An increased incidence of fungal infections caused by Candida species, especially Candida glabrata and Candida krusei, which are less susceptible to azoles, has been observed. Standardized susceptibility testing is essential for clinical management and for monitoring the epidemiology of resistance.
AimsWe evaluated the performance of two different susceptibility testing commercial methods, Vitek 2® and Sensititre YeastOne®, and compared them with the standard broth microdilution method (CLSI).
MethodsA total of 80 isolates of several Candida species (Candida albicans, Candida parapsilosis complex, Candida tropicalis, C. glabrata and C. krusei) were selected for this study.
ResultsWe analyzed the categorical agreement (CA) between the methods, stratifying the disagreements. The average CA between the methods was 96.3% for Vitek 2® and 84% for Sensititre YeastOne®. No very major errors were observed. Major errors and minor errors were found for all the isolates tested. With the azoles, both Vitek 2® and Sensititre YeastOne® had good and similar performance levels, except for C. tropicalis and C. krusei (Sensititre YeastOne® showed low CA, 56.2%). With the echinocandins, both methods showed good performance for C. albicans, C. parapsilosis and C. tropicalis. However, we observed important discrepancies for C. krusei with caspofungin: Vitek 2® had 100% CA while Sensititre YeastOne® had only 25%. With amphotericin B, both Vitek 2® and Sensititre YeastOne® had good performance with high CA.
ConclusionsDespite the limited isolates tested, we concluded that both methods have good performance and are reliable for antifungal susceptibility testing. However, caspofungin activity against C. krusei and C. glabrata should be interpreted carefully when using Sensititre YeastOne® because we observed a low CA.
La incidencia de infecciones fúngicas provocadas por especies de Candida, especialmente por Candida glabrata y Candida krusei, menos sensibles a los azoles, ha ido en aumento. Los métodos estandarizados de estudio de la sensibilidad a los antifúngicos son fundamentales para el manejo clínico y para un mejor seguimiento de la epidemiología de la resistencia.
ObjetivosSe evaluó la actividad de dos métodos comerciales diferentes para el estudio de la sensibilidad in vitro a los antifúngicos, Vitek 2® y Sensititre YeastOne®, y se compararon con la técnica estándar de microdilución en caldo del CLSI.
MétodosPara este estudio se seleccionó un total de 80 cepas aisladas de varias especies de Candida (Candida albicans, Candida parapsilosis, Candida tropicalis, C. glabrata y C. krusei).
ResultadosSe analizó la concordancia categórica (CC) entre los métodos y se estratificaron los desacuerdos. La CC media entre los métodos fue del 96,3% para Vitek 2® y del 84% para Sensititre YeastOne®. No se observaron errores muy altos. Se encontraron errores mayores y menores en todos los aislamientos probados. Con los azoles, tanto Vitek 2® como YeastOne® presentaron rendimientos buenos y similares, excepto para C. tropicalis y C. krusei (Sensititre YeastOne® mostró baja CC, el 56,2%). Con las equinocandinas, los dos métodos mostraron buen rendimiento para C. albicans, C. parapsilosis y C. tropicalis. Sin embargo, se observaron discrepancias importantes para C. krusei con la caspofungina: Vitek 2® presentó el 100% de CC, mientras que Sensititre YeastOne® solo el 25%. Para la anfotericina B, Vitek 2® y Sensititre YeastOne® presentaron un buen rendimiento con una CC alta.
ConclusionesAunque el número de cepas aisladas probadas fue limitado, concluimos que los dos métodos tienen un buen rendimiento y son fiables para la prueba de sensibilidad antifúngica. Sin embargo, la actividad de la caspofungina frente a C. krusei y C. glabrata mediante el método Sensititre YeastOne® debe interpretarse cuidadosamente, ya que se observa un valor bajo de CC.
Fungal infections have emerged as a major cause of disease in humans, especially in health care settings. Such infections have gained great importance in critically ill patients, patients with degenerative or neoplastic diseases and those undergoing transplantation.
Among the opportunistic fungi, Candida is the most common agent of invasive fungal infections in hospitalized patients.11,22 In recent years, the epidemiology of infections caused by Candida has changed; we have seen an increase in the prevalence of bloodstream infections caused by Candida species, especially non-Candida albicans-Candida species, as reported in many series, including Candida glabrata and Candida krusei, which are intrinsically less susceptible to fluconazole.6,10,13-15,19,21 In Brazil, from 2007 to 2010, non-C. albicans species accounted for 65.7% of the total of yeasts isolated from blood.8 In addition, different studies have shown increasing rates of azole resistance among Candida tropicalis and Candida parapsilosis isolates.9,12,19,20,23 Therefore, susceptibility testing to antifungal agents is crucial for an accurate therapy.
