Although in the past decade the management of invasive fungal infections has improved, a number of controversies persist regarding empirical antifungal treatment in critically-ill hematology patients.
AimsTo identify key clinical knowledge to elaborate a set of recommendations, with a high level of consensus, necessary for the approach to fungal infections in critically-ill hematology patients.
MethodsA Spanish prospective questionnaire, which measures consensus through the Delphi technique, was anonymously answered and e-mailed by 30 multidisciplinary national experts, all specialists in fungal invasive infections from six scientific national societies; intensivists, anesthesiologists, microbiologists, pharmacologists and specialists in infectious diseases. They responded to 10 questions prepared by the coordination group after a thorough review of the literature published in the last few years. For a category to be selected, the level of agreement among the experts in each category must be equal to or greater than 70%. In a second round, 73 specialists attended a face-to-face meeting held after extracting the recommendations from the chosen topics, and validated the pre-selected recommendations and derived algorithm.
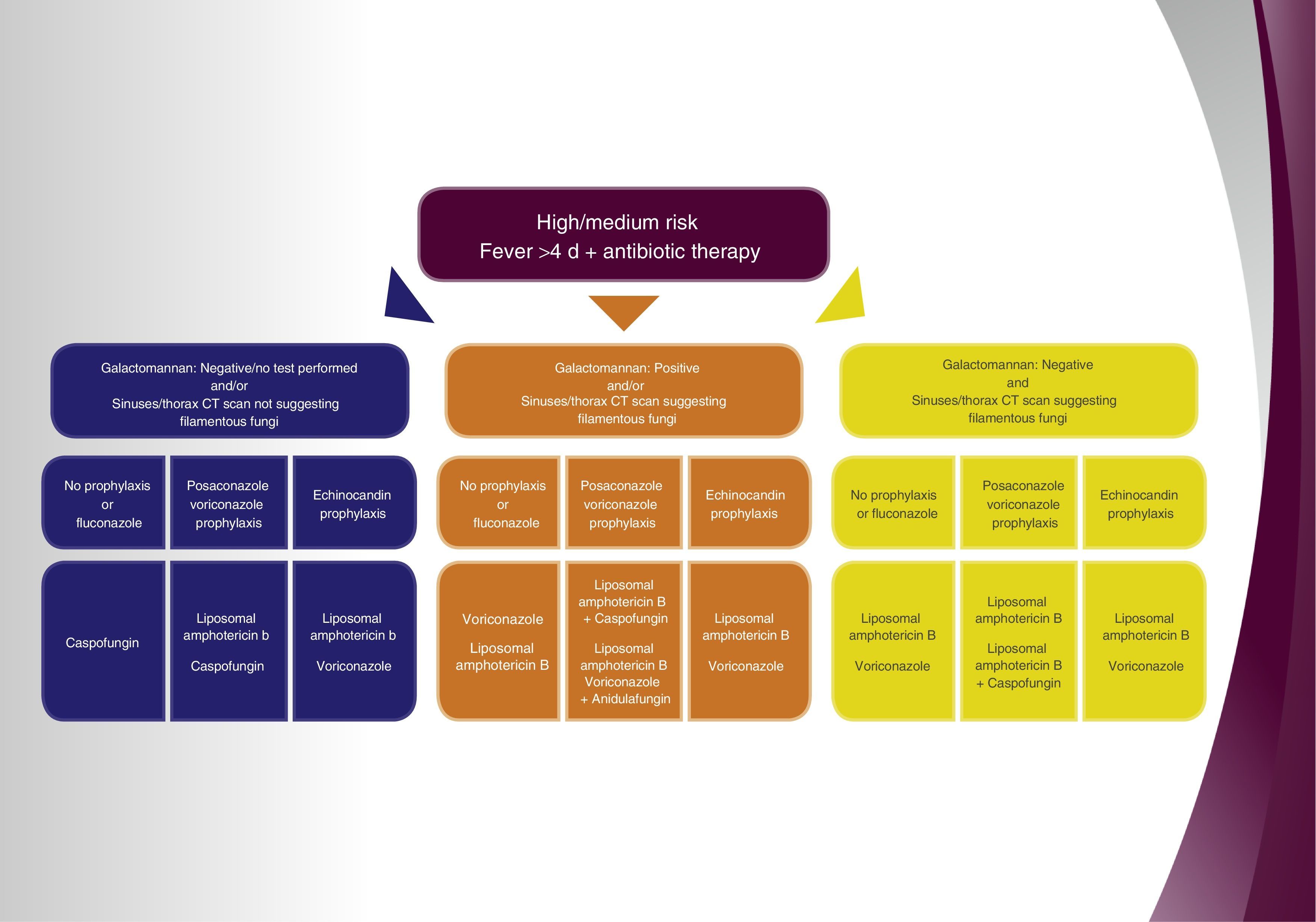
ResultsAssess administering antifungal treatment to patients with high/medium risk factors and fever for over 4 days after onset of antibiotic therapy, and in the event of negative galactomannan or if no detection analysis has been performed and no relevant findings in the sinus and chest computed tomography (CT) have been detected, (1) in the case the patient did not receive prophylaxis, or was administered fluconazole, caspofungin treatment is recommended; (2) in the event the patient received prophylaxis with an azole with activity against filamentous fungi, the administration of liposomal amphotericin B is recommended and caspofungin as second choice therapy; (3) in the event that the prophylaxis received was an echinocandin, liposomal amphotericin B therapy is recommended and voriconazole as second choice. Assess administering antifungal treatment in patients with high/medium risk factors and fever for more than 4 days after onset of antibiotic therapy, and in the event of a positive galactomannan and/or sinus and chest CT suggests fungal infection caused by filamentous fungi, (1) in the event the patient did not receive antifungal prophylaxis or was administered fluconazole, the recommended treatment of choice is voriconazole or liposomal amphotericin B; (2) if the patient received prophylaxis with an azole with activity against filamentous fungi, the administration of liposomal amphotericin B with caspofungin is recommended and monotherapy with liposomal amphotericin B or the combination of voriconazole and anidulafungin are recommended as second choice therapies; (3) in the event an echinocandin was administered as prophylaxis, liposomal amphotericin B or voriconazole are the recommended treatments of choice. Consider the administration of antifungal treatment in patients with high/medium risk factors and fever for more than 4 days after onset of antibiotic therapy, and in the event of a negative galactomannan and the sinus and chest CT suggests fungal infection caused by filamentous fungi, (1) if the patient did not receive prophylaxis or was administered fluconazole, the recommended treatment of choice is liposomal amphotericin B or voriconazole; (2) in the case the patient received prophylaxis with an azole with activity against filamentous fungi, the administration of liposomal amphotericin B is recommended as first choice therapy and liposomal amphotericin B combined with caspofungin as second choice; (3) in the event an echinocandin was administered as prophylaxis, liposomal amphotericin B or voriconazole are the recommended treatments of choice.
