Cryptococcus neoformans is an encapsulated yeast causing mainly opportunistic infections. The virulence factors involved in cryptococcosis pathogenesis include the presence and the size of the polysaccharide capsule, the production of melanin by phenoloxidase, the growth at 37°C and the enzyme secretion like proteinase, phospholipase and urease. Many other enzymes are secreted by C. neoformans but their role in the fungus virulence is not yet known.
AimsIn order to investigate this topic, we compared the phospholipase production between strains from patients and from bird droppings, and we examined its relationship to phenoloxidase production. We further characterized the strains by determining the activity of 19 different extracellular enzymes.
MethodsTwo hundred and five Italian C. neoformans clinical isolates and 32 environmental isolates were tested. Phenoloxidase production was determined by the development of brown colonies on Staib's agar. Extracellular phospholipase activity was performed using the semiquantitative egg-yolk plate method. API ZYM commercial kit was used to observe the production and the activity of 19 different extracellular enzymes.
ResultsStatistical analysis of the results showed a significantly higher phospholipase activity in the clinical isolates than in the environmental isolates. No significant difference about the phenoloxidase production between both groups was found. Regarding the 19 extracellular enzymes tested using the API ZYM commercial kit, acid phosphatase showed the highest enzymatic activity in both groups. Concerning the enzyme α-glucosidase, the clinical isolates presented a significantly higher positivity percentage than the environmental isolates. A hundred percent positivity in the enzyme leucine arylamidase production was observed in both groups, but the clinical isolates metabolized a significantly greater amount of substrate.
ConclusionsThe higher phospholipase production in the clinical isolates group confirms the possible role of this enzyme in the cryptococcosis pathogenesis. The extracellular activities of the enzymes acid phosphatase, α-glucosidase and leucine arylamidase, tested by means of the API ZYM commercial kit, appear to be very interesting. Many studies indicate that these enzymes are involved in the virulence of bacteria and parasites; our results suggest their possible role as virulence factors in Cryptococcus infections too.
Cryptococcus neoformans es una levadura encapsulada que produce infecciones oportunistas. Los factores de virulencia involucrados en la patogénesis de la criptococosis incluyen la existencia y el tamaño de la cápsula polisacarídica, la producción de melanina por medio de la enzima fenoloxidasa, el crecimiento a 37°C y la secreción de ciertas enzimas como proteinasa, fosfolipasa y ureasa. Existen otras enzimas que son secretadas por C. neoformans, pero su papel en la virulencia de este hongo aún no es conocido.
ObjetivosSe investigó la producción de fosfolipasa tanto en aislamientos de C. neoformans obtenidos de pacientes como de aislamientos recuperados de deposiciones de aves, y se comparó el grado de producción con el de la síntesis de la enzima fenoloxidasa. Además, distingue las cepas mediante la definición de la actividad de 19 enzimas extracelulares diferentes.
MétodosSe estudiaron 205 aislamientos clínicos de C. neoformans y 32 ambientales. La producción de fenoloxidasa se determinó por el crecimiento de colonias de color marrón en medio de Staib. Para determinar la actividad fosfolipasa extracelular se utilizó el método semicuantitativo en placa con yema de huevo. Con el método comercial API ZYM se determinó la producción de otras 19 enzimas extracelulares.
ResultadosEl análisis estadístico de los resultados mostró una producción de fosfolipasa significativamente mayor entre los aislamientos clínicos en comparación con los ambientales. No se encontraron diferencias significativas entre ambos grupos en la producción de fenoloxidasa. En lo referente a las 19 enzimas extracelulares valoradas mediante el sistema API ZYM, la fosfatasa ácida mostró la mayor actividad en ambos grupos. Respecto a la enzima α-glucosidasa, nuevamente los aislamientos clínicos presentaron una actividad significativamente mayor. Todos los aislamientos de ambos grupos presentaron actividad leucina-arilamidasa, si bien los aislamientos clínicos procesaron mayor cantidad de sustrato de manera significativa.
ConclusionesLa mayor producción de enzima fosfolipasa entre los aislamientos clínicos evidencia que esta enzima puede estar implicada en la patogénesis de la criptococosis. También es interesante la actividad extracelular de las enzimas fosfatasa ácida, α-glucosidasa y leucina-arilamidasa, valorada por medio del sistema comercial API ZYM. Diversos estudios apuntan a que estas enzimas están implicadas en la virulencia de bacterias y parásitos; nuestros resultados muestran también su posible implicación como factores de virulencia en las infecciones por Cryptococcus.