In vitro standardized susceptibility testing is essential not only for clinical management but also for monitoring the epidemiology of resistance. In this study, several methods have been evaluated in a clinical laboratory. The broth microdilution (BMD) is considered the gold standard for the evaluation of the susceptibility to antifungal agents in Candida species.1,3 However, this is an expensive and laborious method not available in most microbiology laboratories.
The purpose of this study is to evaluate the performance of two different methods of susceptibility testing for Candida species, Vitek 2 (bioMérieux SA, Marcy l’Etoile, France) and the colorimetric test Sensititre YeastOne (Thermo Fisher), comparing them to the BMD method according to the Clinical Laboratory Standards Institute (CLSI).
Materials and methodsA total of 80 isolates of Candida were selected from patients with candidemia and isolated from sterile sites such as pleural and peritoneal fluid. The strains were recovered from our microorganism collection, and only one episode of candidemia in a patient was included. We evaluated the following isolates and species: 16 C. albicans, 16 C. parapsilosis species complex, 16 C. tropicalis, 16 C. glabrata and 16C. krusei. All isolates were identified using chromogenic medium and mass spectrometry using MALDI-TOF-LT Microflex, Bruker Daltonics (Bruker, Germany). For this study we used the software Biotyper 3.0. According to the manufacturer's recommendations, we accepted a genus and species score above 2.0 as reliable.
The BMD was performed according to document M27-A3 of the Clinical and Laboratory Standards Institute (CLSI).3 The antifungal agents tested and the concentrations used are the following: 0.125–64μg/ml for fluconazole (Pfizer Inc., New York, NY USA®) and 0.03–16μg/ml for voriconazole (Pfizer Inc., New York, NY USA®), amphotericin B (Sigma Chemical Corporation, St. Louis, MO, USA®) and caspofungin (Merck & Co., Inc., Rahway, NJ, USA®). For the interpretation of the results we carried out readings after 24h of incubation at 35°C using two distinct readings: for the amphotericin B MIC was defined as the lowest drug concentration able to inhibit 90% or more of growth compared to the control, and for the other antifungal agents the MIC was the lowest concentration able to significantly inhibit growth (50%) compared to the positive control.
For quality assessment of susceptibility testing, the following reference strains from ATCC® were used: C. parapsilosis (ATCC 22019) and C. krusei (ATCC 6258), according to CLSI recommendations M27-A3 (2008). The variations in the MIC of the quality control strains was consistent with those recommended by CLSI M27-S4 document 2012.4
Susceptibility testing by commercial methodsVitek 2We carried out the susceptibility testing using Vitek XL (bioMérieux SA). A standard suspension of yeasts (2.0 McFarland) was inoculated in the AST-YS07 card containing three different concentrations of fluconazole (1–64μg/ml), voriconazole (0.125–8μg/ml), caspofungin (0.25–4μg/ml) and amphotericin B (0.25–16μg/ml). These cards were incubated at a temperature of 35°C, and readings were performed automatically after approximately 24h. The MIC values were interpreted according to the breakpoints stated in CLSI M27-S4.4
Sensititre YeastOneSensititre YeastOne (Thermo Fisher) is a commercial colorimetric test for the determination of minimum inhibitory concentrations. The manufacturer provides a frozen panel with different antifungal concentrations suitable for broth microdilution. To perform the commercial test using Sensititre YeastOne, an inoculum suspension (0.5 McFarland) was inoculated into a culture medium provided by the manufacturer. Plates containing lyophilized antifungals were rehydrated with inoculum suspension using a multichannel pipette (100μl in each well). The Sensititre YeastOne panels had the following serial dilutions: 0.125–8μg/ml for amphotericin B, 0.125–256μg/ml for fluconazole, and 0.008–8μg/ml for voriconazole and caspofungin. Plates were covered with adhesive and incubated at 35°C for 24h. Sensititre YeastOne uses a colorimetric assay for MIC evaluation: the minimum inhibitory concentration is that with which there is no growth of the fungus, and the color changes from blue to pink when growth is detected.
