Candida tropicalis is an increasingly important human pathogen which usually affects neutropenic oncology patients with common hematogenous seeding to peripheral organs and high mortality rates. Candida pathogenicity is facilitated by several virulence attributes, including secretion of hydrolytic enzymes; however, little is known regarding the C. tropicalis ability to secrete them and their role in the disease.
AimsTo confirm by molecular means the identification of 187 clinical isolates (127 from blood, 52 from urine, and 8 from diverse clinical origins) phenotypically identified as C. tropicalis, and to investigate their in vitro aspartyl proteinase, phospholipase, esterase, hemolysin, DNase and coagulase activities.
MethodsThe molecular confirmation was performed by ITS sequencing, and the enzymatic determinations were conducted using plate assays with specific substrates, with the exception of coagulase, which was determined by the classical tube test.
ResultsThe majority of the strains exhibited a very strong or strong activity of aspartyl proteinase, phospholipase and esterase. A 4.7% of the bloodstream isolates were hemolysin producers, and all were negative for the coagulase and DNase assays.
ConclusionsVery strong activities of aspartyl proteinase, phospholipase and esterase profiles were detected, and a statistical association between phospholipase production and blood and urine isolates was found.
Candida tropicalis es un patógeno del ser humano cada vez más importante que afecta especialmente a pacientes oncológicos neutropénicos, en los cuales es frecuente la diseminación hematógena del microorganismo a órganos periféricos, lo que conlleva elevadas tasas de mortalidad. La patogenicidad de Candida es facilitada por diversos factores de virulencia, incluyendo la secreción de enzimas hidrolíticas; sin embargo, poco se sabe respecto a la habilidad de C. tropicalis para su secreción, así como el papel que desempeña en la enfermedad.
ObjetivosConfirmar por un método molecular la identidad de 187 aislamientos clínicos (127 de sangre, 52 de orina y 8 de orígenes diversos) fenotípicamente identificados como C. tropicalis y estudiar la actividad in vitro de las enzimas proteinasa aspártica, fosfolipasa, esterasa, hemolisina, DNasa y coagulasa.
MétodosLa confirmación molecular se llevó a cabo mediante secuenciación del ITS y las determinaciones enzimáticas se llevaron a cabo mediante ensayos en placa con sustratos específicos, a excepción de la coagulasa, que se determinó mediante la clásica prueba en tubo.
ResultadosLa mayoría de los aislamientos analizados mostraron un perfil de actividad muy fuerte o fuerte de proteinasa aspártica, fosfolipasa y esterasa. El 4,7% de las cepas sanguíneas fue productora de hemolisinas y todas fueron negativas para coagulasa y DNasa.
ConclusionesSe detectaron perfiles con una actividad proteinasa aspártica, fosfolipasa y esterasa muy fuerte entre los aislamientos clínicos analizados, así como también se encontró asociación estadística entre la producción de fosfolipasa y aquellos aislamientos obtenidos de sangre y orina.
Candida infection has become one of the most important healthcare-associated opportunistic infections due in part to an increased number of patients with major surgical procedures, central venous catheters (CVC), long hospitalization periods in the intensive care unit (ICU), total parenteral nutrition (TPN), broad spectrum antibiotics, and use of immunosuppressive therapy; all of these are clearly known as risk factors for invasive candidiasis.2,36
There has been an emergence of non-Candida albicans Candida species (NAC), which are clinically indistinguishable, but exhibit varying degrees of virulence and resistance to antifungal drugs; due to it a rapid and correct identification of the Candida species is extremely important in the clinical laboratory.7 In recent years, the number of studies related to NAC has increased. These report the activity of extracellular hydrolytic enzymes, such as phospholipase, proteinase and esterases along with biofilm formation and the expression of hemolysins as the main virulence factors. Candida tropicalis is one of the most common NAC species, which in some countries is even more prevalent than C. albicans. It has been associated with patients with oncological malignancies and has been described as having the ability to develop rapid resistance to fluconazole, even though its virulence remains poorly investigated.8,19,20 This makes the study of its virulence factors a necessity to understand its pathogenicity and improve the development of novel antifungal agents to find new therapeutic targets.35 In the present study, we describe the enzymatic profiles of a Mexican culture collection of 187 clinical isolates of C. tropicalis to aspartyl proteinase, phospholipase, esterase, hemolysin, coagulase, and DNase production.