ConclusionsThe empirical antifungal approach in critically-ill hematology patients requires the application of the broad range of knowledge and skills described in our recommendations and algorithm. These recommendations, based on the DELPHI methodology, may help to identify potential patients, standardize their management and improve overall prognosis.
Aunque en la última década se ha observado una mejora en el tratamiento de la micosis invasiva, todavía existen numerosas controversias en el tratamiento antifúngico empírico del paciente hematológico en estado crítico.
ObjetivosIdentificar los principales conocimientos clínicos y elaborar recomendaciones con un alto grado de consenso, necesarias para el abordaje de la micosis invasiva en el paciente hematológico en estado crítico.
MétodosSe ha empleado un cuestionario prospectivo español, que mide el consenso mediante la técnica Delphi. Se llevó a cabo de forma anónima y por correo electrónico con 30 expertos multidisciplinarios nacionales, especialistas en micosis invasivas de seis sociedades científicas nacionales, incluyendo intensivistas, anestesistas, microbiólogos, farmacólogos y especialistas en enfermedades infecciosas, los cuales respondieron a 10 preguntas preparadas por el grupo de coordinación, tras una revisión exhaustiva de la bibliografía de los últimos años. El grado de acuerdo alcanzado entre los expertos en cada una de las categorías debería ser igual o superior al 70% para ser seleccionada. En una segunda fase, después de extraer las recomendaciones de los temas seleccionados, se celebró una reunión presencial con 73 especialistas y se les solicitó la validación de las recomendaciones preseleccionadas y del algoritmo derivado de estas.
ResultadosSe evalúa la administración de tratamiento antifúngico en pacientes hematológicos con factores de riesgo alto/medio y fiebre de más de 4 días después del inicio del tratamiento antibiótico si el galactomanano es negativo o no se ha realizado, y la TC de senos y tórax no aporta datos relevantes: 1) si no recibían profilaxis o era con fluconazol, se recomienda realizar un tratamiento con caspofungina; 2) en caso de que el paciente reciba profilaxis con un azol con actividad contra hongos filamentosos, la recomendación es la utilización de anfotericina B liposómica y como segunda opción, caspofungina, o 3) en el supuesto de que la profilaxis que reciba sea una equinocandina, la recomendación de tratamiento sería la anfotericina B liposómica y como alternativa el voriconazol. En pacientes con factores de riesgo alto/medio y fiebre de más de 4 días después del inicio del tratamiento antibiótico, si el galactomanano es positivo y/o la TC de senos y tórax sugiere infección por hongos filamentosos: 1) si el paciente no recibe profilaxis o es esta es con fluconazol, la recomendación es utilizar como tratamiento voriconazol o anfotericina B liposómica; 2) si la profilaxis que recibe es con un azol con acción contra hongos filamentosos, la recomendación es la utilización de anfotericina B liposómica con caspofungina y como alternativa anfotericina B liposómica en monoterapia o la combinación voriconazol con anidulafungina, o 3) si la profilaxis es con una equinocandina, la recomendación de tratamiento es emplear anfotericina B liposómica o voriconazol. En pacientes con factores de riesgo alto/medio y fiebre de más de 4 días después del inicio del tratamiento antibiótico, si el galactomanano es negativo y la TC de senos y tórax sugiere infección por hongos filamentosos: 1) si el paciente no recibe profilaxis o es con fluconazol, la recomendación es utilizar como tratamiento anfotericina B liposómica o voriconazol; 2) si la profilaxis que recibe es con un azol con acción contra hongos filamentosos, la recomendación es utilizar anfotericina B liposómica y como alternativa anfotericina B liposómica combinada con caspofungina, o 3) si la profilaxis es con una equinocandina, la recomendación de tratamiento es anfotericina B liposómica o voriconazol.
ConclusionesEl abordaje del tratamiento antifúngico empírico del paciente crítico hematológico requiere la aplicación de los conocimientos y destrezas que se detallan en nuestras recomendaciones y en el algoritmo desarrollado. Estas recomendaciones basadas en la metodología Delphi pueden ayudar a identificar a los potenciales pacientes, estandarizar su tratamiento en conjunto y mejorar su pronóstico.