Analysis of resultsWe evaluated the categorical agreement (CA) between the methods tested. We also analyzed the categorical analysis error as recommended by CLSI, as follows:
- •
Very Major Error (VME): when the result of the method classified the isolate tested as susceptible but the gold standard method classified the isolate as resistant to the drug.
- •
Major Error (ME): when the method classified the isolate as resistant but the gold standard method classified the same isolate as susceptible to the drug.
- •
Minor Error (MiE): when the method tested classified the isolate as intermediate but the gold standard method classified the same isolate as susceptible or resistant. The opposite situation can also be considered a MiE.
According to the CLSI document (M23-ED4), the acceptable rates of error of the methodology tested when compared with the gold standard were the following: <1.5% of VME, <3% of ME and < 5% of MiE.5
Essential agreement was defined as when the gradient test, Vitek 2 and the colorimetric test results agreed within 1 log2 dilution compared to the reference broth microdilution method. Discrepant results were defined if there were 2 log2 or more dilution differences between the methods. The target intermethod correlation should be greater than 90% according to the CLSI document (M23-ED4).5
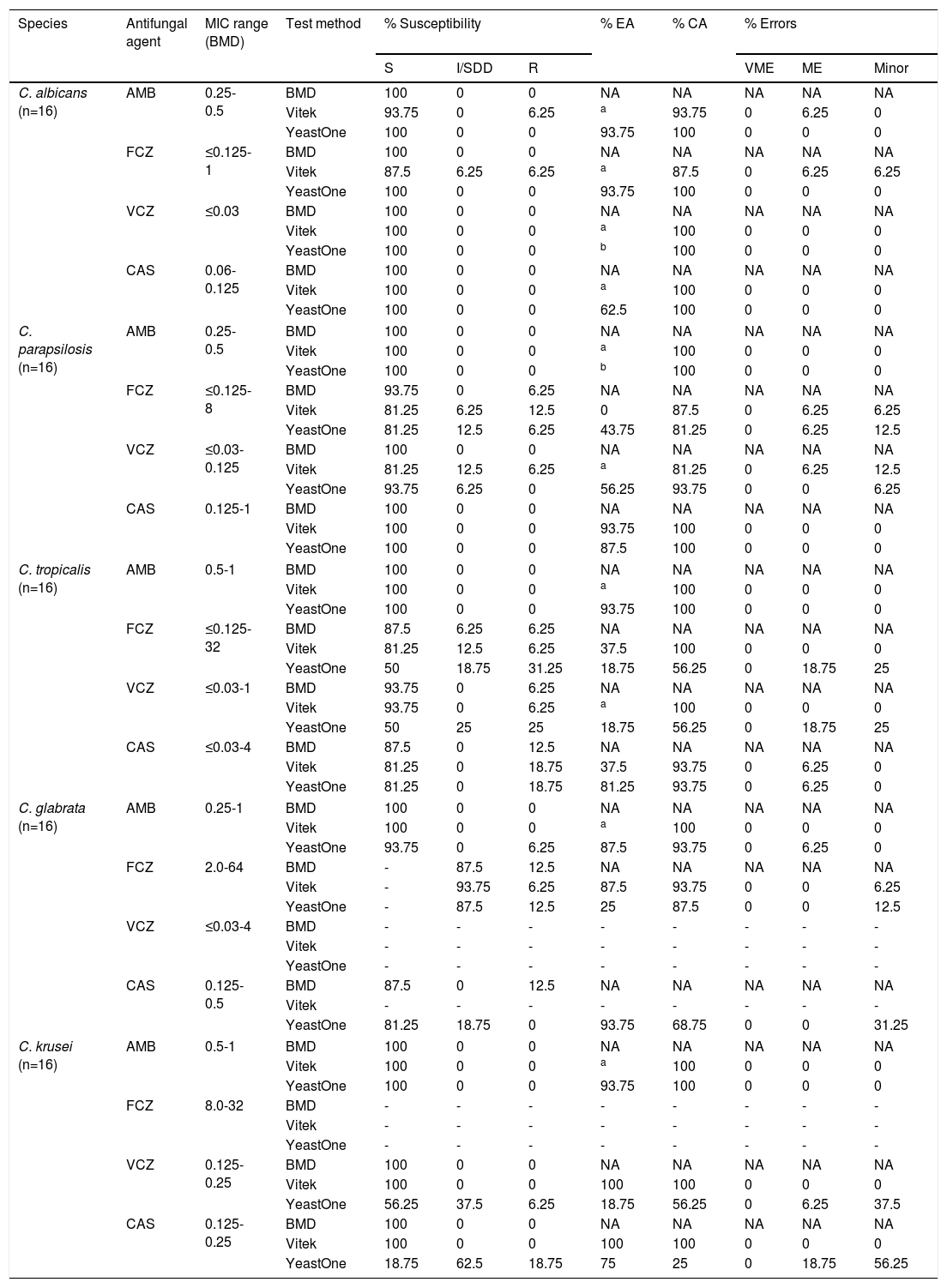
ResultsWe comparatively analyzed the CA between the methods, stratifying the disagreements according to the CLSI recommendations. Table 1 summarizes the agreement between the three methods assayed with different Candida species. The MIC ranges determined by BMD for each combination of species/antifungal agent are also shown in Table 1 For C. albicans and C. tropicalis, no resistance was observed for all of the antifungals tested. For C. krusei and C. glabrata we observed higher MICs for fluconazole. No resistance to echinocandins or amphotericin B were observed in these isolates. One isolate of C. tropicalis (MIC=32μg/ml) and one isolate of C. parapsilosis (MIC=8μg/ml) were resistant to fluconazole.
