Sporotrichosis is a fungal infection caused by the Sporothrix schenckii complex. The adhesion of the fungus to the host tissue has been considered the key step in the colonization and invasion, but little is known about the early events in the host–parasite interaction.
AimsTo evaluate the proteolytic activity of S. schenckii on epithelial cells.
MethodsThe proteolytic system (at pH 5 and 7) was evaluated using azocoll and zymograms. The host–parasite interaction and epithelial cell response were also analyzed by examining the microfilament cytoskeleton using phalloidin-FITC and transmission electron microscopy. Finally, the metabolic activity was determined using an XTT assay.
ResultsThe zymograms showed that S. schenckii yeast cells possess high intracellular and extracellular proteolytic activities (Mr≥200, 116, 97, and 70kDa) that are pH dependent and are inhibited by PMSF and E64, which act on serine and cysteine-type proteases. During the epithelial cell–protease interaction, the cells showed alterations in the microfilament distribution, as well as in the plasma membrane structure. Moreover, the metabolic activity of the epithelial cells decreased 60% without a protease inhibitor.
ConclusionsOur data demonstrate the complexity of the cellular responses during the infection process. This process is somehow counteracted by the action of proteases inhibitors. Furthermore, the results provide critical information for understanding the nature of host–fungus interactions and for searching a new effective antifungal therapy, which includes protease inhibitors.
La esporotricosis es una infección fúngica causada por el complejo Sporothrix schenckii. La adhesión del hongo al tejido hospedero se ha considerado un paso clave en la colonización e invasión, sin embargo poco se conoce de los eventos tempranos en la interacción hospedero-parasito.
ObjetivosEvaluar la actividad proteolítica de S. schenckii en células epiteliales.
MétodosEl sistema proteolítico (bajo los valores pH 5 y 7) fue evaluado mediante azocoll y zimogramas. Además, la interacción hospedero-parasito y la respuesta celular fueron analizadas con el examen de los microfilamentos del citoesqueleto mediante faloidina-FITC y microscopia electrónica de transmisión. Finalmente, la actividad metabólica (viabilidad celular) fue determinada por un ensayo de XTT.
ResultadosLos zimogramas de S. schenckii muestran que posee una alta actividad proteolítica intracelular y extracelular (Mr≥200, 116, 97 y 70kD) dependientes de pH e inhibidas por PMSF y E64, que actúan sobre serin- y cistein proteasas. Durante la interacción de las células epiteliales-proteasas, las células mostraron alteraciones en la distribución de los microfilamentos y la estructura de la membrana plasmática. Además, la actividad metabólica (viabilidad celular) de las células epiteliales disminuyó un 60% sin inhibidores de proteasas.
ConclusionesNuestros datos demuestran la complejidad de la respuesta celular durante el proceso de infección, proceso que puede ser en parte contrarrestado por la acción de los inhibidores de proteasas. Además, los resultados proporcionan información crítica para el entendimiento de la naturaleza en la interacción hospedero-hongo y para una nueva terapia antifúngica eficaz que incluya inhibidores de proteasas.
Sporothrix schenckii is a dimorphic and ubiquitous ascomycetous fungus found worldwide in soil and decomposing vegetable materials.3S. schenckii causes human infection by inhalation or after traumatic subcutaneous implantation, allowing hyphal fragments, yeast and conidial forms to penetrate the skin.7,10,12 The clinical manifestations of S. schenckii infections are collectively termed sporotrichosis. The most common manifestation is a chronic infection of the skin and subcutaneous tissue with lymphatic complications. Moreover, the systemic form, associated with immunocompromised patients, affects bones, lungs and the central nervous system.17
Cell adherence is one of the most important events during the early invasion of a host. In fungal pathogens, this process involves mannoproteins of the cell wall9,23 and the peptido-rhamnomannan, the major component of the S. schenckii cell wall.16 Moreover, a large number of adhesins have been described in different parasites, most of which are glycoproteins involved in the specific adhesion of microorganisms and are considered to be virulence factors.1,30 Therefore, extracellular proteases of pathogenic fungi play an important role in cell invasion and growth.2,14,19,20,31 Extracellular proteases I and II, both hydrolyzing natural substrates (e.g., type I and IV collagens, elastin, stratum corneum), have been isolated from S. schenckii, suggesting an important role in the cell invasion and growth.1,21,31–33
Pathogenic microorganisms utilize a variety of molecular strategies that subvert host cell mechanisms, thereby enabling the pathogens to invade susceptible host cells.13 The cytoskeleton is vital for the formation and maintenance of a functional physical barrier between the cell and its surroundings, and some fungal pathogens induce a host actin-based cytoskeleton reorganization, which directs membrane engulfment of the pathogen.23,28 However, the alteration of the actin cytoskeleton following adherence to the cell surface has not yet been investigated in the S. schenckii–epithelial cell interaction. We recently reported alterations in epithelial cells (i.e., blebs on the surface) during the adhesion of pathogens, suggesting the participation of proteases.25 We analyze now the proteolytic effect of S. schenckii on the host cells using an in vitro model of epithelial cells; cytopathological effects, including alterations in the epithelial cell actin cytoskeleton, were observed.
