Fungal peritonitis is a relatively uncommon infection in peritoneal dialysis patients. However, it can be associated with significant morbimortality. In recent reports, Candida species and other filamentous fungi have been reported as being aetiological agents. Thermoascus species are ubiquitous, thermophilic fungi, with an anamorph in the Paecilomyces genus. Here we present the first report of fungal peritonitis by Thermoascus crustaceus from Chile.
Case reportWe present the case of an 83-year-old female patient, with a history of cholecystectomy, hernia repair, severe arterial hypertension, hip and knee osteoarthritis and several episodes of peritoneal dialysis with a cloudy exudate. Bacterial cultures were negative. In addition, a history of two months with intermittent fever peaks mainly in the evening was reported. Blood culture bottles inoculated with peritoneal fluid revealed the presence of fungal growth. Morphological and molecular studies allowed us to identify the aetiological agent as Thermoascus crustaceus. An antifungal susceptibility test was performed using the M38-A2 method, developed by the Clinical and Laboratory Standards Institute (CLSI). The MIC values to amphotericin B, itraconazole, voriconazole and echinochandins were 0.5, 0.25, 0.25 and 0.125μg/ml, respectively. Antifungal treatment with amphotericin B was prescribed, with good patient progress.
ConclusionsFungal peritonitis is a very rare entity. Moreover, the spectrum of fungal pathogens continues to expand, a reason for which morphological and molecular studies are necessary for a rapid diagnosis.
La peritonitis fúngica es una infección bastante infrecuente en pacientes en diálisis peritoneal. Sin embargo, puede estar relacionada con una morbimortalidad considerable. En informes recientes, se han registrado las especies de Candida y algunos hongos filamentosos como agentes etiológicos. Thermoascus es un género de hongos ubicuos, termófilos, que tienen su anamorfo en el género Paecilomyces. A continuación presentamos el primer caso de peritonitis fúngica por Thermoascus crustaceous de Chile.
Caso clínicoPresentamos el caso de una mujer de 83 años con antecedentes de colecistectomía, hernia, hipertensión arterial grave, artrosis de cadera y rodilla, y varios episodios de diálisis peritoneal con una efusión turbia. Los cultivos bacterianos fueron negativos. Además, la paciente había presentado durante los dos últimos meses picos febriles intermitentes, principalmente por la noche. Los hemocultivos inoculados con líquido peritoneal revelaron el crecimiento de un micelio. Los estudios morfológicos y moleculares permitieron identificar el agente etiológico como Thermoascus crustaceous. Para el estudio de sensibilidad antifúngica se utilizó el método M38-A2, desarrollado por el Clinical and Laboratory Standards Institute. Las CMI obtenidas de anfotericina B, itraconazol, voriconazol y equinocandinas fueron 0,5; 0,25; 0,25, y 0,125 μg/ml, respectivamente. El tratamiento antifúngico con anfotericina B fue el indicado, con una buena evolución de la paciente.
ConclusionesLa peritonitis fúngica es una entidad muy poco frecuente. Además, el espectro de hongos patógenos continúa en expansión, razón por la cual los estudios morfológicos y moleculares son necesarios para el diagnóstico rápido.
An 83-year-old female patient, with a history of cholecystectomy, hernia repair, and hip and knee osteoarthritis was admitted to our hospital. In 1990 a parathyroidectomy was performed, evolving with postoperative hypoparathyroidism, which was treated with calcitriol and oral calcium. Moreover, the patient had a history of severe arterial hypertension since 1992, managed with diuretics, calcium channel blockers and angiotensin-converting enzyme inhibitors. In 1995 the patient developed chronic renal failure. After 10 years she progressed to chronic kidney disease stage 5, opting for peritoneal dialysis. A Tenkhoff catheter was put in 2002. During the first 2 years of peritoneal dialysis the patient developed two episodes of Gram-positive bacterial peritonitis, both completely cured.
In 2012, a permanent pacemaker was implanted due to symptomatic congenital atrioventricular block. In addition, a new bacterial peritonitis due to Corynebacterium spp. was observed. The patient was successfully treated.
On January 2014 the patient presented with pain and cloudy peritoneal fluid. The study of the dialysate fluid showed 80 white blood cells/mm3. No differential count was performed. Bacterial cultures were negative after 7 days of incubation at 37°C. The patient received routine intraperitoneal antibiotics.