Patients with hematologic malignancies have a high risk of acquiring invasive fungal infection (IFI).22Aspergillus and Candida are the most prevalent pathogens in oncohematological patients, and thus, the cause of more than 95% of IFIs in this population.2 Despite the improvement in early diagnosis and the use of new antifungal drugs, the mortality rate associated to invasive candidiasis (IC) and to invasive aspergillosis (IA) in patients suffering from malignant blood diseases and in transplant hematopoietic cell recipients continues significantly high.10,12,16
Since the nineties, due to the use of fluconazole as a prophylaxis therapy, the incidence of IC has decreased significantly in hematology patients.20 However, mortality associated to IC has remained stable, ranging between 20% and 50%.21 On the other hand, the incidence of probable or proven IFI caused by filamentous fungi, especially IA in high-risk hematology patients, has been established between 4% and 22%.23 This is certainly a relevant aspect, as the rate of mortality due to IA is superior to 70% in cases of delayed diagnoses.27 The precise diagnosis of IFI, particularly IA, continues to be quite problematic in patients with malignant blood disease, with unspecific signs and symptoms that may appear late4. Prevention strategies against IFI in hematology patients include the combination of environmental measures and pharmacological prophylaxis.8 Although the incidence of IA in high-risk patients has decreased thanks to the use of highly effective prophylactic treatments, it is difficult to diagnose due to the low sensitivity that biomarkers achieve in this patient population undergoing prophylaxis. Therefore, empirical antifungal therapies are a common practice in critically-ill patients while a precise diagnosis is confirmed.18 In this context, IFIs in critically-ill hematology patients are an excellent target for using empirical antifungal therapies.31 This research study mainly aims to analyze the current situation of empirical antifungal treatment in critically-ill hematology patients admitted to hospitals in Spain, and also to obtain a set of therapeutic recommendations for different scenarios through the DELPHI methodology. For this purpose, a panel including specialists from six scientific societies was formed; Spanish Society of Mycology (AEM), as the promoter; the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC); the Spanish Society of Anesthesiology, Reanimation and Pain Therapeutics (SEDAR); the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC); the Spanish Society of Chemotherapy (SEQ); the Spanish Society of Hospital Pharmacies (SEFH) – all with broad experience in the treatment of critically-ill patients. They were requested to answer a questionnaire drafted by the seven coordinators responsible for this research, who had previously conducted a thorough review of the existing literature, as was performed in the two previous editions of this project.29,30 In a second round, and after the elaboration of the resulting recommendations by the group of coordinators, a second analysis was requested in a face-to-face meeting in which 73 specialists from all around the country who care for critically-ill hematology patients, validated the pre-selected recommendations and the derived algorithm through a voting procedure. The panel was made up of 30 specialists from different geographic locations in our country from six scientific societies involved in the research. The criteria followed for their inclusion was their experience in the investigation of IFIs, as well as their experience in antifungal treatment in critically-ill hematology patients. The Delphi methodology was used for the development of this study to optimize the consultation process of the 30 panel members. More specifically, thanks to the Delphi methodology, we were able to learn the groups’ opinions; not only those of a given individual, but the opinion of each of the experts in different areas of information suggested by the coordinators. The level of consensus required was equal or higher than 70% (21 out of 30) in Top 4 (score of 7 points or more) of the total number of experts consulted in each question. A total of 10 questions, all posed by the coordinators (Annex 1), were evaluated by the experts using a metric scale. The study methodology was based on the development of two phases aimed to discover the level of consensus of each question. In the first phase, on May 19 and 26, 2014, 30 specialists (Annex 2) participating in the research anonymously answered the 8 questions included in the online questionnaire. The second phase consisted of a meeting held on June 26, 2014 in which 23 of the 28 participants anonymously answered the 2 remaining questions on the questionnaire. The coordinators, who were responsible for the systematic research of the literature to elaborate the questions, did not answer the questionnaire. Thereafter, as mentioned above, recommendations and an algorithm were produced and validated by 73 experts in a face-to-face meeting held on September 25, 2014 (Annex 3).
Results1. Assess administering antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment, and in the event of a negative galactomannan or if no detection analysis was performed and no relevant findings were detected in the paranasal sinuses and thorax computed tomography (CT). In this scenario azole prophylaxis against filamentous fungi was administered.
In this respect, the following answers were provided by the coordinators: Amphotericin B lipid complex; none, in the event the patients were stable; micafungin; voriconazole; liposomal amphotericin B; caspofungin.
Rationale. The clinical practice guidelines of the 3rd European Conference on Infections in Leukemia (ECIL-3) recommend the administration of antifungal empirical treatment in this situation with a moderate degree of evidence. Even though caspofungin, and liposomal amphotericin B are the antifungal drugs with the highest degree of scientific evidence,24 the administration of caspofungin is the empirical treatment of choice due to its better tolerability. Most of the panel members (75%) agreed it is appropriate to administer caspofungin as part of an empirical antifungal therapy in this situation. More specifically, on a scale of 0 to 10, where 10 stands for the maximum degree of agreement, 21 out of a total 28 specialists granted 7 points or more to the use of caspofungin, whereas a consensual agreement was reached (Top≥70%). In contrast, no agreement was reached (Top<70%) when considering not to administer an antifungal therapy when the patients were stable (12 answers with 7 points or more; Top 4: 42%) or the use of liposomal amphotericin B (18; 64.3%), voriconazole/17; 60.7%), micafungin (15; 53.6%) and amphotericin B lipid complex (8; 28.6%).
2. Assess administering antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment, and in the event of a negative galactomannan or if no detection analysis was performed and no relevant findings were detected in the sinuses and thorax computed tomography (CT). In this scenario azole prophylaxis against filamentous fungi was administered.
In this respect, the following answers were provided by the coordinators: Amphotericin B lipid complex; none, in the event the patients were stable; micafungin; voriconazole; liposomal amphotericin B; caspofungin.
Rationale. IFIs in patients receiving posaconazole prophylaxis are not very frequent and are associated to a worse prognosis.11 In case of a negative result for galactomannan antigen (a situation which may be due to its lower susceptibility to azoles) and with no radiological evidence to justify IFI in a critically-ill patient treated with broad spectrum azole prophylaxis, the antifungal therapy must be effective against a possible azole resistant Candida, Aspergillus or other fungi.11,13 Although, there is a lack of randomized studies in which this situation has been analyzed, liposomal amphotericin B could be considered the treatment of choice. In patients with a low incidence of non-Aspergillus filamentous fungi, caspofungin could be an alternative therapy.13,24,28 Under the above mentioned circumstances, the great majority of the experts agreed on the appropriateness of administering empirical antifungal therapy with liposomal amphotericin B or with caspofungin. Specifically, on a scale of 0–10, in which 10 stands for the highest level of agreement, 23 out of 28 specialists (82.1%) granted 7 points or more to the use of liposomal amphotericin B, and 21 specialists (75%) achieved the same level of agreement with respect to the use of caspofungin. Thus, a high level of consensus for both antifungal therapies was achieved (Top 4≥70%).