Materials and methodsStrain and growth conditionsS. schenckii sensu strictu, strain MP102 was used in this study (the strain was kindly provided by PhD. Haydee Torres Guerrero, Universidad Nacional Autónoma de México). In order to obtain the yeast form, 1×106conidiaml−1 were inoculated in YPG medium [0.3% (w/v) yeast extract, 1% (w/v) peptone, 2% (w/v) dextrose], pH 7.2, and incubated at 37°C, for 3 days with constant shaking.24
Hydrolysis of azocollThe fungus culture was centrifuged at 10,000×g and 4°C; after 10min, the supernatant and pellet were collected. Proteolytic activity was measured in a 50μg sample of fungi (supernatant or pellet) or with collagenase type IV (Sigma Chemical) used as a control. Reaction mix, Tris 10mM, CaCl2 1mM, NaCl 25mM, pH 8.0, and 3mg/ml of Azocoll (Sigma A-9404) as substrate, were incubated for 2h at 37°C. The released azo group was measured at 540nm.2,6,22,31 The results were expressed as the amount of enzyme which released a μmol of azo group per minute, using the extinction coefficient ¿=98/M/cm. The results represent the average of three independent experiments.
Zymogram analysisExtracellular and intracellular protease activity of the isolate (50μg), as well as a control treated with a protease inhibitor (Roche Cocktail, used for the inhibition of serine, cysteine and metalloproteases) were analyzed. SDS polyacrylamide gels were made at 10% containing copolymerized gelatin 0.2%.6 After electrophoresis (125 Volts for 2h), the gels were washed with Triton X-100 at 2.5% for 1h to eliminate the SDS. Subsequently, the gels were incubated with 50mM citric acid, 100mM disodium phosphate pH 5 or with a Tris–Cl buffer 50mM, 1mM CaCl2 pH 7 for 2h. Gels were immersed in a solution of Tris–Cl 10mM pH 7.5, β-mercaptoethanol at 0.1% for 15min at 37°C.15 The bands were visualized by staining with Coomassie blue for at least 3h and destaining in 10% (v/v) acetic acid. The molecular weights of the proteases were determined by comparing with protein standards (Sigma–Aldrich, St. Louis, MO).
Culture of epithelial cellsThe epithelial cell line L929 (ATCC CCL-1) was used for this study. 2×106cells/ml were grown on sterile coverslips previously placed on six well cell culture plates (Corning, N.Y.USA) with D-MEM medium (GIBCO, Grand Island, NY. USA), enriched with 10% (v/v) decomplemented fetal bovine serum (GIBCO). The culture cells were incubated at 37°C in constant humidity with 5% CO2 for 36h until cell culture confluence.
S. schenckii yeasts–epithelial cells interaction assayThe yeasts (1×104/ml) were added to the epithelial monolayer. After 3, 12 and 24h of interaction the samples were fixed with 4% (w/v) formaldehyde (Polysciences, USA) for 30min at room temperature. Subsequently, the supernatant was withdrawn to eliminate the unadhered cells and the samples were fixed again for 15min. As a control for the experiment, epithelial cultures without the yeast isolate were included. After the interaction, the adhesion percentage was determined at 3, 12 and 24h.8
Inhibition of the extracellular proteases activityEpithelial cells in monolayer were grown on coverslips (described above) and incubated with extracellular proteases of S. schenckii at 37°C in a 5% CO2 atmosphere. The interaction was carried out for 45 and 90min. Additionally, an assay to inhibit the already mentioned enzyme activity of S. schenckii over actin cytoskeleton was performed with PMSF (1mM, Sigma) or E64 (1μmol/l, Calbiochem) at 90min of incubation. The controls consisted of untreated host cells. After the interaction, actin cytoskeleton from epithelial cells was analyzed.
Microfilaments and nuclei stainingThe preparations were fixed with 4% (w/v) formaldehyde (Polysciences, USA), for 15min at room temperature and permeabilized with 0.5% (v/v) Triton X-100 for 3min. Actin filaments were stained with phalloidin-FITC (Sigma, St. Louis, MO, USA; 1:100 dil) for 20min at room temperature. The samples were mounted on coverslips using VECTASHIELD – DAPI (4′,6-diamidino-2-phenylindole), a fluorescent stain that binds strongly to A-T rich regions in DNA (Vector Laboratories Inc., Burlingame, CA) for analysis with a fluorescence microscope (Nikon HFX-II, Japan) equipped with a UV filter (Exc=400–420nm).
Metabolic activity determinationThe XTT assay is a colorimetric assay that measures cellular metabolic activity. During the assay, the yellow tetrazolium salt XTT (2,3-bis-[2-methoxy-4-nitro-5-sylfophenyl]-2H-tetrazolium-5-carboxanilide, disodium salt) is reduced to a highly colored formazan dye by dehydrogenase enzymes (mitochondria) in metabolically active cells.27 The cells (2×106/ml) were placed on 96 well cell culture plates (Corning, N.Y.,USA) and incubated with extracellular proteases from S. schenckii at 37°C in a 5% CO2 atmosphere for 90min with and without proteases inhibitors: PMSF (1mM) or E64 (1μmol/l). Later, 100μl of XTT solution were added (0.25mg/ml in 0.1mM menadione, Sigma) to each well and the plate was returned to the incubator for 90min. The formazan dye formed in the assay was quantified by measuring the absorbance at wavelength 450nm in a spectrophotometer (Epoch Biotek).