Materials and methodsA subset of 187 clinical isolates of C. tropicalis was collected over a 16-year period (2000–2016) at the Microbiology Reference Center of the School of Medicine, Universidad Autónoma de Nuevo León, in Monterrey, Mexico. Each isolate was obtained from a different patient and was grown at 37°C for 24h on Sabouraud-dextrose agar slants (Difco, Detroit, MI, USA). The isolates were initially identified as C. tropicalis using API 20C AUX strips (bioMérieux, Mexico) and standard morphological methods, and then were confirmed to the species level by molecular amplification and sequencing of the non-coding ITS region.33 The clinical origin of the isolates was as follows: 127 (67.9%) from blood, 52 (27.8%) from urine and 8 (4.3%) from diverse sites, such as bronchial secretion (n=2), trachea (n=1), nail (n=1), pleural (n=1), bile (n=1), abdominal (n=1), and peritoneal (n=1) fluids. The isolates were stored as suspensions in sterile water at room temperature and on agar slants at −20°C.
Aspartyl proteinase activity was assayed using YCB-BSA test medium. An aqueous solution of 1.17% w/v yeast carbon base (Difco, Detroit, MI, USA), 0.01% w/v yeast extract (Merck, Darmstadt, Germany) and 0.2% w/v bovine serum albumin (Bio Basic, USA) was first prepared, adjusted to pH 5.0, sterilized by filtration and finally added to an autoclaved cooled solution of 1.5% w/v agar. Cleavage of bovine serum albumin by aspartyl proteinases results in a clearance zone that is detected using 1% amido black staining (Bio Basic, Inc., Amherst NY, USA).4
Phospholipase activity was evaluated using Sabouraud dextrose agar (Difco Mexico, Becton Dickinson, Mexico) supplemented with 3% glucose, 1M NaCl, and 5mM CaCl2.24 The solution was autoclaved, and 8% sterile egg yolk emulsion (Difco, USA) was added later. Phospholipase activity produces a dense zone of precipitation around the enzyme-expressing colony.12
Esterase activity was determined using Tween 80 opacity test medium, which was prepared with 10g Bacto Peptone (Difco, USA), 5g NaCl, 0.1g CaCl2, and 15g agar in 1 l distilled water (pH adjusted to 6.8) and then autoclaved. When the medium cooled down (50°C), 5ml of Tween 80 (Sigma–Aldrich, USA) were added. The lipolytic enzymes hydrolyze the medium, releasing fatty acids, which then bind to the calcium, forming a precipitation halo around the enzyme-expressing colony.16,30
Hemolysin activity was examined using the method described by Luo et al., employing Sabouraud dextrose agar supplemented with 3% glucose and 7% blood. A translucent halo around the enzyme-expressing colony indicates positive hemolysin activity.17
Enzymatic activity was determined using plate assays with each specific test medium (10ml in 90-mm Petri dishes). An aliquot of 5μl of yeast suspension (107 CFU/ml) was spotted onto each specific medium, except for the hemolysin assays in which 10μl were used. The plates were incubated at 37°C for 2, 5, 7, and 10 days for the hemolysin, phospholipase, aspartyl proteinase and esterase assays, respectively. Activity was expressed according to the Pz index, i.e., colony diameter/total diameter of the colony plus the precipitation halo. The following ranges of activity were established according to the Pz index: very strong, Pz<0.69; strong, Pz=0.70–0.79; mild, Pz=0.80–0.89; weak, Pz=0.90–0.99; and negative, Pz=1.15
Additional enzymatic tests were performed and included DNase activity utilizing the commercial test medium with methylene green dye (Difco, Detroit, MI, USA) and coagulase activity using human and rabbit anticoagulated plasma (Difco, Detroit, MI, USA). DNase activity was expressed as positive or negative by the detection of a clearing halo without defined borders around the colonies, while the presence of a clot that could not be resuspended by gentle shaking indicated a positive coagulase test.