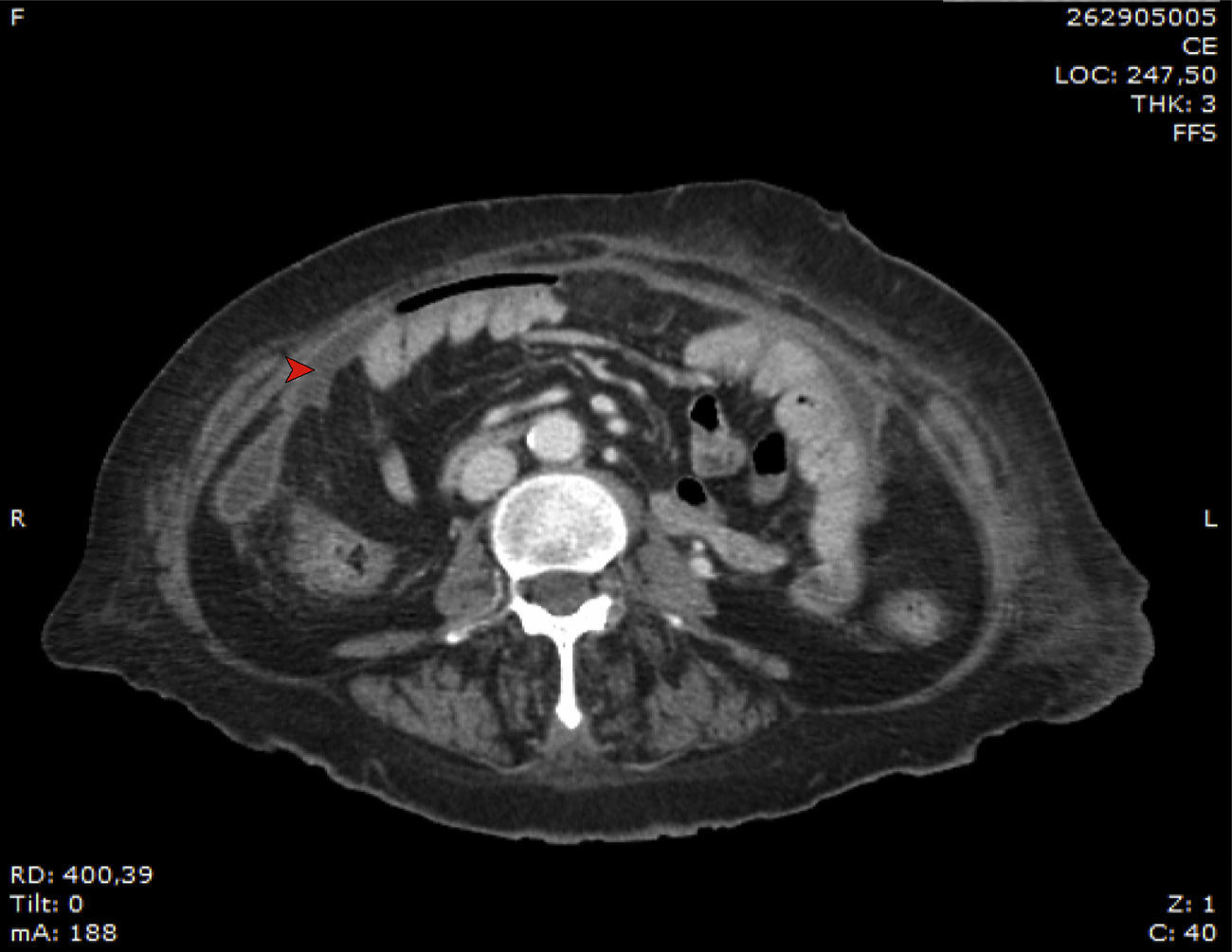
In June 2014, after a mild story of two months of intermittent fever peaking mainly in the evening, with occasional cloudy effluent outlet, new microbiological studies were performed. Clinically, the patient presented no abdominal pain. Again, bacterial cultures were negative after 7 days of incubation. Gram staining did not show any microbiological evidence. However, dialysate fluid cytology showed 260 white blood cells/mm3 (68% mononuclear cells). In addition, blood culture bottles inoculated with peritoneal fluid, indicated the presence of fungal growth. Growth on Sabouraud Dextrose Agar (SDA) showed the presence of yellow-light brown colonies. Fig. 1 shows the radiographic findings.
Antifungal treatment was started with intraperitoneal amphotericin B 50mg/day for 20 days. The patient completed 10 days of treatment after which the indwelling Tenckhoff catheter was removed. Following antifungal treatment, the patient was discharged in good condition.
