based processes66 as well astolerance to antifungal drugs.65,73,131,242,266,290 Notably, this pathway is highly conserved throughout the fungal kingdom.238 Calcineurin is a heterodimeric protein formed by a catalytic subunit A, and a calcium-dependent regulatory subunit B. Steinbach et al.265 demonstrated that the calA/cnaA gene, which codes for the calcineurin subunit A, is implicated in virulence. A ΔcnaA mutant strain exhibited decreased filamentation, morphological conidial defects and attenuation of pathogenicity compared to infection with the wild-type in several different animal models. In agreement with these results, Da Silva Ferreira et al.67 showed that the calA gene is not essential in A. fumigatus, but its deletion results in severe defects in branching and conidial architecture and limited growth. A recent study has also suggested that calcineurin is involved in septum formation and conidiophore development.122 Indeed, calcineurin may be an excellent target for adjuvant in combination with other cell wall inhibitors against A. fumigatus.266 A key target of calcineurin is the zinc finger transcription factor CrzA, a homologue of the S. cerevisiae transcription factor Crz1.258 The ΔcrzA mutant of A. fumigatus resulted in a strain with significant defects in conidial germination, polarized hyphal growth, cell wall structure, and asexual development62 and produced a significantly lower mortality rate in a neutropenic murine model of invasive pulmonary aspergillosis.62,258 Fortwendel et al.89 have obtained data suggesting that the Ras and calcineurin pathways act in parallel to regulate cell wall formation and hyphal growth, and additionally, that the calcineurin pathway elements cnaA and crzA play a major role in proper chitin and glucan incorporation into the A. fumigatus cell wall. Soriani et al.258 also demonstrated a role of crzA in the mediation of cellular tolerance to increased concentrations of calcium and manganese. Thus, crzA is an attractive fungus-specific antifungal target for the treatment of IA.62
A conserved signal transduction cascade linking environmental stress to amino acid homeostasis is the CPC system that acts via phosphorylation of the translation initiation factor eIF2 by a sensor kinase. As noted before, the cpcA gene encodes the transcriptional activator of the CPC-system of amino acid biosynthesis and ΔcpcA strains displayed attenuated virulence in a murine model of IA.134 On the other hand, the cpcC gene encodes the CPC eIF2a kinase. The ΔcpcC deletion mutant showed increased sensitivity towards amino acid starvation but it was not impaired in virulence in a murine model of pulmonary aspergillosis.243
The transcription factor Ace2 influences virulence in other fungi. A. fumigatus contains an ortholog of this gene, ace2, which governs pigment production, conidiation, and virulence82. Mice immunosuppressed with cortisone acetate and infected with the Δace2 mutant showed accelerated mortality, greater pulmonary fungal burden, and increased pulmonary inflammatory responses than mice infected with wild type strain. This hypervirulence of the Δace2 strain was related to reduced expression of ppoC, ecm33, and ags3 detected in this mutant. It is known that A. fumigatus mutants with null or reduced expression of these genes have increased virulence in mice.
MedA is a development regulated protein that governs adherence, host interactions, and virulence in A. fumigatus.103 These authors studied a ΔmedA strain and demonstrated a dramatic reduced conidiation, and impaired biofilm production and adherence to plastic, as well as adherence to pulmonary epithelial cells, endothelial cells, and fibronectin in vitro. This mutant also exhibited reduced virulence in both invertebrate and mammalian models of IA. These results suggest that MedA downstream targets mediate virulence and might provide novel therapeutic targets for IA.
The presence of A. fumigatus causes significant inflammation in the sites of infection. It is known that levels of oxygen are significantly lower at sites of inflammation.298 Accordingly, during infection, A. fumigatus may be exposed to rapid changes in oxygen concentration, even reaching extremely low levels, depending upon the tissue infected and current immune response. The mechanisms of hypoxic adaptation of the aerobic A. fumigatus are currently unknown. Willger et al.298,299 have hypothesized that a putative Sre1 homolog in A. fumigatus (SrbA), related to the sterol regulatory element-binding proteins (SREBPs), could also act as an indirect sensor of oxygen levels and could regulate the transcription of genes required for adaptation to hypoxic environments. These authors have demonstrated that the srbA gene plays a critical role in ergosterol biosynthesis, azole resistance, and the maintenance of cell polarity in A. fumigatus.299 The ΔsrbA strain was almost avirulent in mouse models of IA, and loss of this gene, affects the expression of 87 genes related sterol biosynthesis and hyphal morphology, as demonstrated by expression analysis using DNA microarrays.299 Hypoxia adaptation is likely an important virulence attribute of pathogenic molds.