Cryptococcus neoformans is an encapsulated yeast that causes opportunistic infections, mostly meningoencephalitis in immunocompromised patients. Conventional nomenclature classified C. neoformans into five serotypes (A, B, C, D, and AD) and three varieties: C. neoformans var. neoformans (serotype D), C. neoformans var. grubii (serotype A), and C. neoformans var. gattii (serotypes B and C).13,18 The yeast has been recently reclassified into two species, C. neoformans and Cryptococcus gattii, based on genetic variability and lack of evidence for genetic recombination.20 Moreover, C. gattii differs from C. neoformans in phenotypic characters, natural habitat, epidemiology, clinical manifestations of the disease, and response to antifungal treatment. While bird droppings have been associated with the spread of C. neoformans in the environment, eucalyptus trees and decaying woods have been associated with C. gattii.5 The infection caused by C. neoformans is particularly observed in patients infected with human immunodeficiency virus.5 The most common variety, C. neoformans var. grubii, primarily infects immunocompromised individuals and it causes the majority of cryptococcal infections.4 The second variety, C. neoformans var. neoformans, causing infections mainly in Europe, has been reported to be less virulent and more susceptible to fluconazole than C. neoformans var. grubii.5,37 Habitat of C. gattii was primarily restricted to tropical and subtropical regions until an outbreak on Vancouver Island, Canada, started in 1999.3 This species has a lower susceptibility to several antifungal agents, needs larger treatments, and is responsible for higher mortality rates.38 Until recently it was believed that C. neoformans caused disease mainly in immunocompromised patients while C. gattii was found to cause disease preferentially in immunocompetent individuals.4,34 However several studies observed that a substantial number of cryptococcal infections in African HIV infected patients are caused by C. gattii.26,36
Various factors contribute to cause Cryptococcus infections in hosts. The virulence factors of Cryptococcus spp. involved in pathogenesis include the presence and size of the polysaccharide capsule, the production of melanin, growth at 37°C and the secretion of various enzymes, as proteinase, phospholipase, phenoloxidase and urease.5
The phospholipases are a heterogeneous group of enzymes able to hydrolyze one or more ester linkages in glycerophospholipids. The action of phospholipases can result in the membrane destabilization and cell lysis.5 The phospholipase secreted by C. neoformans can be responsible for the pulmonary infection beginning, by favoring cryptococci adhesion to the lung epithelial cells.9,22 In the present study we compared the phospholipase production among the strains from patients and from bird droppings and we examined its relationship to the phenoloxidase production which is regarded as one of the main virulence factors for its protection against the external physical agents and against the immune defenses in the host.6,17C. neoformans produces many other extracellular enzymes. In order to further characterize its enzymatic profiles and to observe the different enzymatic activity between the strains from humans and from the environment, API ZYM commercial kit system was used.
Material and methodsStrainsTwo hundred and five Italian Cryptococcus clinical isolates (CI) and 32 environmental isolates (EI) were tested. The human strains, collected in the Microbiology laboratory of Florence University from 1985 to 1996, were isolated from 174 patients, hospitalized mainly in northern and central Italy.
The environmental isolates were from soil and bird dried excreta, mostly from pigeons, mainly in central Italy. Identification was performed by classical methods based on the urease production on Christensen's Urea Agar, the presence of a capsule, the melanin production, and the assimilation of nitrogen and carbon sources. The differentiation between C. neoformans and C. gattii was performed on canavanine-glycine-bromothymol blue (CGB) medium19 and on a medium containing d-proline,11 while the serotype was determined by a slide agglutination test with specific monoclonal antibodies for capsular polysaccharide, Crypto-check kit (Iatron Laboratories Inc., Tokyo, Japan). All identifications were confirmed using the ID32C system (bioMérieux AS, Marcy l’Etoile, France).
Phenoloxidase activityPhenoloxidase production was tested after 6 days of incubation at 28°C and 37°C in Staib's medium35 containing the extract of Guizotia abyssinica seeds. Melanin production was qualitatively assayed by visually determining the color of the colonies. According to the color intensity of the colony, the phenoloxidase activity was scored as follows: 4+, very high activity; 3+, high activity; 2+, moderate activity; 1+, low activity; and 0, no activity. A Candida albicans (ATCC 90018) strain was used as negative control. Phenoloxidase activity of each strain was tested in triplicate.