All enzymatic determinations were assayed twice. Reference strains C. albicans ATCC 90028 and C. tropicalis ATCC 750 were used as quality controls for aspartyl proteinase, phospholipase, esterase and hemolysin determinations, and Staphylococcus aureus ATCC 29213 for DNase and coagulase assays. Enzymatic profiles were compared to the clinical origin of the isolates using the Chi-square and Fisher's exact tests in the SPSS package (SPSS v17.0 for Windows; SPSS Inc., Chicago, IL); a p-value ≤0.05 was considered significant. Finally, a comprehensive literature review was performed in order to compare our results with those published elsewhere. The platform used was NCBI-PubMed (www.ncbi.nlm.nih.gov/pubmed/) and the search criterion was: ‘Candida tropicalis in vitro enzymes’.
ResultsThe sequence analysis of the non-coding ITS region allowed the species confirmation of the clinical isolates. All of them were identified as C. tropicalis. The sequences were deposited in GenBank under the following accession numbers: KX664484–KX664670 and KX664664–KX664670.
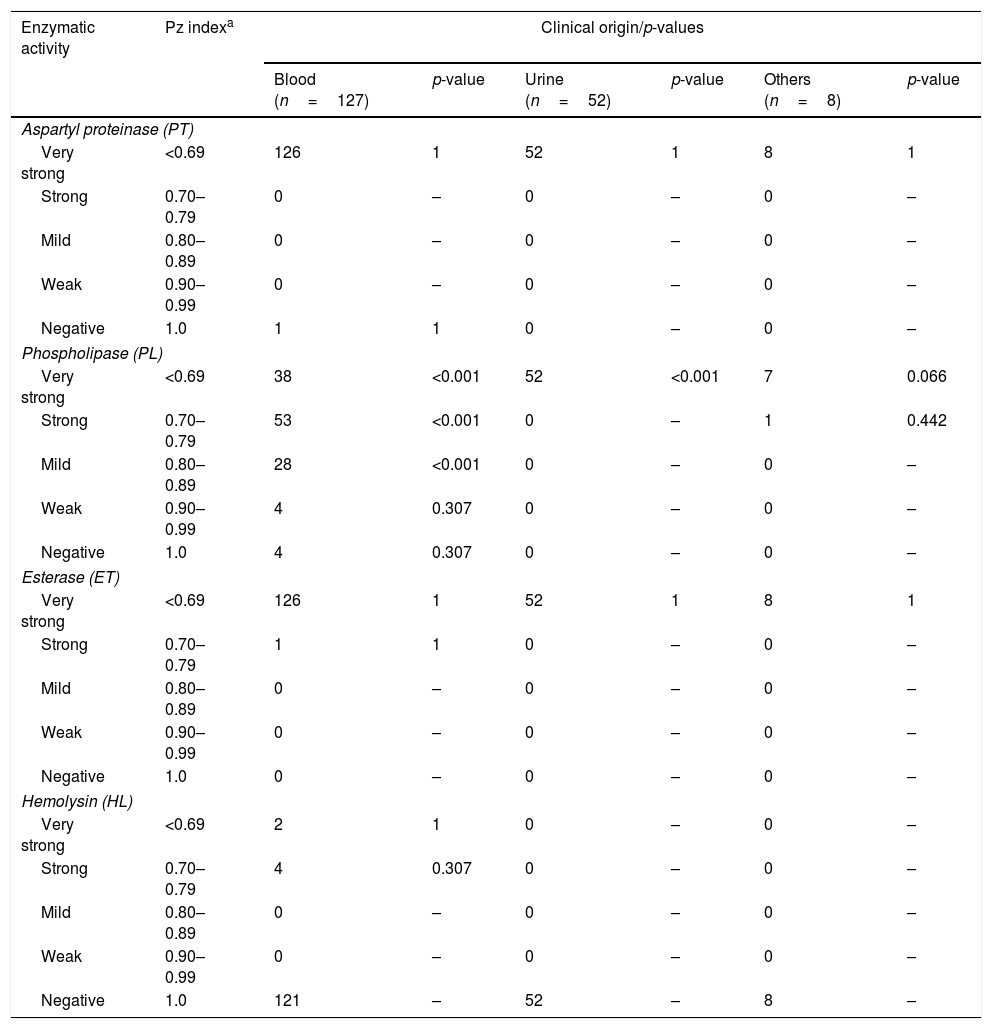
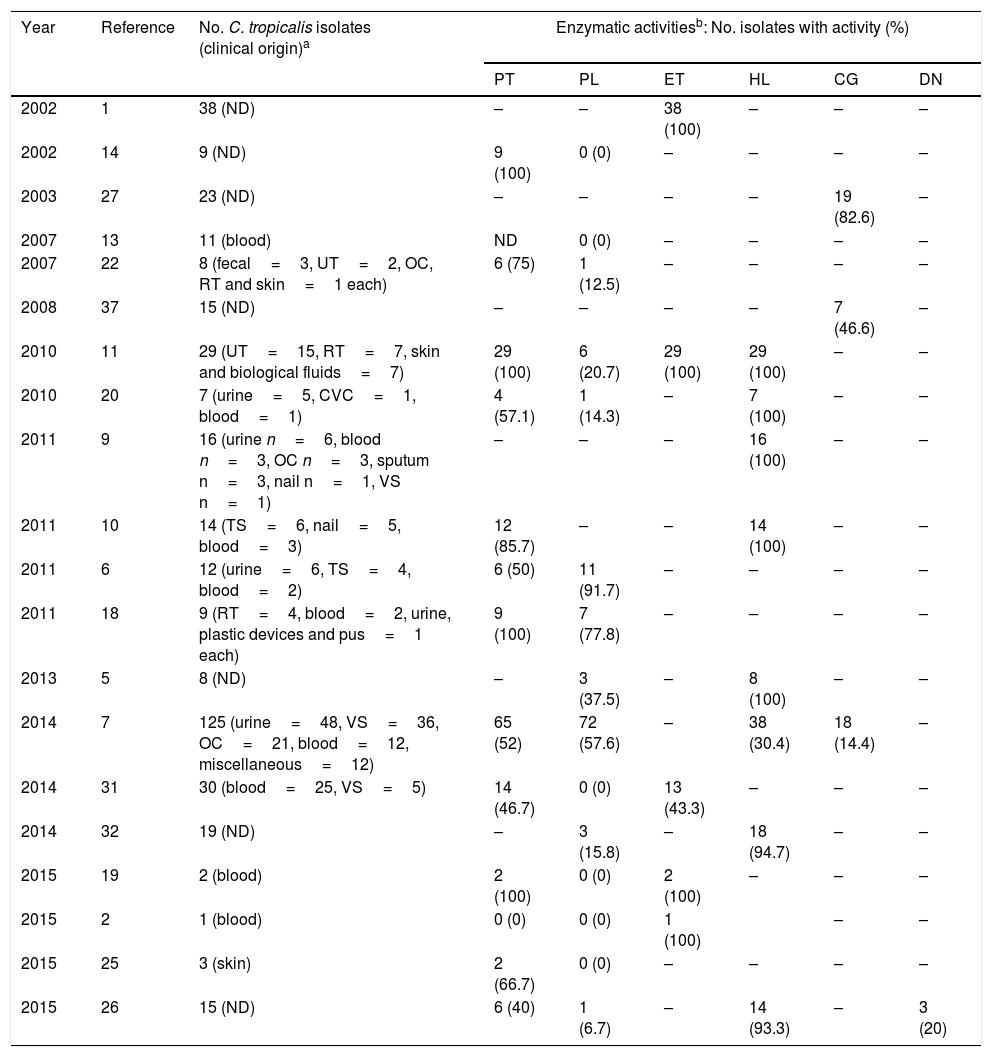