In contrast, no consensus was reached (Top 4<70%) when considering not to administer an antifungal therapy when the patients were stable (8 answers with 7 or more points; Top 4: 28.6%) or the use of micafungin (14; 50%) and amphotericin B lipid complex (13; 46.4%).
3. Consider the administration of antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment, and in the event of a negative galactomannan or if no detection analysis was performed and no relevant information from sinuses and thorax CT was detected. Under these circumstances, echinocandin prophylaxis against filamentous fungi was administered.
The following answers were provided by the coordinators: amphotericin B lipid complex, none in the event the patients were stable, voriconazole, liposomal amphotericin B.
Rationale. The administration of prophylactic echinocandin antifungal therapy implies the risk of echinocandin resistance.19 In the case of a negative galactomannan antigen (a situation which may be due to its variable sensitivity or to the use of candins) and in the absence of radiological evidence which justifies IFI in critically-ill patients on an echinocandin prophylactic treatment, the antifungal therapy must be effective against a possible azole resistant to Candida, Aspergillus or other filamentous fungus.5,13 Although there is a lack of randomized studies analyzing this situation, the treatment of choice could be liposomal amphotericin B.13,24,28 The great majority of the panel members believed that it is appropriate to prescribe liposomal amphotericin B and voriconazole as a suitable antifungal therapy in this population. Specifically, on a scale of 0–10, in which 10 stands for the highest level of agreement, 22 out of the 28 specialists (78.6%) granted 7 or more points to the use of liposomal amphotericin B, and 20 specialists (71.4%) achieved the same level of agreement with respect to the use of voriconazole, Thus, a high level of consensus for both antifungal therapies was achieved (Top 4≥70%). In contrast, consensus was not reached (Top 4<70%) when evaluating not to administer an antifungal treatment when the patients were stable (7 answers with 7 or more points; Top 4: 25%) or the use of amphotericin B lipid complex (13; 46,4%).
4. Evaluate administering antifungal treatment in patients with high/medium risk factors on antibiotic treatment and with a positive galactomannan and/or presence of filamentous fungi infection evidenced from sinuses and thorax CT. In this scenario fluconazole or no prophylaxis was administered.
The following answers were provided by the coordinators: amphotericin B lipid complex, voriconazole, amphotericin B liposomal, caspofungin.
Rationale. The situation described in the heading outlines a clinical situation that requires early therapy and compulsory treatment against Aspergillus.1,14 Therapeutic guidelines and documents of consensus establish the administration of voriconazole as the treatment of choice in the presence of evidence in this situation.3,9,24,25 The great majority of the specialists considered that it is appropriate to prescribe voriconazole and liposomal amphotericin B as an empirical antifungal therapy in this population of patients. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 26 out of the 28 specialists (92.9%) granted 7 or more points to the use of voriconazole in this situation; an opinion shared by 25 specialists (89.3%) in the case of liposomal amphotericin B. Therefore, high consensus was reached regarding both antifungal drugs (Top 4≥0%). In contrast, consensus was not reached (Top 4<70%) when evaluating antifungal treatment with caspofungin (18 responses with 7 or more points; Top 4: 64.3%) or with amphotericin B lipid complex (16; 57.1%) in this situation.
5. Assess administering antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and with a positive galactomannan and/or presence of filamentous fungi infection evidenced by sinuses and thorax CT. In this situation azole prophylaxis against filamentous fungi was administered.
The following answers were provided by the coordinators: liposomal amphotericin B, liposomal amphotericin B+caspofungin, voriconazole+anidulafungin, voriconazole+caspofungin.
Rationale. Breakthrough invasive aspergillosis in patients receiving broad spectrum azoles is not common (<3%). In this situation, in the event of a positive galactomannan, IA may be due to a lack of appropriate antifungal concentrations in serum.7 Although there are no randomized studies analyzing this situation, the treatment of choice could be liposomal amphotericin B, whereas the combination of 2 antifungals is considered an alternative therapy while awaiting a confirmed etiological diagnosis.3,9,24,25 The great majority of the experts agreed on the appropriateness of administering liposomal amphotericin B, either in monotherapy or in combination with caspofungin; or also the combined therapy of voriconazole+anidulafungin. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 23 out of the 25 specialists (92%) granted 7 points or more to the appropriateness of the combination of liposomal amphotericin B+caspofungin in this situation; an opinion shared by 19 experts (76.0%) for the administration of liposomal amphotericin B in monotherapy or the combination of voriconazole+anidulafungin. High consensus regarding the three above mentioned therapeutic alternatives was achieved (Top 4≥70%). In contrast, no consensus was reached (Top 4<70%) when evaluating the administration of a combined therapy of voriconazole+caspofungin (16 out of 25 answers with 7 points or more; Top 4: 64%) or monotherapy with voriconazole (7; 28%) in this situation.
6. Assess administering antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and with a positive galactomannan and/or suspicion of filamentous fungi infection evidenced from sinuses and thorax CT. In this scenario, echinocandin prophylaxis was administered.
The following answers were provided by the coordinators: amphotericin B lipid complex, voriconazole, liposomal amphotericin B.
Rationale. Although there are no randomized studies in which breakthrough IFI in patients receiving echinocandin prophylaxis treatment or empirical treatment has been analyzed, voriconazole17 may be considered the treatment of choice in case of a breakthrough IFI with evidence of aspergillosis in this population.3,9,24,25 Liposomal amphotericin B may also be an alternative and the first therapeutic choice in case of suspicion of the presence of non-Aspergillus filamentous fungi.3,9,24,25 The great majority of the experts participating in the research positively valued the administration of liposomal amphotericin B and voriconazole as empirical antifungal treatment in this situation. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 26 out of the 28 members of the panel (92.9%) granted 7 points or more to the appropriateness of a treatment with liposomal amphotericin B in this situation; an opinion shared by 25 specialists (89.3%) in the case of voriconazole. High consensus was achieved regarding both antifungal drugs (Top 4≥70%). In contrast, no consensus was reached (Top 4<70%) when considering the use of amphotericin B lipid complex (16 out of 28 responses with 7 or more points; Top 4: 57.1%) in this situation.