Susceptibility testing with amphotericin B, fluconazole, voriconazole and caspofungin for 5 Candida species. The categorical agreement, essential agreement and the analysis of error as recommended by CLSI between Vitek 2, Yeast One and BMD are also shown in this table.
| Species | Antifungal agent | MIC range (BMD) | Test method | % Susceptibility | % EA | % CA | % Errors | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I/SDD | R | VME | ME | Minor | ||||||
| C. albicans (n=16) | AMB | 0.25-0.5 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA |
| Vitek | 93.75 | 0 | 6.25 | a | 93.75 | 0 | 6.25 | 0 | |||
| YeastOne | 100 | 0 | 0 | 93.75 | 100 | 0 | 0 | 0 | |||
| FCZ | ≤0.125-1 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 87.5 | 6.25 | 6.25 | a | 87.5 | 0 | 6.25 | 6.25 | |||
| YeastOne | 100 | 0 | 0 | 93.75 | 100 | 0 | 0 | 0 | |||
| VCZ | ≤0.03 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | b | 100 | 0 | 0 | 0 | |||
| CAS | 0.06-0.125 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | 62.5 | 100 | 0 | 0 | 0 | |||
| C. parapsilosis (n=16) | AMB | 0.25-0.5 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | b | 100 | 0 | 0 | 0 | |||
| FCZ | ≤0.125-8 | BMD | 93.75 | 0 | 6.25 | NA | NA | NA | NA | NA | |
| Vitek | 81.25 | 6.25 | 12.5 | 0 | 87.5 | 0 | 6.25 | 6.25 | |||
| YeastOne | 81.25 | 12.5 | 6.25 | 43.75 | 81.25 | 0 | 6.25 | 12.5 | |||
| VCZ | ≤0.03-0.125 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 81.25 | 12.5 | 6.25 | a | 81.25 | 0 | 6.25 | 12.5 | |||
| YeastOne | 93.75 | 6.25 | 0 | 56.25 | 93.75 | 0 | 0 | 6.25 | |||
| CAS | 0.125-1 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 100 | 0 | 0 | 93.75 | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | 87.5 | 100 | 0 | 0 | 0 | |||
| C. tropicalis (n=16) | AMB | 0.5-1 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | 93.75 | 100 | 0 | 0 | 0 | |||
| FCZ | ≤0.125-32 | BMD | 87.5 | 6.25 | 6.25 | NA | NA | NA | NA | NA | |
| Vitek | 81.25 | 12.5 | 6.25 | 37.5 | 100 | 0 | 0 | 0 | |||
| YeastOne | 50 | 18.75 | 31.25 | 18.75 | 56.25 | 0 | 18.75 | 25 | |||
| VCZ | ≤0.03-1 | BMD | 93.75 | 0 | 6.25 | NA | NA | NA | NA | NA | |
| Vitek | 93.75 | 0 | 6.25 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 50 | 25 | 25 | 18.75 | 56.25 | 0 | 18.75 | 25 | |||
| CAS | ≤0.03-4 | BMD | 87.5 | 0 | 12.5 | NA | NA | NA | NA | NA | |
| Vitek | 81.25 | 0 | 18.75 | 37.5 | 93.75 | 0 | 6.25 | 0 | |||
| YeastOne | 81.25 | 0 | 18.75 | 81.25 | 93.75 | 0 | 6.25 | 0 | |||
| C. glabrata (n=16) | AMB | 0.25-1 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 93.75 | 0 | 6.25 | 87.5 | 93.75 | 0 | 6.25 | 0 | |||
| FCZ | 2.0-64 | BMD | - | 87.5 | 12.5 | NA | NA | NA | NA | NA | |
| Vitek | - | 93.75 | 6.25 | 87.5 | 93.75 | 0 | 0 | 6.25 | |||
| YeastOne | - | 87.5 | 12.5 | 25 | 87.5 | 0 | 0 | 12.5 | |||
| VCZ | ≤0.03-4 | BMD | - | - | - | - | - | - | - | - | |
| Vitek | - | - | - | - | - | - | - | - | |||
| YeastOne | - | - | - | - | - | - | - | - | |||