The aspartyl proteinase, phospholipase, esterase and hemolytic activities of the analyzed strains are presented in Table 1. In general, the majority of the strains exhibited a very strong or strong activity of aspartyl proteinase (99%), phospholipase (81%) and esterase (100%). Hemolytic activity was exhibited in just 6 strains (4.7%) from blood, showing a total erythrocyte lysis (β-hemolysis). According to the clinical origin, the strains from blood were statistically associated with a very strong (Chi2=76.392, p-value <0.001), strong (Chi2=31.849, p-value <0.001) and mild (Chi2=15.558, p-value <0.001) activity of phospholipase, while those strains isolated from urine were associated with very strong production of the aforementioned enzyme (Chi2=66.832, p-value <0.001). On the other hand, all strains were negative in DNase and coagulase (human and rabbit plasma) tests. Table 2 summarizes the comprehensive literature review performed, which included a total of 20 publications, being aspartyl proteinase and phospholipase the hydrolytic enzymes more widely studied.
Enzymatic profiles of the Candida tropicalis isolates evaluated in this study.
| Enzymatic activity | Pz indexa | Clinical origin/p-values | |||||
|---|---|---|---|---|---|---|---|
| Blood (n=127) | p-value | Urine (n=52) | p-value | Others (n=8) | p-value | ||
| Aspartyl proteinase (PT) | |||||||
| Very strong | <0.69 | 126 | 1 | 52 | 1 | 8 | 1 |