Transmission electron microscopy (TEM)At 3h and 12h the samples of yeast adhesion to epithelial cells were fixed with 2.5% glutaraldehyde (Electron Microscopy Sciences), 4% paraformaldehyde (Polyscience) and 10% calcium chloride in 100mM cacodylate buffer, pH 7.4, for 1h at 25°C, washed in cacodylate buffer, and then postfixed with 1% OsO4 in 100mM cacodylate buffer for 1h. Samples were then washed in cacodylate buffer, dehydrated in a graded series of ethanol and embedded in Epon resin (Electron Microscopy Sciences). Ultrathin sections were obtained, stained with uranyl acetate and lead citrate, and examined in a Carl Zeiss 1010 Transmission Electron Microscope.
Statistical analysisThe experiments of the interaction S. schenckii–epithelial cells and those of metabolic activity were performed with 9 samples, and data are expressed as median. Statistical analysis was performed using Kruskal–Wallis nonparametric test to determine significant differences between treatments (p<0.05), and Dunn's post-test was used for multiple comparisons. Minitab 17.0.1 software was used to perform data analysis.
The results of percentage of fungus adherence at 3, 12 and 24h of interaction were expressed as values of the median (n=9) of yeast adhesion to epithelial cells at different time interaction. The metabolic activity was expressed as the median (n=9) of untreated cells (control) in the presence of proteases of S. schenckii, without and with protease inhibitors (E-64 1μmol/l and PMSF 1mM).
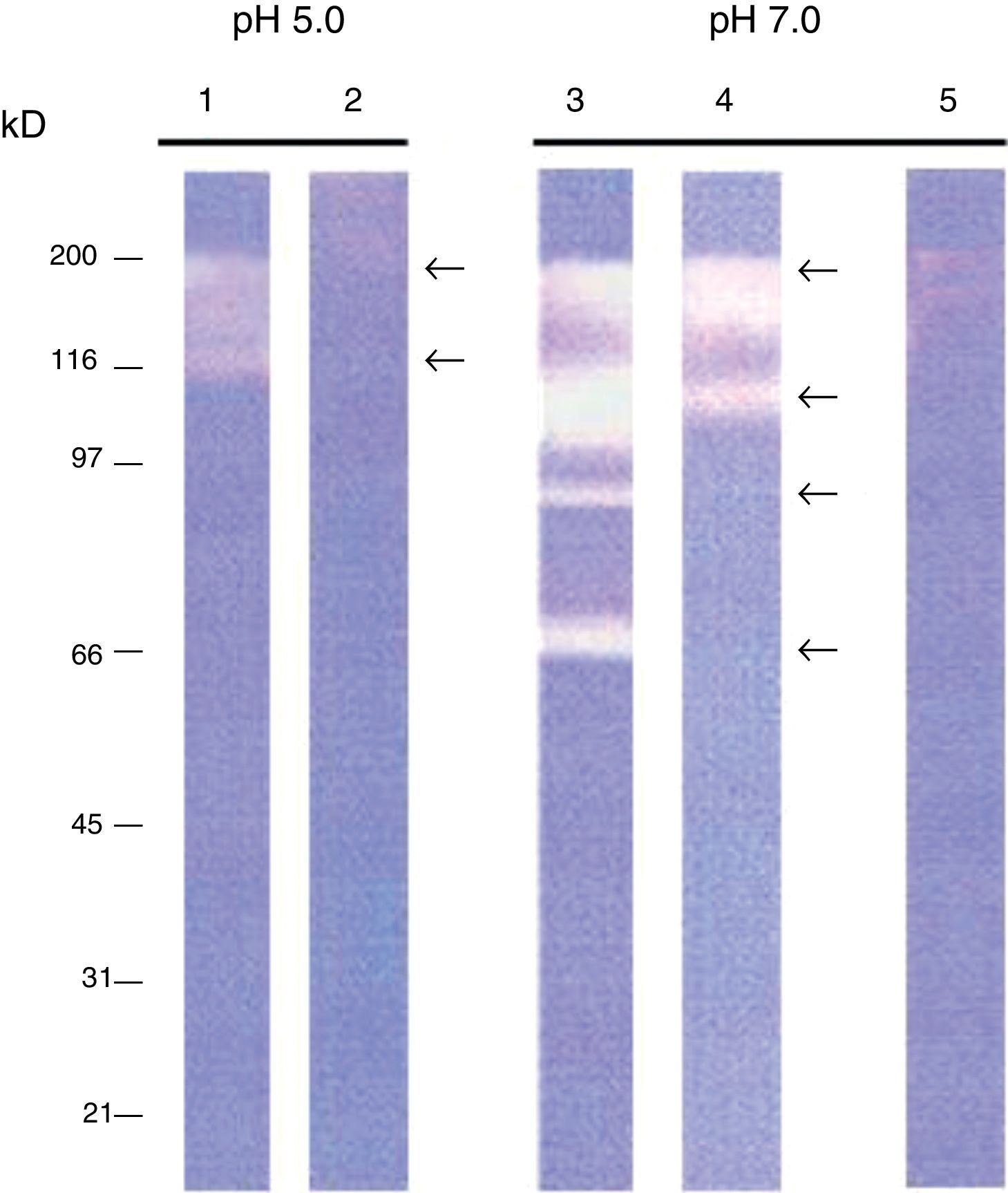
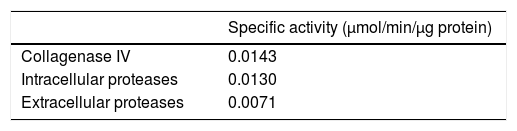
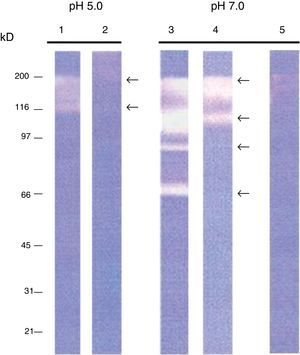