Phospholipase activityExtracellular phospholipase activity of Cryptococcus was performed according to Polak28 using the semi-quantitative egg-yolk plate method of Price et al.29 Four percent of egg-yolk (Fluka-Sigma Aldrich, Milan, Italy) was added to Sabouraud dextrose agar (Difco-Becton, Dickinson and Company, Milan, Italy) with 1M sodium chloride and 5mM calcium chloride. A drop (2 microliters) of a 108 cel/ml yeast suspension was placed on the egg-yolk agar plate surface. The plates were incubated for 10 days at 30°C in plastic boxes with a layer of paper soaked in water on the bottom to prevent desiccation. The formation of precipitation zones (Pz) around the colony was considered indicative of the enzyme production. Phospholipase production was expressed as the ratio between the colony diameter and the colony diameter plus the precipitation zone, as described previously.29 The average Pz value of each strain was obtained with two separate tests in which each strain was repeated twice. C. albicans (ATCC 90018), C. neoformans var. grubii (ATCC 10415) and C. neoformans var. neoformans (ATCC 34874) strains were used as controls.
Enzymatic profileUsing the semi-quantitative API ZYM system (bioMérieux) 75 clinical and 25 environmental C. neoformans isolates were checked for 19 different enzyme activities.
Fresh culture cells of each C. neoformans strain were suspended in distilled water to reach an optical density of 0.1 at 550nm. Sixty-five microliters of the yeast suspension were dispensed into each cupule, as recommended by the manufacturer. After incubation at 37°C for 4h in the dark, 1 drop each of Zym A and Zym B reagent was added to each cupule. The color development in the cupule was recorded after 5min. The results were determined in nanomoles (nmol) of the hydrolyzed substrate according to the intensity of the color developed in the reaction, on a scale which ranges from 1 (negative reaction) to 5 (maximum positive reaction), i.e., 1=5nmol, 2=10nmol, 3=20nmol, 4=30nmol and 5≥40nmol of API ZYM substrate metabolized. Each strain was tested in duplicate to confirm the results obtained.
The serotype and the phenoloxidase activity (melanin production) were determined right away after arrival at the laboratory. The strains were stored for a long period in water at room temperature and then put back in culture to determine their phospholipase activity and API ZYM profile.
Statistical analysisThe statistical analysis was carried out to compare the enzymatic activities tested in this study; the comparison was performed between serotype A and serotype D and between the two groups of strains, CI and EI. The chi-square test was used to compare the frequency of melanin or phospholipase high and intermediate strain producers. To contrast the Pz values, the Student t-test and the Mann–Whitney test were used. The frequency of strains producers of API enzymes was analyzed by chi-square test, while to compare the amount of substrate metabolized by each API enzyme the Student t-test and the Mann–Whitney test were used. For all the tests, a p-value of ≤0.05 was considered statistically significant. The possible correlation between the different enzymatic activities of the tested strains was investigated by Pearson's and Spearman's correlation coefficient.
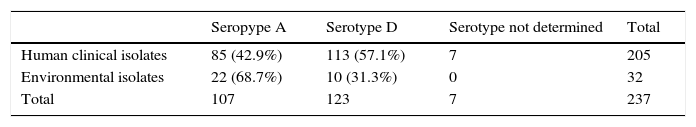
ResultsAll the strains, tested on the CGB agar and in the medium containing d-proline, belonged to the species C. neoformans. The serotype of the strains examined is reported in Table 1. The serotype D was prevalent among the CI (57.1%), while the serotype A was significantly prevalent among the EI (68.7%).