AllergensA. fumigatus produces a significant number of allergenic molecules which show reactions with IgE in asthmatic patients and patients with allergic bronchopulmonary aspergillosis (ABPA). Data concerning all known A. fumigatus allergens are collected by “Allergome, a platform for allergen knowledge”1 and the Allergen nomenclature website,2 and are summarized in Table 7 of this review. Only 23 molecules currently hold an official name of allergen, and have names in the range Asp f 1–Asp f 34. One of these, Asp f 15, has been proposed to be removed from the list due to it having been demonstrated that it is identical to Asp f 13, and the Asp f 6 allergen has shown a high degree of homology with Asp f 9.64 On the other hand, there are three candidates to be considered as allergens, Asp f 56kDa (a protease), Asp f AfCalAp, and Asp f GST (related to glutathione-S-transferase). However, the sequence of Asp f 56kDa is not predicted to be encoded in any of the sequenced Aspergillus genomes.64 Some of these allergens have known structural, toxic or enzymatic functions, and their relationship with virulence has been discussed in previous sections of this review. However, other allergenic components do not have virulence activities except as allergens. All Aspergillus allergens reacted with IgE in asthmatic patients and with ABPA.129 Some A. fumigatus allergens showed cross-reactivity with various conserved proteins including some human proteins. Among these, Asp f 6 (Mn-Sod), Asp f 8 (P2 acidic ribosomal protein), Asp f 11 and Asp f 27 (cyclophilins), and Asp f 28 and Asp f 29 (thioredoxins) have been shown to belong to families of cross-reactive pan-allergens.64 This fact could imply autoimmunity problems in human patients.
Allergens of A. fumigatus related with activation of Type I hypersensitivity
| Genes | Gene product | Function | Pathogenesis related activities | Effect of deletiona | Uses | References |
| asp f 1/mitF/res | Restrictocin, mitogillin | Ribotoxin | Protein biosynthesis inhibition | Normal virulence | Diagnosis | 12,224,257 |
| Cytotoxin | ||||||
| Apoptosis | ||||||
| Type I hypersensitivity | ||||||
| asp f 2 | Asp f 2 | Fibrinogen binding protein | Adhesion | Diagnosis | 9,18 | |
| Zn metabolisms? | Type I hypersensitivity | |||||
| asp f 3 | Asp f 3, peroxisomal protein (PMP, redoxin) | Peroxisomal membrane Protein | Type I hypersensitivity | Diagnosis | 109 | |
| asp f 4 | Type I hypersensitivity | Diagnosis | 64,140 | |||
| Confirm ABPA | ||||||
| mep/asp f 5 | Mep/Asp f 5 (extracellular metalloprotease glycosylated) | Protein degradation (collagen and elastin) | Tissue destruction/invasion | 63,121,172,255 | ||
| Type I hypersensitivity | ||||||
| sod3/asp f 6 | Sod3/Asp f 6 (cytoplasmic Mn superoxide dismutase, Cross-reactive pan-allergen) | O2− degradation | ROS protection | Diagnosis | 63,64,147 | |
| Type I hypersensitivity | Confirm ABPAb | |||||
| Autoimmunity | ||||||
| Antigen | ||||||
| asp f 7 | Asp f 7 | Type I hypersensitivity | Diagnosis | 63 | ||
| asp f 8 | Asp f 8, 60S acidic ribosomal protein P2 (cross-reactive pan-allergen) | Protein synthesis (elongation step) | Type I hypersensitivity | Diagnosis | 141 | |
| Autoimmunity | ||||||
| asp f 9/crf1 | Asp f 9/Crf1 (cell wall glucanase) | Cell wall assembly | Type I hypersensitivity | Diagnosis | 63 | |
| pep/asp f 10 | Pep/Asp f 10, aspergillopepsine F (aspatic endopeptidase) | Protein degradation (collagen) | Tissue destruction/invasion | Diagnosis | 63,157,231 | |
| Type I hypersensitivity | ||||||
| asp f 11 | Asp f 11 (peptidyl-prolyl cis-trans isomerase, cyclophilin, cross-reactive pan-allergen) | Peptide synthesis | Type I hypersensitivity | Diagnosis | 87 | |
| Chaperone and cell signaling function | Autoimmunity | |||||
| hsp1/asp f 12 | Hsp1/Asp f 2 (heat shock protein, Hsp90 family) | Chaperone | Chaperone activity and protein transport in growth at 37°C | 136 | ||
| Stress response during inflammation | ||||||
| Autoimmunity | ||||||
| Type I hypersensitivity | ||||||
| alp/asp f 13 | Alp/Asp f 13, orycine (alkaline serine protease, Elastase) | Protein degradation (elastin, collagen, fibrinogen and casein) | Tissue destruction/invasion | Hypovirulent | 121,130,228,271 | |
| Type I hypersensitivity | ||||||
| asp f15 | Asp f 15, (serine protease, homolog Asp f 13) | Tissue destruction/invasion | Diagnosis | 64 | ||
| Type I hypersensitivity | ||||||
| asp f 16 | Asp f 16 | Putative glycosylhidrolase | Type I hypersensitivity | 20 | ||
| mp1/asp f 17 | Mp1/Asp f 17 (relation with Afmp1?) | Cell wall galactomannoprotein | Adhesion | Diagnosis | 64 | |
| Type I hypersensitivity | ||||||
| asp f 18 | Asp f 18 (vacuolar serine protease, related with Alp2?) | Protein degradation | Tissue destruction/invasion | Normal virulence | 249,251 | |
| Type I hypersensitivity | ||||||
| asp f 22 | Asp f 22, Enolase | General metabolism | Type I hypersensitivity | 144 | ||