ResultsIntracellular and extracellular proteases of S. schenckiiAzocoll assays and zymograms were performed to evaluate the proteolytic activity of S. schenckii (Table 1 and Fig. 1). The amount of the free azo group revealed a higher intracellular proteolytic activity of the yeast cells in comparison to the extracellular activity (0.0130 vs. 0.0071μmol/min/μg protein). Collagenase type IV was used as a control (Table 1). The zymogram revealed both intracellular and extracellular proteolytic activities. At an acidic pH of 5 (Fig. 1), the yeast revealed an intracellular proteolytic activity associated with bands of Mr≤200 and 116kDa (lane 1), and unrevealed extracellular proteolytic activity (lane 2). In contrast, at a pH of 7, the yeast cells showed a higher intracellular activity (lane 3) associated with bands at Mr≤200, 116, 97 and 70kDa. Some of these bands (Mr≤200 and 116kDa) also corresponded to extracellular activity (lane 4). Finally, light bands were observed when the higher intracellular proteolytic activity was exposed to protease inhibitor cocktails (lane 5), thus revealing the inhibition of proteolytic activity.
Proteolytic activity of S. schenckii. Determination of specific enzyme activity at pH 7.0 using azocoll as substrate. It shows the activity of type IV collagenase used as a control and intracellular and extracellular proteolytic activities of S. schenckii.
| Specific activity (μmol/min/μg protein) | |
|---|---|
| Collagenase IV | 0.0143 |
| Intracellular proteases | 0.0130 |
| Extracellular proteases | 0.0071 |
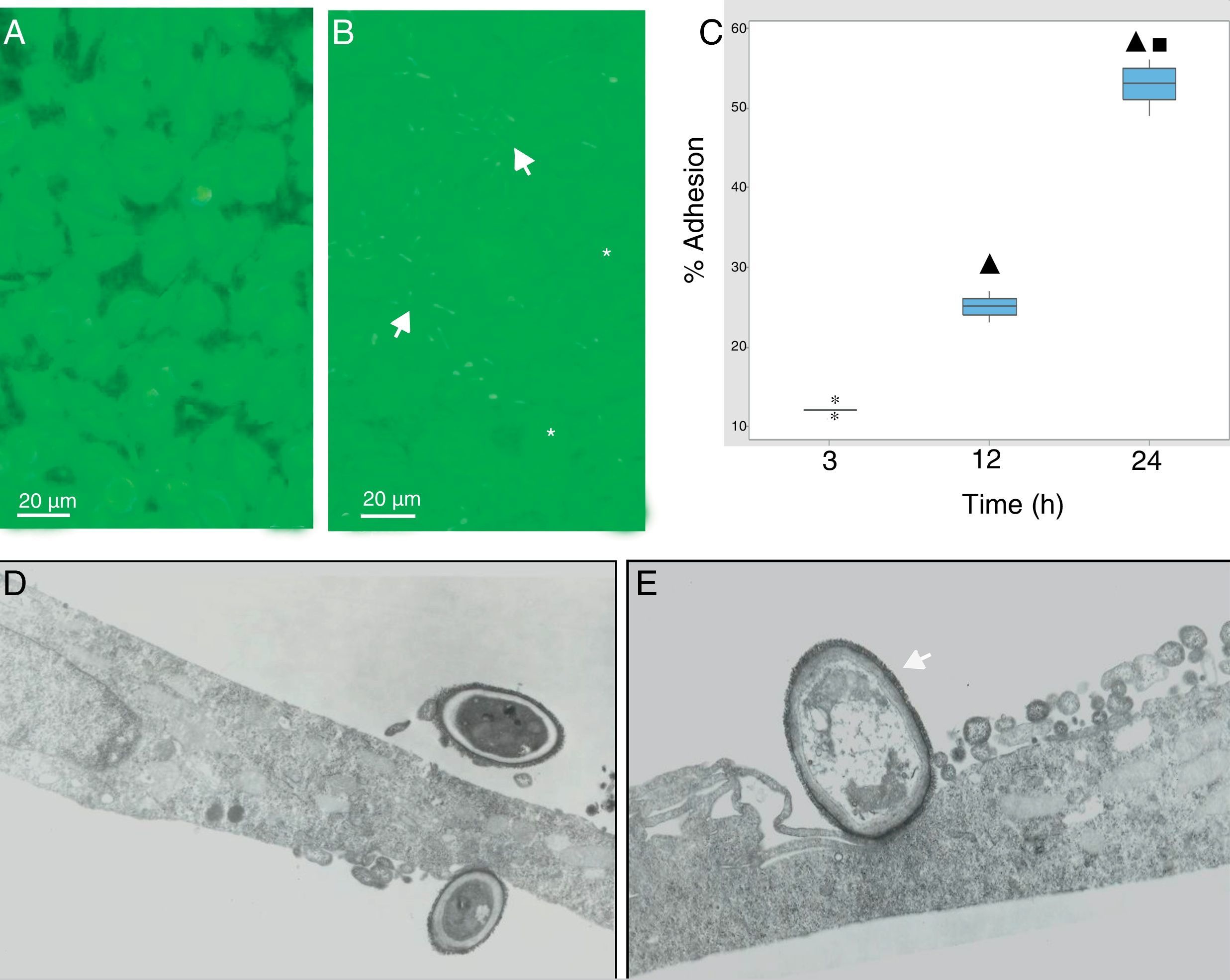
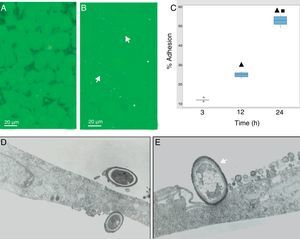
During the host–pathogen interaction, S. schenckii initially bound to the apical region of the epithelium. At 24h post-infection, structural changes were observed in the monolayer integrity (Fig. 2B), with significant areas of damage, whereas the control monolayer displayed its complete integrity (Fig. 2A). The adhesion of the yeasts to the epithelial monolayer was examined after 3, 12 and 24h (Fig. 2C), and significant differences (p<0.05) were shown (12%, 25% and 55%, respectively), according to the cytopathological effects.