| CAS | 0.125-0.5 | BMD | 87.5 | 0 | 12.5 | NA | NA | NA | NA | NA | |
| Vitek | - | - | - | - | - | - | - | - | |||
| YeastOne | 81.25 | 18.75 | 0 | 93.75 | 68.75 | 0 | 0 | 31.25 | |||
| C. krusei (n=16) | AMB | 0.5-1 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA |
| Vitek | 100 | 0 | 0 | a | 100 | 0 | 0 | 0 | |||
| YeastOne | 100 | 0 | 0 | 93.75 | 100 | 0 | 0 | 0 | |||
| FCZ | 8.0-32 | BMD | - | - | - | - | - | - | - | - | |
| Vitek | - | - | - | - | - | - | - | - | |||
| YeastOne | - | - | - | - | - | - | - | - | |||
| VCZ | 0.125-0.25 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 100 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | |||
| YeastOne | 56.25 | 37.5 | 6.25 | 18.75 | 56.25 | 0 | 6.25 | 37.5 | |||
| CAS | 0.125-0.25 | BMD | 100 | 0 | 0 | NA | NA | NA | NA | NA | |
| Vitek | 100 | 0 | 0 | 100 | 100 | 0 | 0 | 0 | |||
| YeastOne | 18.75 | 62.5 | 18.75 | 75 | 25 | 0 | 18.75 | 56.25 | |||
AMB, amphotericin B; FCZ, fluconazole; VCZ, voriconazole; CAS, caspofungin; S, susceptible; I, intermediate; SDD, susceptible dose dependent; R, resistant; EA, essential agreement; CA, categorical agreement; VME, very major error; ME, major error; MiE, minor error; NA, not applicable.
The general categorical agreement between the methods was 96.3% for the automated method (Vitek 2) and 84% for the colorimetric method (Sensititre YeastOne). No VMEs were observed. However, MEs and MiEs were found for all the isolates tested. For C. albicans, a 6.25% (1 isolate) ME was observed with fluconazole and amphotericin B using Vitek 2. By the same method, we noted a 6.25% MiE only for fluconazole. For C. parapsilosis and fluconazole we observed a 6.25% ME and 6.25% MiE with Vitek 2. Using the Sensititre YeastOne method, we also observed a 6.25% ME but a 12.5% MiE for the same drug. Regarding voriconazole, Vitek 2 showed a 6.25% ME and 12.5% MiE. With Sensititre YeastOne, only 6.25% showed a MiE for this drug.
When we analyzed C. tropicalis, only Sensititre YeastOne had errors regarding azoles. A 18.75% ME and 25% MiE with fluconazole and voriconazole were observed, respectively. Both methodologies showed a 6.25% ME when we analyzed caspofungin. For C. glabrata, Sensititre YeastOne had only a 6.25% ME with amphotericin B. Concerning fluconazole, both methods showed only MiEs, at 6.25% and 12.5% with Vitek 2 and Sensititre Yeast One, respectively. A methodological limitation with caspofungin and C. glabrata was observed using Vitek 2 when the lowest concentration of the drug available in the panel was above the breakpoint for susceptibility according to the CLSI document (the lower concentration is 0.25μg/ml and the breakpoint for susceptibility is less than or equal to 0.125μg/ml). Thus, the data using this method could not be analyzed. For C. krusei, only Sensititre YeastOne had a ME with voriconazole and caspofungin, 6.25% and 18.75%, respectively. The same method also showed a MiE with these drugs, 37.5% with voriconazole and 56.25% with caspofungin.