7. Assess the administration of antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and with a negative galactomannan and presence of filamentous fungi infection evidenced from sinuses and/or thorax CT. Under these circumstances, fluconazole or no prophylaxis was administered.
The following answers were provided by the coordinators: amphotericin B lipid complex, voriconazole, liposomal amphotericin B, caspofungin.
Rationale. In this situation, and in cases in which the galactomannan technique used complied with the established standards, the possibility of IFI caused by non-Aspergillus filamentous fungi should be taken into account. Thus, the best therapeutic choice is the administration of liposomal amphotericin B.3,9,15,24,25 The great majority of the specialists believed that it is appropriate to prescribe voriconazole and liposomal amphotericin B as an empirical antifungal treatment in this population of patients. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 25 out of the 28 members of the panel (89.3%) granted 7 points or more to the use of voriconazole or liposomal amphotericin B in this population, whereas consensus was reached regarding the use of both antifungal drugs (Top 4≥70%). In contrast, no consensus was reached when evaluating antifungal treatment with caspofungin (16 answers with 7 points or more; Top 4: 57.1%) or with amphotericin B lipid complex (15; 53.6%) in this situation.
8. Evaluate the administration of antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and in the event of a negative galactomannan and suspicion of filamentous fungi infection evidenced from sinuses and/or thorax CT. In this scenario, azole prophylaxis against filamentous fungi was administered.
The following answers were provided by the coordinators: liposomal amphotericin B, liposomal amphotericin B+caspofungin, voriconazole+caspofungin, voriconazole+anidulafungin, voriconazole.
Rationale. In this situation, in the event the patient has received azoles with activity against Aspergillus, the possibility of IFI caused by non-Aspergillus filamentous fungi3,9,15 should be taken into account. The galactomannan technique could be negative in patients receiving azoles against Aspergillus or negative because of its low sensitivity, therefore IA is not totally excluded. In this situation, the treatment of choice is liposomal amphotericin B,6 although the combined therapy of liposomal amphotericin B+caspofungin could also be considered due to the additive/synergistic effect it shows in vitro against mucorales.26 The great majority of the experts (96.0%) positively valued the administration of liposomal amphotericin B in this situation. Specifically, on a scale of 0–10 in which 10 stands for the highest level of agreement, 24 out of the 25 specialists granted 7 points or more to the appropriateness of a therapy with liposomal amphotericin B in this population; 80% of the experts validated the combination of liposomal amphotericin+caspofungin. (Top 4>70%) In contrast, consensus was not reached (Top 4<70%) when evaluating treatment with caspofungin (17 answers out of 25 with 7 points or more; Top 4: 68.0%) in this situation.
9. Assess administering antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and with a negative galactomannan, as well as suspicion of filamentous fungi infection evidenced by sinuses and/or thorax CT. Under these circumstances, echinocandin prophylaxis was administered.
The following answers were provided by the coordinators: amphotericin B lipid complex, voriconazole, liposomal amphotericin B.
Rationale. In this situation in which patients have been treated with echinocandins the possibility of IFI caused by non-Aspergillus filamentous fungi should be taken into account. Therefore, the administration of liposomal amphotericin B is thought to be the best therapeutic choice for this population.3,6,9,15 The vast majority of the experts participating in this research positively valued the administration of liposomal amphotericin B and voriconazole as an empirical antifungal therapy for this population. More specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 25 out of the 28 members of the panel (89.3%) granted 7 points or more to the appropriateness of a therapy with liposomal amphotericin B in this situation; an opinion shared by 23 specialists in the case of a treatment with voriconazole (82.1%). Hence, consensus was reached regarding both antifungal drugs (Top 4≥70%). In contrast, no consensus was reached (Top 4<70%) when evaluating the use of amphotericin B lipid complex (18 out of 28 answers with 7 or more points; Top 4: 64.3%) in this situation.
10. Assess the administration of antifungal treatment in patients with high/medium risk factors, fever for more than 4 days after initiating antibiotic treatment and in the event of a positive galactomannan and/or suspicion of infection caused by filamentous fungi, evidenced by the results of sinuses or thorax CT. In this situation, the combined treatment with caspofungin (together with either voriconazole or liposomal amphotericin B) was considered.
The following answers were provided by the coordinators: always, almost always, only in special situations, almost never, never.
Rationale. Combination treatment with caspofungin must be considered in special situations. This is the case of the combination with liposomal amphotericin B as a therapeutic alternative in patients receiving a prophylactic azole treatment against filamentous fungi, with positive galactomannan and/or sinuses and thorax CT suggesting an infection caused by filamentous fungi.3,9,24,25 This combination could also be beneficial for patients with negative galactomannan and/or sinuses and thorax CT suggesting infection caused by filamentous fungi, receiving a prophylactic azole treatment against filamentous fungi due to the in vitro synergistic/additive effect against mucorales.26 All panel members disagreed that the administration of a combined treatment of caspofungin with voriconazole or with liposomal amphotericin B can never be used in this population – “never” –. In fact, most of the experts (71.4%) considered that the above mentioned combined treatment with caspofungin is only indicated in special situations. Specifically, on a scale of 0–10 points, in which 10 stands for the highest level of agreement, 20 of the 28 members of the panel granted 7 points or more to this regard, therefore consensus was reached (Top 4≥70%) On the other hand, consensus was not achieved when considering the appropriateness of administering combined therapy with caspofungin in every situation in this population – “always” – (1 out of 28 answers with 7 points or more; Top 4: 3.6%), in nearly all situations – “almost always” – Top 4: 3.6%), or in a number of limited situations – “almost never” – (2; 7.1%).