S. schenckii yeast–epithelial cell interaction. (A) Epithelial cell control. (B) Fungus–epithelial cell interactions after 24h, showing adhered yeasts (arrow) to epithelial cells and areas (asterisk) of monolayer damage. (C) Percentage of fungus adherence at 3, 12 and 24h of interaction. Box plot indicates the median (n=9) of yeast adhesion to the epithelial cells at different interaction time. ▴ and ■ indicate differences with 3 and 12h, respectively (p<0.05). * indicates outliers. (D) TEM of yeast adhesion to epithelial cells at 3h and (E) 12h. Note the close interaction of fungus cell wall (arrow) with the membrane of host cells.
Transmission electron microscopy was used to analyze the interaction between S. schenckii and the epithelial cells at an ultrastructural level. After 3h of interaction, the yeasts were intimately adhered to the epithelial cell surfaces (Fig. 2D). Additionally, at 12h epithelial cells showed various alterations i.e. diminished lamellipodia, and retracted cell processes associated to loss of intercellular contacts, mainly as a consequence of the fungus interaction on the epithelium (Fig. 2E).
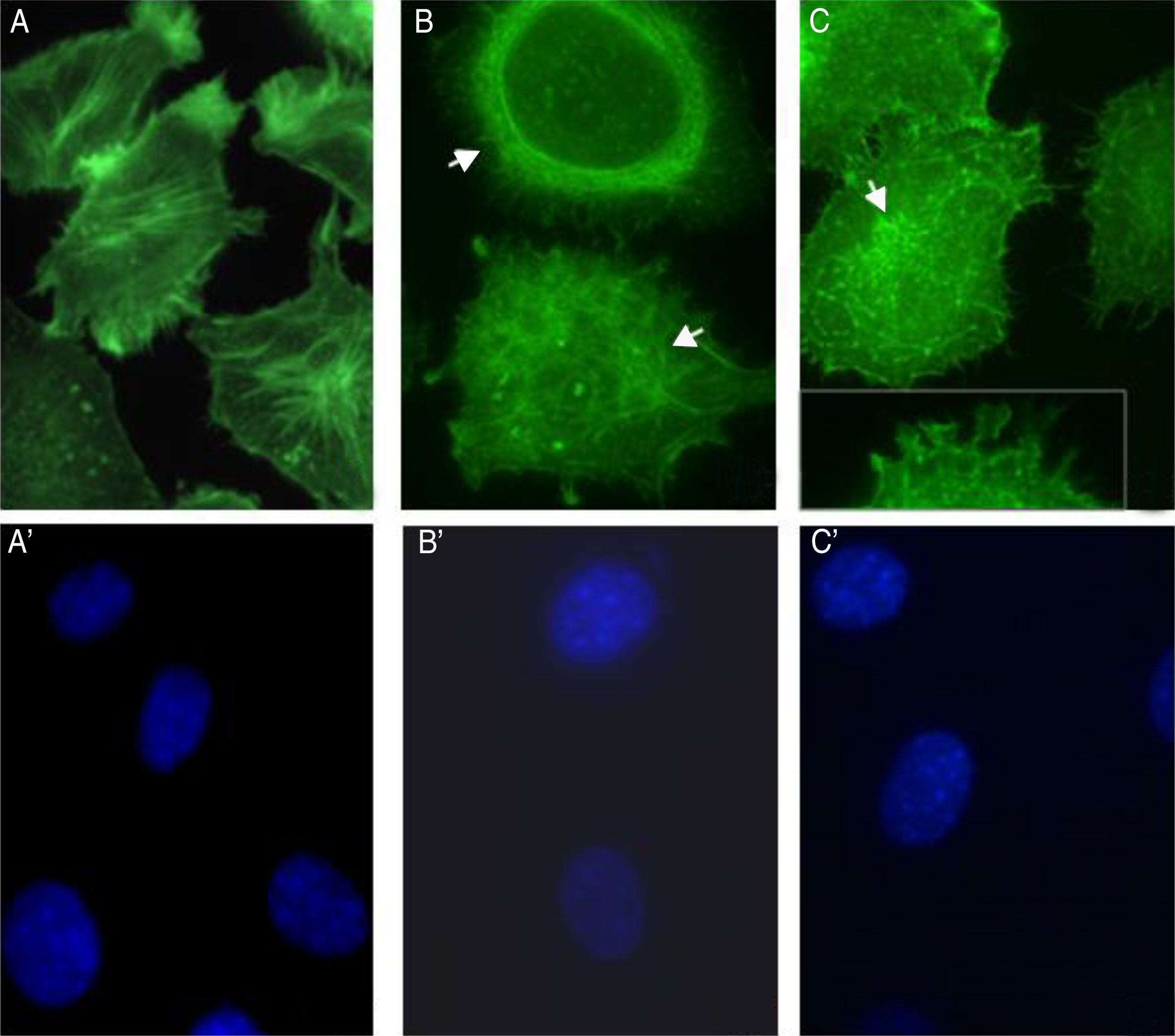
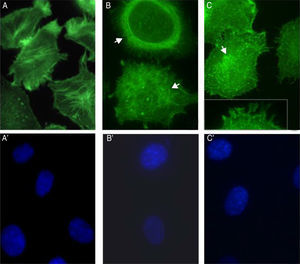
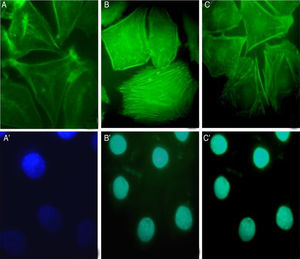
S. schenckii proteases induce actin cytoskeleton alterations in epithelial cellsWe characterized the changes in the cytoskeleton to explore the participation of S. schenckii proteases in the epithelial cell damage. The untreated host cells showed pronounced actin stress fibers and a concentrated actin “mesh” near the plasma membrane (Fig. 3A). At 45min and 90min of incubation with S. schenckii proteases (Fig. 3B and C), the cytoplasmic actin stress fibers were markedly decreased, with an accumulation of the label under the cell membrane and aggregates appearing in the cytoplasm (Fig. 3B). The depolymerized cytoskeleton produced spherical-shaped epithelial cells. Furthermore, membrane blebbing and cytoplasmic actin filament alteration (Fig. 3C) was observed after 90min. The nuclear analysis of epithelial cells treated with proteases (Fig. 3B′ and C′) showed that DNA packaging into heterochromatin was homogeneous, as was the control nuclei (Fig. 3A′).