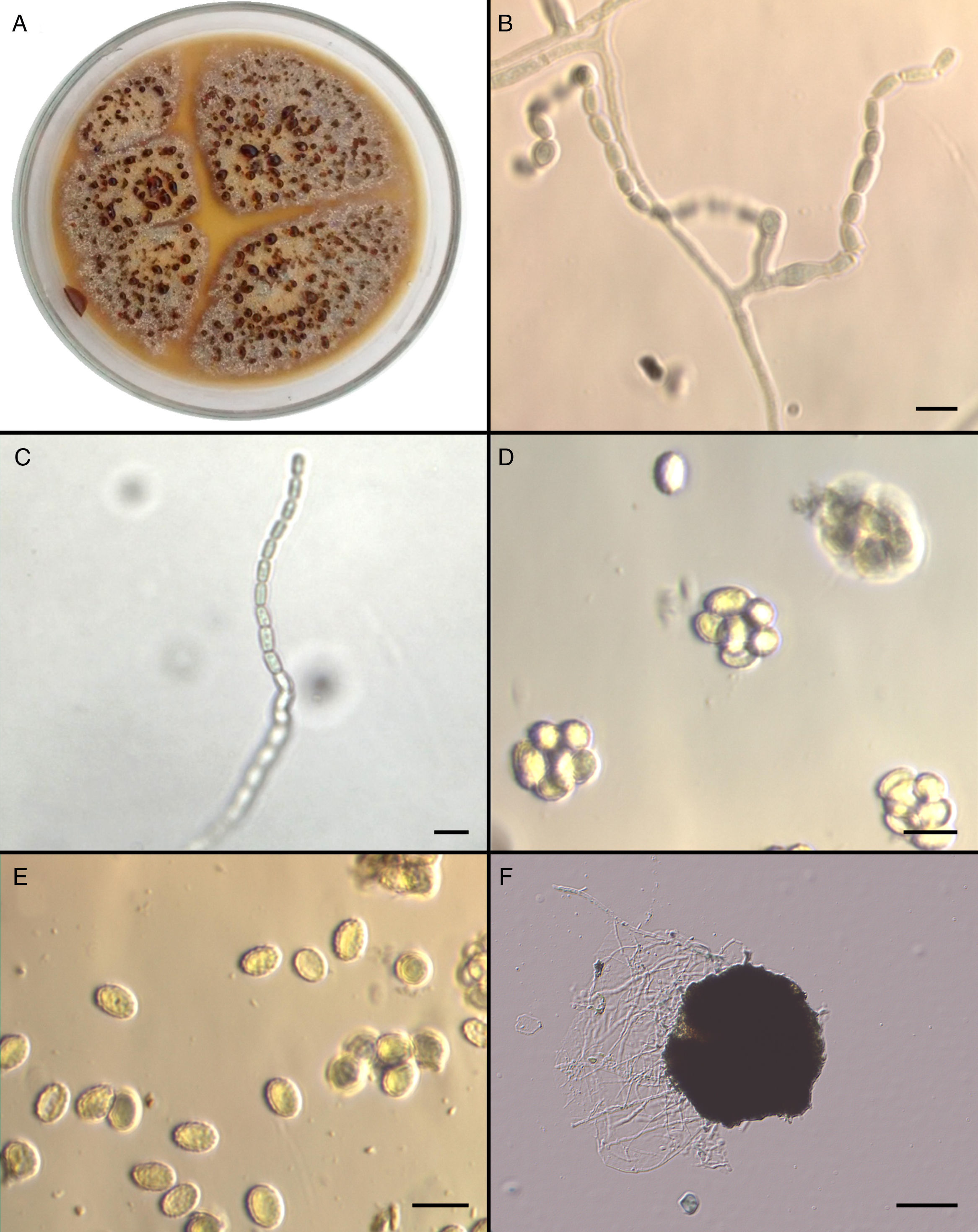
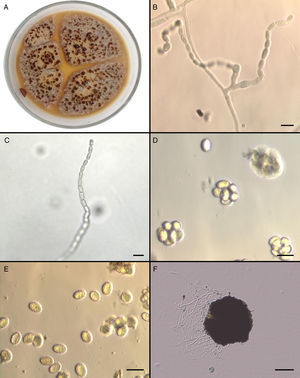
Mycological studiesThe isolate was referred to the Fungal Reference Centre at Universidad de Chile. The mould was cultured on Potato Dextrose Agar (PDA) and Corn Meal Agar (CMA) for 5 days at 25°C and 37°C. The growth of the isolate on PDA (Fig. 2A) and CMA revealed colonies that were initially flat and buff coloured but that quickly (within 6 days) became yellowish orange with a brownish yellow reverse. Sparse conidial structures of the anamorph Paecilomyces were evident in 3–4 days at 25°C on PDA and CMA. Branched conidiophores measuring between 350 and 400μm were observed. Conidia were elliptical to subglobose, approximately 7–7.8 by 4.5–5.3μm, smooth-walled in long chains (Fig. 2B and C). In order to induce the formation of sexual stage, additional subcultures onto malt extract agar (MEA) (Becton Dickinson) at 37°C were performed. Colonies on MEA became brown-orange and granular to crust-like with a brownish orange reverse. Subspherical, nonostiolate ascomata occurred within 7 days and contained asci that were approximately 600 by 750μm (Fig. 2D). Ascospores were elliptical, pale yellow, thick walled, approximately 4–5 by 6.5–7μm, and predominately echinulate by light microscopy (Fig. 2E). Temperature studies were also performed by inoculating five plates of MEA in duplicate with a 1mm portion of the isolate. Plates were incubated at 15, 25, 37, and 42°C for 6 days, and zone sizes were measured. Optimal growth was observed at 37°C. The ascocarp formation occurred mainly at 37°C (Fig. 2F).