Recommendations and algorithmOnce the results from the Delphi methodology applied to empirical antifungal treatment in critically-ill hematology patients were known, the 9 recommendations exhibited in Table 1 were extracted as conclusions. They were based on the questions that achieved a degree of agreement equal or over 70%. Thereafter, the recommendations and derived algorithm (Fig. 1) were validated in a face-to-face meeting with the hospital experts.
Recommendations for the empirical antifungal treatment of critically-ill hematology patients.
| 1) The administration of empirical antifungal treatment against yeasts and Aspergillus must be considered in critically-ill patients with high/medium IFI risk, persistent fever in spite of the administration of broad spectrum antibiotics and who have not received prophylaxis against filamentous fungi, and in the event of a negative galactomannan or if no analysis was performed and no relevant information from sinuses and thorax CT have been detected. In this situation caspofungin is the treatment of choice. |
| 2) The administration of empirical antifungal treatment with liposomal amphotericin B or caspofungin may be considered for critically-ill patients with high/medium IFI risk, persistent fever despite the administration of broad spectrum antibiotics, in the event of a negative galactomannan or if no analysis was performed, and no relevant information from thorax and sinuses CT has been detected, as well as receiving antifungal azole prophylaxis against filamentous fungi. |
| 3) The administration of empirical antifungal treatment with liposomal amphotericin B may be taken into consideration in critically-ill patients with high/medium IFI risk, persistent fever in spite of the administration of broad spectrum antibiotics, with a negative galactomannan or no analysis performed, and in the event no relevant information from thorax and sinuses CT has been detected, as well as receiving echinocandin prophylaxis. |
| 4) Voriconazole or liposomal amphotericin B are the treatment of choice for critically-ill patients with persistent fever in spite of the administration of broad spectrum antibiotics, with positive galactomannan and/or suspicion of filamentous fungi infection evidenced by sinuses and thorax CT and who are not receiving antifungal prophylaxis against filamentous fungi. |
| 5) Liposomal amphotericin B could be the treatment of choice for critically-ill patients with high/medium IFI risk, receiving antifungal prophylaxis against filamentous fungi, and in the event of a positive galactomannan antigen and/or suspicion of infection caused by filamentous fungi. Antifungal combined therapy could be taken into account in some situations. |
| 6) In critically-ill patients with high/medium IFI risk receiving antifungal prophylaxis with echinocandins meanwhile an etiologic diagnosis is made, and in the case of a positive galactomannan antigen and/or suspicion of infection caused by filamentous fungi evidenced by the CT, voriconazole is the treatment of choice in patients suffering from fungal breakthrough infection suspicious of aspergillosis. Liposomal amphotericin B would be the alternative and first choice if there was suspicion of the presence of non-Aspergillus filamentous fungi. |
| 7) Liposomal amphotericin B or voriconazole is the treatment of choice for critically-ill patients with high/medium IFI risk, persistent fever despite the administration of broad spectrum antibiotics, with a negative galactomannan antigen and radiological image suggesting IFI, and who are not receiving antifungal prophylactic treatment against filamentous fungi. |
| 8) Liposomal amphotericin B, in monotherapy or in combination, is the treatment of choice for critically-ill patients with a high/medium IFI risk, persistent fever in spite of the administration of broad spectrum antibiotics, with a negative galactomannan and radiological findings that suggest IFI, and who are on extended spectrum prophylactic antifungal treatment against filamentous fungi. |
| 9) Liposomal amphotericin B is the treatment of choice for critically-ill patients with a high/medium IFI risk, persistent fever in spite of the administration of broad spectrum antibiotics, with a negative galactomannan antigen and radiological findings that suggest IFI, and who are receiving echinocandin prophylaxis. |
This consensus has been sponsored by MSD Laboratories, Spain.
Rafael Zaragoza Crespo
Intensive Care Department, Dr. Peset University Hospital. Valencia, Spain
Ricard Ferrer Roca
Intensive Care Department, Vall d¿Hebron University Hospital. Barcelona, Spain
Alejandro Hugo Rodríguez
Intensive Care Department, Joan XXIII University Hospital. Tarragona, Spain
Emilio Maseda Garrido
Department of Anesthesiology and Surgical Critical Care, La Paz University Hospital. Madrid, Spain
Pedro Llinares Mondéjar
Infectious Diseases Department, A Coruña University Complex. A Coruña, Spain
Santiago Grau Cerrato
Pharmacy Department, Hospital del Mar. Barcelona, Spain
José María Aguado García
Infectious Diseases Department, 12 de Octubre University Hospital. Madrid, Spain
Gerardo Aguilar Aguilar
Department of Anesthesiology and Surgical Critical Care, Valencia Clinical University Hospital. Valencia, Spain
Benito Almirante Gragera
Infectious Diseases Department, Vall d’Hebron University Hospital. Barcelona, Spain
Francisco Álvarez Lerma
Intensive Care Department, Hospital del Mar. Barcelona, Spain
César Aragón González
Intensive Care Department, Carlos Haya University Hospital. Málaga, Spain
María Izaskun Azcárate Egaña
Intensive Care Department, Donostia University Hospital. Donostia, Spain