| asp f 23 | Asp f 23, 60S ribosomal protein L3 | Protein synthesis | Type I hypersensitivity | |||
| asp f 27 | Asp f 27 (peptidyl-prolyl cis-trans isomerase, cyclophilin, cross-reactive pan-allergen) | Peptide synthesis | Type I hypersensitivity | Diagnosis | 101 | |
| Chaperone and cell signaling function | Autoimmunity | |||||
| asp f 28 | Asp f 28 (thioredoxin, cross-reactive pan-allergen) | Protein disulfide oxidoreductases | Type I hypersensitivity | Diagnosis | ||
| Autoimmunity | ||||||
| asp f 29 | Asp f 29 (thioredoxin, cross-reactive pan-allergen) | Protein disulfide oxidoreductases | Type I hypersensitivity | Diagnosis | ||
| Autoimmunity | ||||||
| asp f 34 | Asp f 34, PhiA | Cell wall protein | Type I hypersensitivity | Diagnosis | 100 | |
| Asp f 56kD | Protease | Type I hypersensitivity | 202 | |||
| Asp f AfCalAp | Type I hypersensitivity | 282 | ||||
| Asp f GST (glutathione S-transferases) | Detoxification with glutathione | Type I hypersensitivity |
Allergenic behaviour of the aforementioned molecules, due to their presence on conidia, their release by the destruction of the conidia by pulmonary phagocytes, or their production during the growth of fungus is unclear in IA. We were able to identify two different situations, namely, the infections caused by Aspergillus in immunocompetent or immunocompromised patients. In immunocompetent patients Aspergillus can produce several hypersensitivity diseases due to these allergens, such as ABPA, allergic rhinosinusitis, asthma, and aspergilloma. Inhalation of fungal spores, often considered the traditional route of exposure, has been associated with the induction or exacerbation of these respiratory diseases. Large numbers of inhaled fungal spores are removed from the lungs prior to germination,139 but a few conidia could escape phagocytosis and may begin to germinate. Dormant or nonviable A. fumigatus conidia uptake is associated with IFN-γ production and Th1 responses, while hyphae or swollen (germinating) conidia induce IL-4 production and eosinophil recruitment, a hallmark of allergic inflammation and Th2 responses.39,273 Therefore, successful germination is likely to contribute to the development of fungal allergy. Specific structures, factors secreted by fungi or released by killed conidia, can play an important role in allergic sensitization, but the environmental and patient-specific factors (such as the personal history of previous contact in early life immune development) are also critical to acquire tolerance or allergic sensitization in immunocompetent individuals. All Aspergillus allergens appear to activate a Type I hypersensitivity response in sensitized patients with production of high affinity IgG and IgE antibodies.232 In immunocompromised patients with debilitated innate immune responses, these allergenic compounds can increase the risk associated with aspergillosis because they may redirect the immune response to the fungus by the activation of Th2 lymphocytes, a response that does not seem to be efficient in eliminating this fungus.108 Some of these allergens have been studied for their usefulness for diagnosis (see Allergome1 and Table 7).
Gene expression assaysDuring the last years only a few studies have investigated A. fumigatus gene expression during infection. Zhang et al.308 analysed the expression of certain virulence factors in vivo and in vitro concluding that in vitro measurements of transcription compared to transcription in infected lung tissue demonstrated low levels of fos-1 and rhbA genes, and 20–40-fold increases in cpcA, lysF, and pabA genes, while the pksP gene was only detected in vivo. Gravelat et al.102 performed real-time reverse transcription-PCR analysis on lung samples from mice with invasive pulmonary aspergillosis to determine the expression of A. fumigatus genes that are expressed at specific stages of development. This study revealed that in established infections, A. fumigatus exhibited mRNA expression of specific genes to develop competent hyphae, such as stuA. The acquisition of competence is referred to the shift of hyphae from a state in which they cannot undergo asexual reproduction to one in which they can. In contrast, mRNA of genes expressed specifically by conidia and precompetent hyphae was not detected. Many genes required for mycotoxin synthesis, including aspHS, gliP, mitF, and metAP, were expressed at significantly higher levels during invasive infection than in vitro. On the other hand, the expression of gliP mRNA in vitro was found to be highly dependent on culture conditions. Furthermore, this expression was found to be dependent on the transcription factor StuA both in vitro and in vivo. These results highlight the importance of the evaluation of putative virulence factors expressed by competent hyphae and the analysis of gene expression levels during invasive infection rather than in vitro alone.