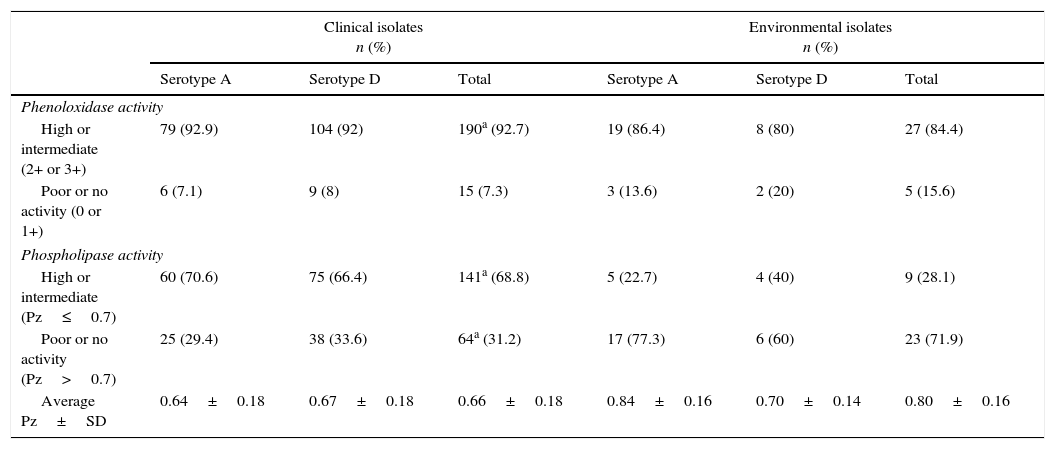
The phenoloxidase and phospholipase activities are shown in Table 2. The 97.6% of the CI and the 96.9% of the EI were positive for the phenoloxidase activity when they were tested on the Staib's medium; 5 (2.4%) strains among the CI and 1(3.1%) among the EI were negative. No strain showed very high melanin production (4+). Analyzing the data by means of the chi-square test, no significant difference in the frequency of the strains with high or intermediate (+++/++) and poor or absent (+/0) melanin production between both the isolate groups and between serotype A and serotype D strains was observed.
Phenoloxidase and phospholipase activity.
| Clinical isolates n (%) | Environmental isolates n (%) | |||||
|---|---|---|---|---|---|---|
| Serotype A | Serotype D | Total | Serotype A | Serotype D | Total | |
| Phenoloxidase activity | ||||||
| High or intermediate (2+ or 3+) | 79 (92.9) | 104 (92) | 190a (92.7) | 19 (86.4) | 8 (80) | 27 (84.4) |
| Poor or no activity (0 or 1+) | 6 (7.1) | 9 (8) | 15 (7.3) | 3 (13.6) | 2 (20) | 5 (15.6) |
| Phospholipase activity | ||||||
| High or intermediate (Pz≤0.7) | 60 (70.6) | 75 (66.4) | 141a (68.8) | 5 (22.7) | 4 (40) | 9 (28.1) |
| Poor or no activity (Pz>0.7) | 25 (29.4) | 38 (33.6) | 64a (31.2) | 17 (77.3) | 6 (60) | 23 (71.9) |
| Average Pz±SD | 0.64±0.18 | 0.67±0.18 | 0.66±0.18 | 0.84±0.16 | 0.70±0.14 | 0.80±0.16 |
a Seven strains were not serotyped.
Concerning the phospholipase activity, the CI Pz value ranged from 0.28 to 1 (average 0.66±0.187); 27 (13.1%) isolates were negative (Pz=1). In the EI group, Pz value ranged from 0.50 to 1 (average 0.80±0.163); 10 (31.2%) isolates were negative (Pz=1). Analyzing the data by means of the chi-square test, the frequency of the strains with high or intermediate phospholipase activity (Pz≤0.7) was significantly higher (p<0.01) among the CI than among the EI. The quantitative data of phospholipase production (Pz value) were analyzed by means of the Student t-test and the Mann–Whitney test: the CI showed a significantly higher (p<0.01) phospholipase activity than the EI, while no significant difference between serotype A and serotype D strains was observed. The Spearman and Pearson correlation tests showed no correlation between Pz value and phenoloxidase production in both the isolate groups.