Marcio Borges Sa
Sepsis Unit Coordinator, Son Llàtzer Hospital. Palma de Mallorca, Spain
Mercedes Bouzada Rodríguez
Anaesthesia, Resuscitation and Pain Therapy Department, University Hospital Clinic of Santiago. Santiago de Compostela, Spain
Juan Carlos del Pozo Laderas
Intensive Care Department, Reina Sofía University Hospital. Córdoba, Spain
Carmen Fariñas Álvarez
Intensive Care Department, Marqués de Valdecilla University Hospital. Santander, Spain
Jesús Fortún Abete
Infectious Diseases Department, Ramón y Cajal University Hospital. Madrid, Spain
Beatriz Galván Guijo
Intensive Care Department, La Paz University Hospital. Madrid, Spain
José Garnacho Montero
Intensive Care Department, Virgen del Rocío University Hospital. Sevilla, Spain
José Ignacio Gómez Herreras
Department of Anesthesiology and Surgical Critical Care, Valladolid Clinical University Hospital. Valladolid, Spain
Rafael Huarte Lacunza
Pharmacy Department, Miguel Servet University Hospital. Zaragoza, Spain
Cristóbal León Gil
Intensive Care Department, Valme University Hospital. Sevilla, Spain
Rafael León López
Intensive Care Department, Reina Sofía University Hospital. Córdoba, Spain
Patricia Muñoz García
Microbiology and Infectious Diseases Department, Gregorio Marañón University Hospital. Madrid, Spain
Jordi Nicolás Picó
Pharmacy Department, Son Llàtzer Hospital. Palma de Mallorca, Spain
Pedro Olaechea Astigarraga
Intensive Care Department, Galdakao Usansolo Hospital. Vizcaya, Spain
Javier Pemán García
Microbiology Unit, La Fe University and Polythecnic Hospital. Valencia, Spain
María Luisa Pérez del Molino Bernal
Microbiology and Parasitology Unit, Santiago de Compostela University Hospital Complex. Santiago de Compostela, Spain
Leonor Periañez Párraga
Pharmacy Department, Son Espases University Hospital. Palma de Mallorca, Spain
Guillermo Quindós Andrés
Microbiology Unit, Faculty of Medicine and Dentistry, Basque Country University. Vizcaya, Spain
Jesús Rico Feijoo
Department of Anesthesiology and Surgical Critical Care, Río Hortega University Hospital. Valladolid, Spain
María Rodríguez Mayo
Microbiology Unit, A Coruña University Hospital Complex. A Coruña, Spain
Eva Romá Sánchez
Pharmacy Department, La Fe University and Polythecnic Hospital. Valencia, Spain
Isabel Ruiz Camps
Infectious Diseases Department, Vall d’Hebron University Hospital. Barcelona, Spain
Miguel Salavert Lleti
Infectious Diseases Department, La Fe University and Polytechnic Hospital. Valencia, Spain
Juan Carlos Valía Vera
Department of Anesthesiology and Surgical Critical Care, General University Hospital Consortium. Valencia, Spain
César Aldecoa Álvarez-Santullano
Department of Anesthesiology and Surgical Critical Care, Río Hortega University Hospital. Valladolid, Spain
Rosa Ana Álvarez Fernández
Department of Anesthesiology and Surgical Critical Care, Asturias Central University Hospital. Asturias, Spain
Rocío Armero Ibáñez
Department of Anesthesiology and Surgical Critical Care, Doctor Peset University Hospital. Valencia, Spain
Fernando Armestar Rodríguez
Intensive Care Department, Germans Trias i Pujol University Hospital. Badalona, Barcelona, Spain
Miguel Ángel Arribas Santamaría
Intensive Care Department, Arnau de Vilanova Hospital. Valencia, Spain
José Ignacio Ayestarán Rota
Intensive Care Department, Son Espases University Hospital. Palma de Mallorca, Spain
María Ángeles Ballesteros Sanz
Intensive Care Department, Marqués de Valdecilla University Hospital. Santander, Spain
María José Bartolomé Pacheco
Department of Anesthesiology and Surgical Critical Care, Marqués de Valdecilla University Hospital. Santander, Spain
Unai Bengoetxea Uriarte
Department of Anesthesiology and Surgical Critical Care, Basurto Hospital. Bilbao, Vizcaya, Spain
Eva Benveniste Pérez
Intensive Care Department, Germans Trias i Pujol University Hospital. Badalona, Barcelona, Spain
José Blanquer Olivas
Intensive Care Department, Valencia Clinical University Hospital. Valencia, Spain
Felipe Bobillo del Amo
Intensive Care Department, San Carlos Clinical University Hospital. Madrid, Spain
Ángel Caballero Sáez
Intensive Care Department, San Millán Hospital Complex- San Pedro Hospital. Logroño, La Rioja, Spain
Andrés Carrillo Alcaraz
Intensive Care Department, Morales Meseguer University General Hospital. Murcia, Spain
José Castaño Pérez
Intensive Care Department, Virgen de las Nieves University Hospital. Granada, Spain
Pedro Castro Rebollo
Intensive Care Department, Clínic i Provincial of Barcelona Hospital. Barcelona, Spain
Milagros Cid Manzano
Department of Anesthesiology and Surgical Critical Care, Complex of Ourense University Hospital. Ourense, Spain
Belén Civantos Martín
Intensive Care Department, La Paz University Hospital. Madrid, Spain
María Victoria de la Torre Prados
Intensive Care Department, Virgen de la Victoria University Hospital. Málaga, Spain
David Domínguez García
Department of Anesthesiology and Surgical Critical Care, Nuestra Señora de la Candelaria University Hospital. Santa Cruz de Tenerife, Spain
Juan Ramón Fernández Villanueva
Intensive Care Department, Complex of Santiago Compostela University Hospital. A Coruña, Spain
Rafael García Hernández
Department of Anesthesiology and Surgical Critical Care, Puerta del Mar University Hospital. Cádiz, Spain
Rafael Franco Llorente
Department of Anesthesiology and Surgical Critical Care, Virgen de las Nieves University Hospital. Granada, Spain
Luis Gajate Martín
Department of Anesthesiology and Surgical Critical Care, Ramón y Cajal University Hospital. Madrid, Spain
Emilio García Prieto
Intensive Care Department, Asturias Central University Hospital. Oviedo, Asturias, Spain