Actin cytoskeleton alterations in epithelial cell induced by proteases of S. schenckii. (A) Epithelial cell control. (B) After 45min or (C) 90min of incubation with proteases; and (A′, B′ and C′) cell nuclei labeled with DAPI respectively. Note the actin disorganization (box (C) and arrow) in the cells.
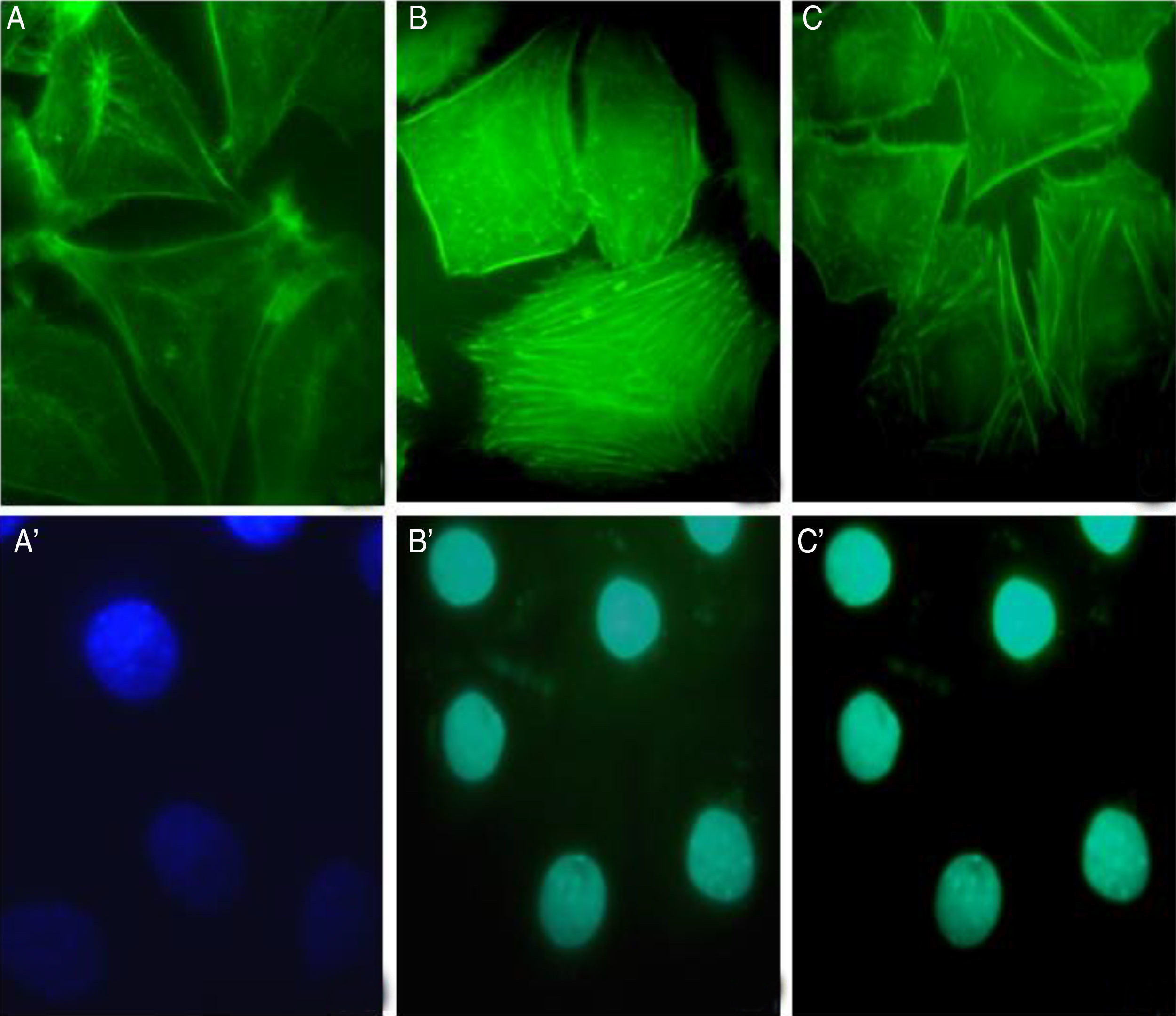
The involvement of S. schenckii protease activity in actin cytoskeleton alterations in epithelial cells was inhibited by the treatment with a cysteine (E64) or serine (PMSF) protease inhibitor. We found that the protease inhibitors were able to block the disruption in the actin stress fibers (Fig. 4B and C) caused by the proteolytic activity of the fungus.
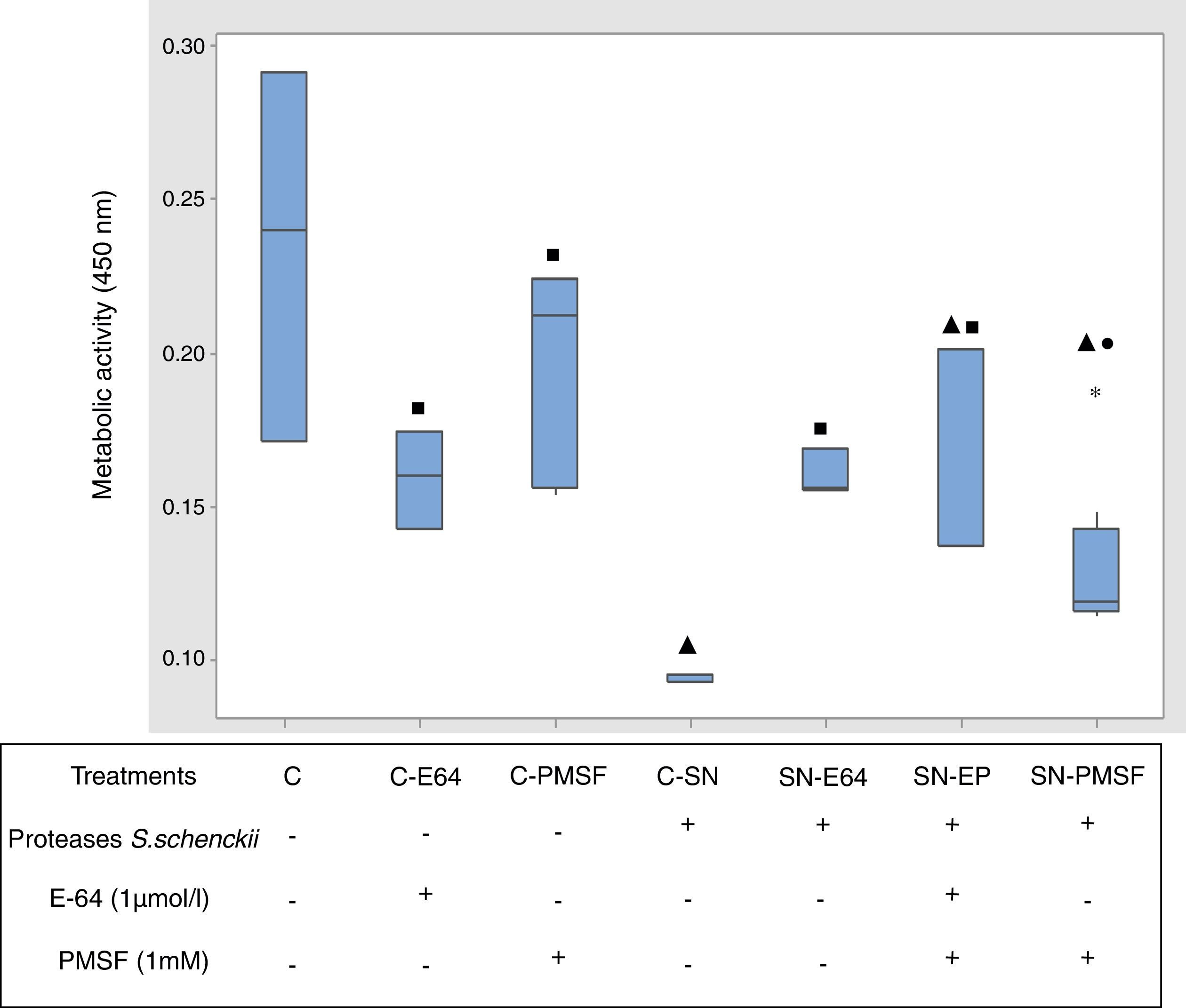
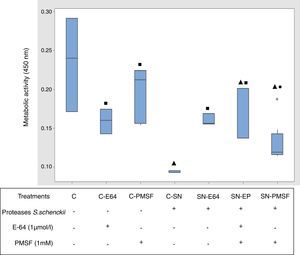
Metabolic activity (cellular viability)Fig. 5 shows the comparative metabolic activity of the epithelial cells incubated with S. schenckii proteases with and without protease inhibitors (E-64 1μmol/l and PMSF 1mM) at 90min. The Kruskal–Wallis test was applied to analyze the data. The epithelial cells exposed to S. schenckii proteases showed a 60% decrease in the metabolic activity, which was significant (p<0.05) with respect to all the treatments. In contrast, the epithelial cells treated with protease inhibitors (E-64 1μmol/l and PMSF 1mM) showed a metabolic activity similar to that of the untreated cells (control). Furthermore, the epithelial cells incubated with S. schenckii proteases and protease inhibitors showed no significant difference with respect to the controls, showing the protective effect of the protease inhibitor.
Proteases of S. schenckii induced loss of metabolic activity (cell viability) on epithelial cells. The metabolic activity was measured by XTT assay. Box plot indicates the median (n=9) of untreated cells (control) and in the presence of proteases of S. schenckii, without and with protease inhibitors (E-64 1μmol/L and PMSF 1mM). ▴, ■ and ● indicate significant differences with treatments C, C-SN and C-PMSF, respectively (p<0.05). * indicates outliers.