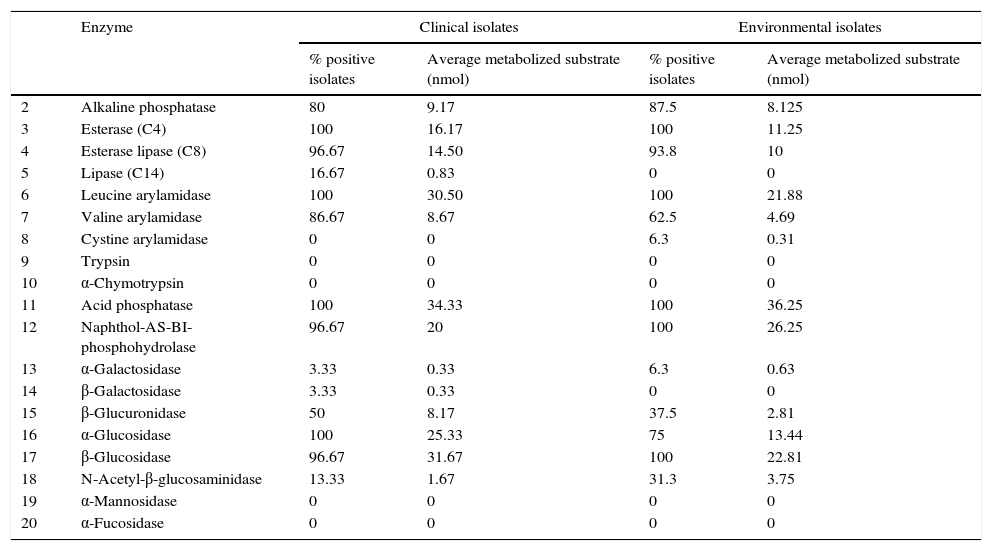
Because of insufficient resources only 100 isolates, randomly selected from the yeast population investigated in this study, were tested to determine the API ZYM profile. Table 3 displays the API ZYM profiles of 75 CI and 25 EI. Both the CI and EI groups showed an activity higher than 90% for the enzymes 3-4-6-11-12-17. All the isolates were negative for the enzymes 9-10-19 and 20, while an activity lower than 10% was found for the enzymes 8-13 and 14. Acid phosphatase (11) showed the highest enzymatic activity in both groups, metabolizing an average of about 35nmol of substrate; the enzymes leucine arylamidase (6) and α and β glucosidase (16, 17) showed high activity too. For the enzyme 16 (α-glucosidase), and using the chi-square test, a significant difference in the number of positive strains between the CI and the EI was observed. For the enzyme 6 (leucine arylamidase), 100% positivity was found in both groups, but the CI metabolized a significantly greater amount of substrate (T Student test and Mann–Whitney test). The quantitative data relating to the API enzymes production (amount of metabolized substrate) were compared with the phenoloxidase or phospholipase production by means of Spearman and Pearson correlation tests, and no correlation between melanin production or Pz value and activity of each of the 19 API enzymes was observed in both the strain groups.
Enzymatic characterization of clinical and environmental isolates of Cryptococcus neoformans by API ZYM analysis.
| Enzyme | Clinical isolates | Environmental isolates | |||
|---|---|---|---|---|---|
| % positive isolates | Average metabolized substrate (nmol) | % positive isolates | Average metabolized substrate (nmol) | ||
| 2 | Alkaline phosphatase | 80 | 9.17 | 87.5 | 8.125 |
| 3 | Esterase (C4) | 100 | 16.17 | 100 | 11.25 |
| 4 | Esterase lipase (C8) | 96.67 | 14.50 | 93.8 | 10 |
| 5 | Lipase (C14) | 16.67 | 0.83 | 0 | 0 |
| 6 | Leucine arylamidase | 100 | 30.50 | 100 | 21.88 |
| 7 | Valine arylamidase | 86.67 | 8.67 | 62.5 | 4.69 |
| 8 | Cystine arylamidase | 0 | 0 | 6.3 | 0.31 |
| 9 | Trypsin | 0 | 0 | 0 | 0 |
| 10 | α-Chymotrypsin | 0 | 0 | 0 | 0 |
| 11 | Acid phosphatase | 100 | 34.33 | 100 | 36.25 |
| 12 | Naphthol-AS-BI-phosphohydrolase | 96.67 | 20 | 100 | 26.25 |
| 13 | α-Galactosidase | 3.33 | 0.33 | 6.3 | 0.63 |
| 14 | β-Galactosidase | 3.33 | 0.33 | 0 | 0 |
| 15 | β-Glucuronidase | 50 | 8.17 | 37.5 | 2.81 |
| 16 | α-Glucosidase | 100 | 25.33 | 75 | 13.44 |
| 17 | β-Glucosidase | 96.67 | 31.67 | 100 | 22.81 |
| 18 | N-Acetyl-β-glucosaminidase | 13.33 | 1.67 | 31.3 | 3.75 |
| 19 | α-Mannosidase | 0 | 0 | 0 | 0 |
| 20 | α-Fucosidase | 0 | 0 | 0 | 0 |
Phenoloxidase was first described by Staib35 as a major virulence factor of C. neoformans. The melanin, generated by phenoloxidase from various exogenous catecholamines, protects C. neoformans against external oxidants and intracellular killing during phagocytosis.6 In this study not all the strains tested developed brown colonies although they were confirmed to be C. neoformans. The finding was consistent with those of Vidotto et al.41 who used Pal's medium, containing the seed extract of Helianthus annuus instead of Guizotia abyssinica. Vidotto et al.,41 as in our case, found no significant difference in the melanin production between CI and EI, but these methods are not fully comparable because of the subjective evaluation of different intensity of the colonies’ brown color.