| Strong | 0.70–0.79 | 0 | – | 0 | – | 0 | – |
| Mild | 0.80–0.89 | 0 | – | 0 | – | 0 | – |
| Weak | 0.90–0.99 | 0 | – | 0 | – | 0 | – |
| Negative | 1.0 | 1 | 1 | 0 | – | 0 | – |
| Phospholipase (PL) | |||||||
| Very strong | <0.69 | 38 | <0.001 | 52 | <0.001 | 7 | 0.066 |
| Strong | 0.70–0.79 | 53 | <0.001 | 0 | – | 1 | 0.442 |
| Mild | 0.80–0.89 | 28 | <0.001 | 0 | – | 0 | – |
| Weak | 0.90–0.99 | 4 | 0.307 | 0 | – | 0 | – |
| Negative | 1.0 | 4 | 0.307 | 0 | – | 0 | – |
| Esterase (ET) | |||||||
| Very strong | <0.69 | 126 | 1 | 52 | 1 | 8 | 1 |
| Strong | 0.70–0.79 | 1 | 1 | 0 | – | 0 | – |
| Mild | 0.80–0.89 | 0 | – | 0 | – | 0 | – |
| Weak | 0.90–0.99 | 0 | – | 0 | – | 0 | – |
| Negative | 1.0 | 0 | – | 0 | – | 0 | – |
| Hemolysin (HL) | |||||||
| Very strong | <0.69 | 2 | 1 | 0 | – | 0 | – |
| Strong | 0.70–0.79 | 4 | 0.307 | 0 | – | 0 | – |
| Mild | 0.80–0.89 | 0 | – | 0 | – | 0 | – |
| Weak | 0.90–0.99 | 0 | – | 0 | – | 0 | – |
| Negative | 1.0 | 121 | – | 52 | – | 8 | – |
C. tropicalis ATCC 750: PT=0.25 (very strong), PL=0.34 (very strong), ET=0.45 (very strong), HL=0 (negative).
C. albicans ATCC 90028: PT=0.31 (very strong), PL=0.29 (very strong), ET=0.46 (very strong), HL=0 (negative).
Comprehensive literature review.