Pau Garro Martínez
Intensive Care Department, Granollers General Hospital. Barcelona, Spain
Carolina Giménez-Esparza Vic
Intensive Care Department, Vega Baja Hospital. Orihuela, Alicante, Spain
Ricardo Gimeno Costa
Intensive Care Department, La Fe University and Polytechnic Hospital. Valencia, Spain
Francisco Javier González de Molina Ortiz
Intensive Care Department, Mutua de Terrassa University Hospital. Barcelona, Spain
Marta Gurpegui Puente
Intensive Care Department, Miguel Servet University Hospital. Zaragoza, Spain
María José Gutiérrez Fernández
Intensive Care Department, San Agustín Hospital. Avilés, Asturias, Spain
Joaquín Lobo Palanco
Intensive Care Department, Navarra Hospital Complex. Pamplona, Navarra, Spain
Mauro Loinaz Bordonabe
Intensive Care Department, Navarra Hospital Complex. Pamplona, Navarra, Spain
Esther María López Ramos
Intensive Care Department, Príncipe de Asturias University Hospital. Alcalá de Henares, Madrid, Spain
María Pilar Luque Gómez
Intensive Care Department, Lozano Blesa Clinic University Hospital. Zaragoza, Spain
Juan Francisco Machado Casas
Intensive Care Department, Jaén Hospital Complex. Jaén, Spain
José Miguel Marcos Vidal
Department of Anesthesiology and Surgical Critical Care, Virgen Blanca Hopital Complex. León, Spain
Fernando Maroto Monserrat
Intensive Care Department, San Juan de Dios del Aljarafe Hospital. Bormujos, Sevilla, Spain
Juan Carlos Martínez Cejudo
Intensive Care Department, Infanta Elena University Hospital. Huelva, Spain
María del Carmen Martínez Ramagge
Intensive Care Department, La Línea Hospital (AGSCampo of Gibraltar). La Línea de la Concepción, Cádiz, Spain
Ignacio Moreno Puigdollers
Department of Anesthesiology and Surgical Critical Care, La Fe University and Polytechnic Hospital. Valencia, Spain
Lorena Mouríz Fernández
Department of Anesthesiology and Surgical Critical Care, Lucus Augusti University Hospital. Lugo, Spain
Luis Alberto López Olaondo
Department of Anesthesiology and Surgical Critical Care, Navarra University Clinic. Pamplona, Navarra, Spain
Sergio Ossa Echeverri
Intensive Care Department, Burgos University Hospital. Burgos, Spain
Juan Carlos Pardo Talavera
Intensive Care Department, Reina Sofía General University Hospital. Murcia, Spain
Inés María Parejo Cabezas
Department of Anesthesiology and Surgical Critical Care, San Pedro de Alcántara Hospital. Cáceres, Spain
Jorge Pereira Tamayo
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Vigo, Pontevedra, Spain
Miguel Ángel Pereira Loureiro
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Vigo, Pontevedra, Spain
Ana Pérez Carbonell
Department of Anesthesiology and Surgical Critical Care, Elche General University Hospital. Alicante, Spain
Marcos Pérez Carrasco
Intensive Care Department, Vall d’Hebron University Hospital. Barcelona, Spain
Demetrio Pérez Civantos
Intensive Care Department, Infanta Cristina University Hospital. Badajoz, Spain
María José Pérez-Pedrero Sánchez-Belmonte
Intensive Care Department, Virgen de la Salud Hospital. Toledo, Spain
David Pestaña Lagunas
Department of Anesthesiology and Surgical Critical Care, Ramón y Cajal University Hospital. Madrid, Spain
Pedro Picatto Hernández
Department of Anesthesiology and Surgical Critical Care, Asturias Central University Hospital. Oviedo, Asturias, Spain
Rosa Poyo-Guerrero Lahoz
Intensive Care Department, Son Llàtzer Hospital. Palma de Mallorca, Spain
Luis Quecedo Gutiérrez
Department of Anesthesiology and Surgical Critical Care, La Princesa University Hospital. Madrid, Spain
Roberto Reig Valero
Intensive Care Department, Castellón General Hospital. Castellón, Spain
Manuel Rodríguez Carvajal
Intensive Care Department, Juan Ramón Jiménez Hospital. Huelva, Spain
Enrique Samsó Sabé
Department of Anesthesiology and Surgical Critical Care, Hospital del Mar. Barcelona, Spain
Catalina Sánchez Ramírez
Intensive Care Department, Doctor Negrín of Gran Canaria University Hospital. Las Palmas de Gran Canaria, Spain
Margarita Sánchez Castilla
Department of Anesthesiology and Surgical Critical Care, Puerta de Hierro-Majadahonda University Hospital. Madrid, Spain
Susana Sancho Chinesta
Intensive Care Department, Doctor Peset University Hospital. Valencia, Spain
Juan Carlos Sotillo Díaz
Intensive Care Department, Gregorio Marañón General University Hospital. Madrid, Spain
José Manuel Soto Blanco
Intensive Care Department, San Cecilio University Hospital. Granada, Spain
Luis Suárez Gonzalo
Department of Anesthesiology and Surgical Critical Care, La Paz University Hospital. Madrid, Spain
Teresa Tabuyo Bello
Intensive Care Department, A Coruña University Hospital. A Coruña, Spain
Eduardo Tamayo Gómez
Department of Anesthesiology and Surgical Critical Care, Valladolid Clinic University Hospital. Valladolid, Spain
Luis Mariano Tamayo Lomas
Intensive Care Department, Río Hortega University Hospital. Valladolid, Spain
Gonzalo Tamayo Medel
Department of Anesthesiology and Surgical Critical Care, Cruces University Hospital. Bilbao, Vizcaya, Spain
Vicente Torres Pedrós
Department of Anesthesiology and Surgical Critical Care, Son Espases University Hospital. Palma de Mallorca, Spain
Montserrat Vallverdú Vidal
Intensive Care Department, Arnau de Vilanova University Hospital. Lleida, Spain
Marina Varela Durán
Department of Anesthesiology and Surgical Critical Care, Pontevedra University Hospital Complex. Pontevedra, Spain
Paula Vera Artazcoz
Intensive Care Department, Santa Creu i Sant Pau Hospital. Barcelona, Spain
María Elena Vilas Otero
Department of Anesthesiology and Surgical Critical Care, Álvaro Cunqueiro University Hospital. Pontevedra, Spain