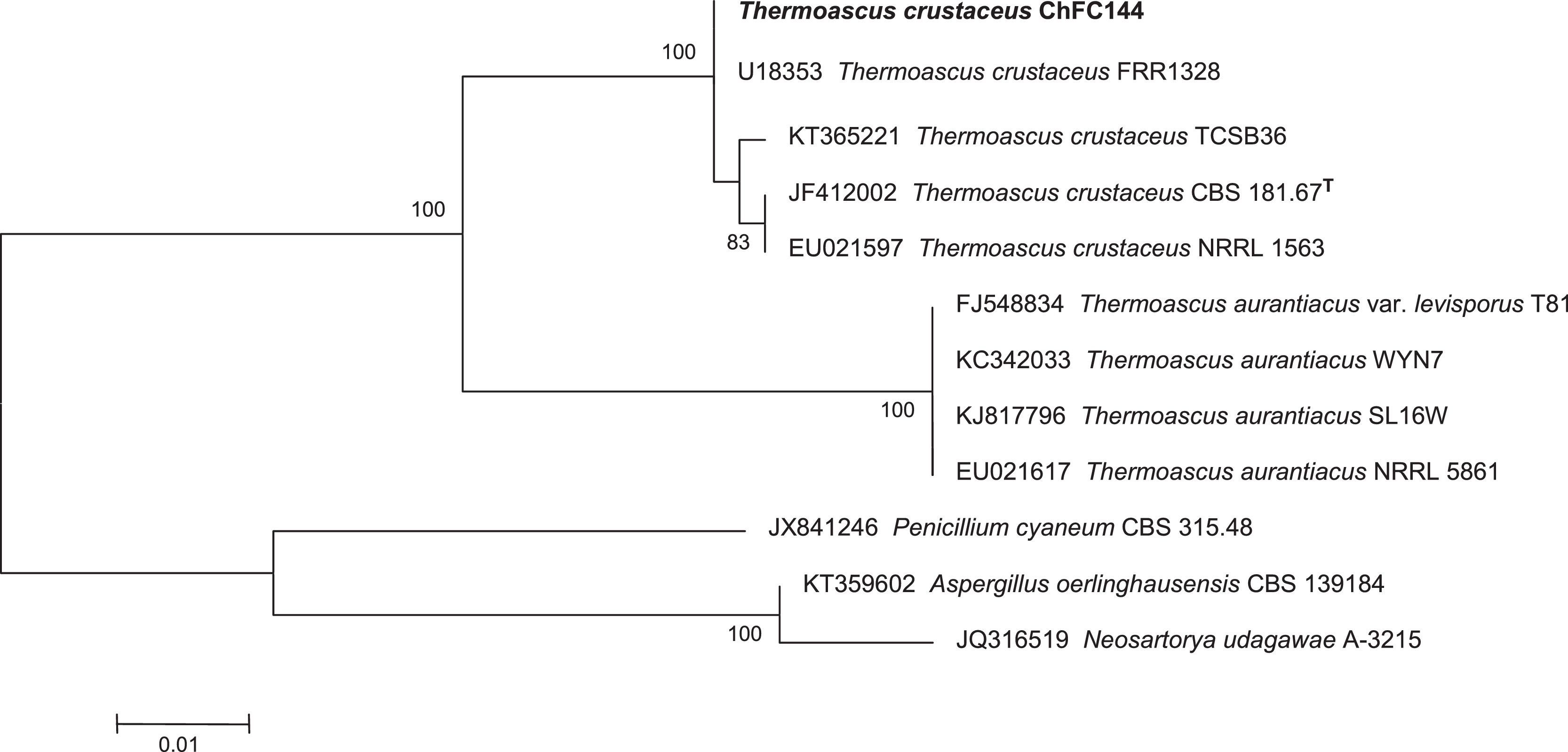
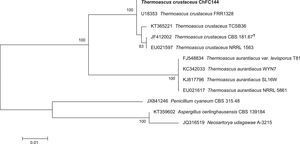
In addition, an in order to corroborate the morphological identification, molecular studies based on sequences of the ITS region were conducted. PCR amplification of fungal genomic DNA was performed using the primers and conditions as described previously.1 The results obtained from GENBANK showed a high level of identity (≥99%) with the sequences (accession number FN675837) from the type strain of Thermoascus crustaceus (T. crustaceus CBS 181.67T). In order to evaluate the genetical relationships between our isolate and those reported previously, phylogenetical analyses were conducted (Fig. 3). Phylogenetical inference was carried out following the protocols reported by Alvarez et al.2 The isolate reported in the present study has been stored in the Chilean Fungal Collection (ChFC) housed at the Mycology Unit, University of Chile, Santiago de Chile, with the unique identifier ChFC EA144. Also, the sequence of ITS region from our isolate was deposited in GENBANK database as KY115690.