| Year | Reference | No. C. tropicalis isolates (clinical origin)a | Enzymatic activitiesb: No. isolates with activity (%) | |||||
|---|---|---|---|---|---|---|---|---|
| PT | PL | ET | HL | CG | DN | |||
| 2002 | 1 | 38 (ND) | – | – | 38 (100) | – | – | – |
| 2002 | 14 | 9 (ND) | 9 (100) | 0 (0) | – | – | – | – |
| 2003 | 27 | 23 (ND) | – | – | – | – | 19 (82.6) | – |
| 2007 | 13 | 11 (blood) | ND | 0 (0) | – | – | – | – |
| 2007 | 22 | 8 (fecal=3, UT=2, OC, RT and skin=1 each) | 6 (75) | 1 (12.5) | – | – | – | – |
| 2008 | 37 | 15 (ND) | – | – | – | – | 7 (46.6) | – |
| 2010 | 11 | 29 (UT=15, RT=7, skin and biological fluids=7) | 29 (100) | 6 (20.7) | 29 (100) | 29 (100) | – | – |
| 2010 | 20 | 7 (urine=5, CVC=1, blood=1) | 4 (57.1) | 1 (14.3) | – | 7 (100) | – | – |
| 2011 | 9 | 16 (urine n=6, blood n=3, OC n=3, sputum n=3, nail n=1, VS n=1) | – | – | – | 16 (100) | – | – |
| 2011 | 10 | 14 (TS=6, nail=5, blood=3) | 12 (85.7) | – | – | 14 (100) | – | – |
| 2011 | 6 | 12 (urine=6, TS=4, blood=2) | 6 (50) | 11 (91.7) | – | – | – | – |
| 2011 | 18 | 9 (RT=4, blood=2, urine, plastic devices and pus=1 each) | 9 (100) | 7 (77.8) | – | – | – | – |
| 2013 | 5 | 8 (ND) | – | 3 (37.5) | – | 8 (100) | – | – |
| 2014 | 7 | 125 (urine=48, VS=36, OC=21, blood=12, miscellaneous=12) | 65 (52) | 72 (57.6) | – | 38 (30.4) | 18 (14.4) | – |
| 2014 | 31 | 30 (blood=25, VS=5) | 14 (46.7) | 0 (0) | 13 (43.3) | – | – | – |
| 2014 | 32 | 19 (ND) | – | 3 (15.8) | – | 18 (94.7) | – | – |
| 2015 | 19 | 2 (blood) | 2 (100) | 0 (0) | 2 (100) | – | – | – |
| 2015 | 2 | 1 (blood) | 0 (0) | 0 (0) | 1 (100) | – | – | |
| 2015 | 25 | 3 (skin) | 2 (66.7) | 0 (0) | – | – | – | – |
| 2015 | 26 | 15 (ND) | 6 (40) | 1 (6.7) | – | 14 (93.3) | – | 3 (20) |
C. tropicalis is a diploid dimorphic opportunistic yeast which colonizes several anatomically distinct sites such as the skin, gastrointestinal and genitourinary tracts.3 This pathogen accounts for 3–66% of all Candida bloodstream infections worldwide29 and it is usually considered the first or second NAC species most frequently isolated from the bloodstream and urinary tract (candiduria).20C. tropicalis candidemia is particularly common in oncology patients; bone marrow transplant recipients have the highest infection rates (11–50%), followed by hematological malignancies (18%), and solid tumors (4–9%).3,29 The mortality rates associated with this disease are unacceptably high (40–70%) and some important predisposing factors are acute leukemia, neutropenia, and anti-neoplastic therapy.3
In order to establish an infectious process prone to dissemination in the immunocompromised host, C. tropicalis possess many virulence attributes which differentially express and assist tissue invasion, such as the capability of adhesion to different surfaces, biofilm production, hyphae formation, and secretion of extracellular enzymes, the latter considered integral to the pathogenic fitness of Candida. Some of the most extensively investigated enzymes in the genus are aspartyl proteinase, phospholipase, esterase and hemolysin. However, as shown in Table 2, the principal limitation of many of these reports1,5,6,8–11,13,14,18,19,21,22,25,26,31,32 are the small number of isolates tested, compromising sternly the statistical support of species-specific associations.