The pathogenic filamentous fungi use enzymes to degrade the structural barriers of the host. In animal tissues, these barriers are mostly composed of proteins; therefore, the fungus requires proteolytic enzymes to invade them. For this reason, it is logical to assume that this class of hydrolytic enzymes could act by making this tissue invasion easier, but they could also participate in the infection by eliminating some mechanisms of the immunological defenses and/or helping in the obtaining of nutrients.5,19–21,32 Despite the importance of proteases in fungus–host interaction, little is known about these molecules particularly in S. schenckii.
The lytic enzyme system in pathogenic fungi includes many hydrolytic activities, such as chitinase, mannanase and a variety of proteases and glucanases.19,20 Two extracellular proteases of low weight have been described and linked to virulence factors in S. schenckii.21,31,32 In Trichophyton rubrum, Aspergillus fumigatus and Candida albicans, the coexistence of more than one extracellular protease with an inherent ability for tissue invasion has been reported,4,20 suggesting that these fungi have fundamental similarities in their proteolytic systems even though the proteinases they produce may have adaptive specificity related to the substrates and tissues normally invaded. Although the protease release mechanisms are not well understood, the adhesion of the fungus to the host may induce a response of protease secretion. In this study, the analyzed proteases produced by S. schenckii showed intracellular and extracellular collagenolytic activities detected by azocoll hydrolysis and gelatin zymograms. These results show a system of both extracellular and intracellular proteolytic enzymes of high molecular weight. Within this context, molecular studies have reported great variability in different strains of S. schenckii,1,7,12 with each of these strains having a specific pattern of proteases contributing to pathogenicity and virulence.