Neighbour-joining tree based on maximum composite likelihood corrected nucleotide distances among the ribosomal internal transcribed spacer (ITS) regions and 5.8S rRNA gene sequences of the strains of Thermoascus spp. Bootstrap support values above 70% are indicated at the nodes. The bar indicates genetic distance. Sequences of Penicillium, Neosartorya and Aspergillus were used to root the phylogram.
In vitro susceptibility testing was performed using broth microdilution for filamentous fungi according to the CLSI document M38-A2.5 The MIC and MEC (minimal effective concentration) values were read at 48 and 72h. Due to the lack of clinical breakpoints for Thermoascus species, the following suggested cut off breakpoints were used: sensible (S), ≤1μg/ml; intermediate (I), 2μg/ml; and resistant (R), ≥4μg/ml.5 Pure active powders of known potency of amphotericin B (AMB) (USP, Rockville, MD), voriconazole (VRC) (Sigma-Aldrich), itraconazole (ITC) (Janssen Pharmaceutica, Beerse, Belgium), caspofungin (CSF) (Merk & Co., Inc., Rahway, USA), micafungin (MCF) (Astellas Pharma), and anidulafungin (ANF) (Pfizer) were tested. All tests were performed in duplicates. Paecilomyces variotii ATCC MYA 3630 was included as quality control.
Results indicated the good activity of the antifungals tested. Amphotericin B and azoles showed MIC values of 0.5 and 0.25μg/ml, respectively. Equinocandins showed the best activity with MIC values of 0.125μg/ml. Based upon achievable drug concentrations using standard dosing regimens, the isolate appeared to be sensible, in vitro, to all the antifungal agents assayed in the present work.
DiscussionFungal peritonitis is a rare complication in patients undergoing peritoneal dialysis. The incidence is around 6%.4,12 Etiological agents reported to date include Aspergillus spp.,3Candida spp.,4,12Curvularia geniculata,11Fusarium spp.,7Penicillium spp.,9 and T. crustaceus,8 among others. Recently, following the Chile's earthquake of 2010, several cases of fungal peritonitis due to Paecilomyces variotti were reported.10 A search of the literature indicates that the present case is the second report describing fungal peritonitis by T. crustaceus in the world,8 and the first in Chile.
In an earlier examination of the fungal cultures, and based on taxonomic information from fungal structures, Paecilomyces species was identified in the plates incubated at room temperature. Teleomorph (Thermoascus) morphological structures were observed from agar plates cultured at 37°C. It was also possible to confirm the preliminary morphological identification with the molecular tools. The ITS sequence of the studied isolate allowed us to identify it as T. crustaceus.
In vitro susceptibility data for T. crustaceus is limited. However, MIC values for azoles (<0.015μg/ml) and amphotericin B (<0.25μg/ml) on Thermoascus taitungiacus have been reported,6 consistent with this study.
Usually fungal peritonitis is a serious disease. In our case the clinical picture was mild, without systemic compromise. Despite the lack of clear treatment guidelines in fungal peritonitis, the early removal of Tenckhoff catheter seems to be essential and timely antifungal treatment must be started in order to have a good prognosis. In the present study, AMB, ITC and CSF, MCF, and ANF were active against the strain of T. crustaceus at low MIC values.
In summary, this is the first report of fungal peritonitis caused by T. crustaceus in Chile. Further studies are needed to elucidate optimal treatment strategies.