Aspartyl proteinases (also known as ‘Saps’) are implicated in the colonization and invasion of host tissues by disruption of mucosal membranes28 and by degrading immunological and structural defense proteins.23 Irrespective of the clinical origin, all the isolates tested in this study were very strong Saps producers (Pz index <0.69), with the exception of one isolate from blood, which was negative. These findings agree with previous reports11,14,18,19 and are in contrast with others.6,8,26,31
Phospholipases are, in addition to Saps, enzymes often considered to be involved with the pathogenic potential of Candida spp. These act through the hydrolysis of phospholipids into fatty acids, contributing to host cell membrane damage, which finally could also expose receptors to facilitate the adhesion.12,14 Of the blood isolates analyzed, 97% were phospholipase producers at different degrees, while all isolates from urine were very strong producers (p<0.0001). The results we obtained are different to those reported by other authors,11,21,22,26,32 who suggest a reduced ability of C. tropicalis to secrete extracellular phospholipases; however, this production is highly strain dependent. On the other hand, although the production of esterase has been documented as a virulence factor in Candida, little is known about its pathogenic role, though recent evidence suggest a cytotoxic effect of this enzyme in host tissues.34 Of all clinical isolates, 99% were very strong esterase producers. Similar results have been reported.1,2,11,19
Hemolysins are a key virulence factor used by Candida species to degrade hemoglobin and facilitate the recovery of elemental iron from host erythrocytes.17 As shown in Table 2, there are several reports in which this extracellular enzyme was studied in C. tropicalis; in many of them, 100% of the tested isolates secreted hemolysins.5,9–11,21 In contrast, we detected only 6 strains (3%) exhibiting hemolytic activity, and all were recovered from blood; however, this enzymatic activity is strain dependent,17 which greatly influences the particular enzymatic profile of each strain, regardless of its clinical origin.
Apart from the abovementioned enzymes, there are other ones described also as virulence factors in Candida, but that have been studied to a lesser extent, such is the case of coagulases and DNases. The former binds to plasma fibrinogen and activates a cascade of reactions that induce clotting of plasma, while the latter participates in the hydrolysis of deoxyribonucleic acid. Coagulase production by Candida was first reported by Rodrigues and colleagues,27 who detected high activity in C. albicans (88.5%) and C. tropicalis (82.6%) using the coagulase tube test with rabbit plasma. Later, it was reported differences in sensitivities between the rabbit, sheep and human plasma used to test the coagulase activity of Candida species37; these authors established that the rabbit plasma is the most appropriate medium for the study of this activity and reported a 46.6% of C. tropicalis isolates coagulase-positive. Recently, it was reported coagulase production in 14.4% of C. tropicalis isolates.8 On the other hand, DNase activity in Candida species has been practically unexplored. One study reported 3 isolates of C. tropicalis DNase positive.26 In contrast with the aforementioned reports, all the isolates we tested were negative for both determinations.
In general, we found very strong activities of aspartyl proteinase, phospholipase and esterase profiles in the clinical isolates tested, highlighting the importance of these virulence attributes in the pathogenesis of the infection due to C. tropicalis. To our knowledge, this is the study with the highest number of C. tropicalis isolates tested. Additionally, this is the first report that examines an extended battery of enzymatic assays. Overall, the present study contributes to expand the knowledge concerning the incidence of in vitro production of extracellular lytic enzymes in C. tropicalis.
Funding sourcesThis work was supported by internal resources of the Department of Microbiology, School of Medicine, UANL.
Conflict of interestThe authors have no conflict of interest to declare. The authors alone are responsible for the content and the writing of the paper.
We are grateful to Sergio Lozano-Rodríguez, M.D. for his review of the manuscript prior to submission.