S. schenckii has an extracellular proteolytic activity that allows the pathogen to penetrate and colonize the epithelium through a paracellular route18 and to act on the cytoskeleton fibers (actin) during the infection process26 in the same way as other fungi pathogens. This suggests the possible action of the proteases on the actin, consequently altering the morphology and integrity of the host cells. Furthermore, during yeast–epithelium interactions, alterations are evident on the cell surface, reflecting the proteolytic activity of the fungus. Similar effects were described for cultured alveolar cells (A549 cells) infected with A. fumigatus: the cells displayed morphological alteration, i.e. membrane blebbing and cytoskeleton structure.11 The action of specific protease inhibitors indicated that serine and cysteine proteases are involved in these structural alterations. However, the nuclei of these cells did not exhibit DNA fragmentation, in contrast to the reports for Paracoccidioides brasiliensis and C. albicans.28
Any future research should be directed toward the investigation of the regulated expression of proteases in the different morphological stages from S. schenckii. Furthermore, Souza Ferreira et al. describe the development and importance of the Th17 response for the host immune response against S. schenckii. It has been showed that both Th17 and Th1/Th17 mixed cells are developed during the systemic mice infection, which also leads to augmented production of IL-17 and IL-22.29 The biochemical and immunological analysis of these aspects will provide critical information in understanding the nature of host–fungus interaction.
Conflict of interest statementThe authors declare there are no conflicts of interest (financial/commercial).
This work was supported by DAIP-Guanajuato University grants (523/2015) to MSL.