Gene expression assays have also been developed to analyse the function of various proteins, comparing the gene expression profiles of the mutant against those of the reference strain. For example, Soriani et al.259 searched the metabolic pathways influenced by A. fumigatus transcription factor AfCrzA after a short pulse of calcium, by determining the transcriptional profile of A. fumigatus wild type in comparison to ΔafcrzA mutant strains. Similarly, Twumasi-Boateng et al.281 described, on the basis of transcriptional profile studies, the role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in A. fumigatus. Using transcriptomic analysis, other authors have investigated the exit from dormancy of A. fumigatus conidia146 and the genes differentially expressed in conidia and hyphae of this fungus upon exposure to human neutrophils.267 Gene expression assays with DNA microarrays are also being used to study the adaptation of A. fumigatus to different stress conditions such as hypoxia,299 heat shock,201 and antifungals activities such as voriconazole68. Finally, DNA microarray-based studies have also been used for the detection and identification of fungal pathogens, including A. fumigatus.49,260
ConclusionsA. fumigatus is an opportunistic pathogen whose ability to produce disease is inextricably linked to the host immune response. The most recent progress in research has revealed how components of the immune system are able to eliminate the fungus and that the weakness of immune system has a role in the development of aspergillosis. Likewise, some of the mechanisms that the fungus uses to evade immune responses, to obtain nutrients and to cause damage to the host and thus generate an IA, have been identified. If we consider only the classical definitions of virulence factors, i.e., a component of a pathogen that allows it to cause disease, we would probably have difficulties in deciding what is or is not a virulence factor in human fungal pathogenesis. In fact, that would exclude, for example, normal or adaptive mechanisms of the fungi to grow in different environmental niches, which are extensively used during the colonization of a human host. In A. fumigatus a great variability of “non-classical” virulence factors have been described, associated with its structure, its capacity to grow and adapt to stress conditions, its mechanisms for evading the immune system and its ability to cause host damage. As detailed in this review, a large number of genes and molecules have been identified and investigated in some depth as potential virulence factors. However, none of them have proven to be sufficiently important to fully explain the virulence of A. fumigatus. In most cases, the experiments based on the loss of gene/function by mutation have shown only small declines in virulence, unless the genes involved regulation of multiple activities of fungal adaptation and growth were eliminated. The pleiotropic effect of certain genes, the function of various genes associated with the virulence in the normal growth of A. fumigatus, and the redundancy due to the existance of several genes with the same activity, complicate the process of studying virulence factors of A. fumigatus with mutant strains. On the other hand, virulence studies use animal models with high levels of immunosuppression, which can also lead to failure to detect the effect on the virulence of the mutant strains. Likewise, the animal immunosuppression used, focusing mainly on causing neutropenia, only simulates the situation in neutropenic patients without providing any data for the other types of patients with aspergillosis. From all this data, the idea has emerged that the pathogenesis of diseases caused by this fungus in immunocompromised patients is very complex. As shown in Fig. 3, we could imagine a complex puzzle, the pieces of which would be virulence factors or the different activities of the fungus, and our task would then be to complete this puzzle to obtain a comprehensive vision of the virulence of A. fumigatus. We begin to understand the intricacies of its metabolism but much remains to be learned concerning the activity of this fungus in vivo. Furthermore, understanding changes in the host microenvironment, including hypoxia, pH, available nutrients, and immune responses, and how these signals are processed by the fungus, could be useful to determine the efficacy and effectiveness of particular antimicrobial strategies. The data so far have helped to improve diagnosis and identify new targets for antifungal development, which in combination with currently available therapies can improve the prognosis for IA patients. Expression studies using DNA microarrays of A. fumigatus during invasion or interaction with immune responses may help to provide a more rapid and profound understanding of the virulence capabilities of this fungus, as well as their adaptation mechanisms based on networks of complex metabolic and genetic regulation systems, in order to find new possible targets for detection and treatment of the disease. In particular, these expression studies using DNA microarrays are being applied to different stress conditions such as heat shock and antifungal activity.
This work was supported by General Grant to Research Groups (GIU08/20) from the UPV/EHU, and SAIOTEK Program Grant (S-PC09UN04) and Consolidated Research Group Grant (IT343-10) from the Basque Government. Jimena Victoria Fernandez Molina was supported with a “Beca de Investigación Predoctoral” from the UPV/EHU.