The performance of the methods with amphotericin was high (CA greater than 93.7%). Only two MEs were observed, one for C. albicans and Vitek 2 and one for C. glabrata and Sensititre YeastOne.
DiscussionSusceptibility testing for antifungal agents in microbiology laboratories has a direct impact on the appropriate treatment of patients with infections caused by Candida species. Recently, several studies have reported the emergence of resistance to antifungals in C. tropicalis and C. parapsilosis complex.9,12,19,20,23 We have observed a high incidence of candidemia caused by non-C. albicans species, mainly C. glabrata, which has a reduced susceptibility to azoles.6,10,13,15,19
In this scenario, different commercial methods are available for susceptibility testing. Vitek 2 is a fully automated system based on a spectrophotometric evaluation of the MIC. This method is easy to perform and requires minimal handling. The reading is automatically determined and interpreted by the software of this system. However, only three dilutions are tested in the susceptibility card and intermediate concentrations are calculated.
Sensititre YeastOne is a commercial BMD method available in panels with several antifungals in different concentrations. The MIC reading is performed visually: the blue color in the wells changes to pink when growth is present. Despite being easy to handle and read, there are manual steps for the dilution and inoculation of the panel. However, both methods are easier than the traditional BMD, which usually requires many steps, including the preparation of the drugs and solutions, manual inoculation and the reading of the MICs. We observed, in general, high categorical agreement (average CA) between the two methods tested when compared to the conventional BMD: 96.3% with the automated method (Vitek 2) and 84% with the colorimetric method (Sensititre YeastOne). However, we noted variations in the CA depending on the species and drugs tested; the CA ranged from 18.7 to 100%. Cuenca-Estrella et al. evaluated in 2010 four different techniques of susceptibility testing, including Sensititre YeastOne and BMD. The authors found a high CA greater than 97% for Candida spp. with all the antifungals tested.7 Bertout et al. compared Sensititre YeastOne and BDM and found a CA ranging from 72.6 to 97.1%.2 In our study, no VMEs were found. However, another study reported 1.6% and 0.2% between BMD and Vitek 2 or Sensititre YeastOne, respectively.7
When we analyzed the results of the azoles, both Vitek 2 and Sensititre YeastOne showed similar performance, except for C. tropicalis and C. krusei, where Sensititre YeastOne had a lower CA (56.2%). Pfaller et al. also showed high agreement for all the species tested when comparing Vitek 2 and BMD.18 With the echinocandins, both methods showed good performance for C. albicans, C. parapsilosis and C. tropicalis. However, we observed important discrepancies for C. krusei and voriconazole: Vitek 2 had 100% CA while Sensititre YeastOne had only 25%. Pfaller et al. also observed a low CA (69.1%) for C. krusei and caspofungin but showed good performance for other species using Sensititre YeastOne when compared to BMD.16 With amphotericin B, as seen in previous studies, Vitek 2 and Sensititre YeastOne had good performance and a high CA.7,17 Our data demonstrate that the results for C. krusei and C. glabrata with caspofungin using Sensititre YeastOne should be interpreted carefully because we observed a low CA. We also emphasize the low CA for C. tropicalis and fluconazole using the same method.
BMD is still the gold standard for the susceptibility testing of Candida species. However, this method is laborious and expensive for clinical microbiology laboratories. In this study we evaluated the performance of Vitek 2 and Sensititre YeastOne in five species of Candida. We concluded that although there was a high CA between the methods for almost all drug/yeast combinations, YeastOne had a low CA with fluconazole and voriconazole for C. tropicalis, and it was the same for C. krusei and voriconazole. In this case, the Vitek 2 showed better results and could be an interesting alternative in clinical laboratories. With caspofungin, Sensititre YeastOne presented a low CA for C. glabrata and C. krusei. Caspofungin is an important therapeutic choice to treat infections caused by these agents, and other methods should be considered when testing this drug. For C. krusei, Vitek 2 is a better option when testing caspofungin. An important limitation of Vitek 2 is the combination of C. glabrata/caspofungin, as previously stated, emphasizing that BMD is a better method because Sensititre YeastOne did not have a good performance for this echinocandin.
Conflict of interestsThe authors declare no conflict of interests.