Extracellular phospholipases are considered virulence factors of many pathogenic bacteria and protozoa and of some fungi such as C. albicans, Aspergillus fumigatus and C. neoformans.15 Barret-Bee et al.2 were the first to correlate the phospholipase production in C. albicans with its pathogenic nature, demonstrating that the isolates with a high pathogenic potential (high level of adhesion to oral epithelial cells and greater pathogenicity for mice) show higher phospholipase activity than yeasts with a low pathogenic potential. In addition, C. albicans blood isolates show greater in vitro phospholipase activity than oral isolates from healthy patients.16
Similarly, it was observed that the C. neoformans isolates from AIDS patients show a higher level of phospholipase activity than isolates from bird droppings or from non AIDS patients.41,42 The higher virulence of C. neoformans isolates producing phospholipases is already demonstrated in animal models where the mutant strain with deletion for the genes encoding this group of enzymes was significantly less virulent than the wild strains.10 It has been postulated that the secreted phospholipase is involved in the beginning and development of pulmonary cryptococcosis and that it is essential for the entry of cryptococci into the pulmonary lymphatics and in the blood.30,31 In this work, the egg-yolk-based assay according to Price29 is used to determine the phospholipase production of C. neoformans strains isolated from patients and from environment. This method is not entirely specific because the egg-yolk contains substrates for both phospholipases (phospholipids) and lipases (triglycerides). However, the Pz value correlates with hydrolysis of phosphatidylcholine,29 furthermore the phospholipase activity produced by cryptococcal isolates, identified by radiometric analysis, correlates with the results from the egg-yolk plate assay.9 This method should be considered a good initial screening suitable for a large number of strains as in our study, but the results require further confirmation with more specific assays such as radiometric or colorimetric methods. Furthermore the phospholipase activity appears to be a stable trait since remains constant despite repeated passages on artificial media.9 In our study, the 86.9% of the CI and the 68.7% of the EI were positive for phospholipase production and a significant difference in Pz value between CI and EI was recognized, in agreement with Vidotto et al.40 and Souza et al.33 for Italian and Brazilian C. neoformans strains respectively. These results confirm the possible correlation between phospholipase activity and the pathogenicity of C. neoformans.
In addition to phenoloxidase and phospholipase production, the detection of other extracellular enzymes in C. neoformans by the API ZYM method allows to highlight differences in enzymatic activities in strains sampled from different sources and different regions.39 This method could also be very important to identify new Cryptococcus virulence factors.39 The C. neoformans strains examined showed a typical enzymatic profile, different from that of other medically important yeasts.14 In agreement with other authors,7,39,40 enzymes 3 (esterase-C4), 4 (esterase lipase-C8) and 11 (acid phosphatase), showed a high positivity percentage in the tested strains, regardless of their origin. For the enzyme 6 (leucine arylamidase), the CI metabolized significantly higher amount of substrate than the EI. Leucine arylamidase belongs to the group of aminopeptidases which play a critical role in cellular metabolism and catalyze the removal of N-terminal amino acid residues from peptides. Emerging experimental evidences suggest the role for leucine aminopeptidases (LAPs) in the survival of pathogens under different conditions. The LAP from Plasmodium vivax catalyzes the removal of amino acids from the peptide generated during the hemoglobin degradation so they can be used for the growth and development of the pathogen.21 Furthermore, secreted LAP appears to be essential in the process of biofilm formation associated with the Staphylococcus aureus virulence.32 For the yeasts, little is known about this enzyme but this data may provide a starting point for future researches. Concerning enzyme 16 (α-glucosidase), the CI showed a significantly higher percentage of positivity compared to EI and, on average, metabolized a significantly higher amount of substrate. Studies performed on Saccharomyces cerevisiae and C. albicans demonstrate that the enzymes belonging to the group of α-glucosidase are essential in the reaction of mannose attachment to the proteins and so they are indispensable for the mannoproteins synthesis. These latter have an important role for the adhesion and the antigenicity; indeed the lack of this enzyme causes a difficult adhesion to host cells.12,25 The amount of substrate metabolized by the acid phosphatase (11) was very high, confirming earlier reports of this activity in C. neoformans.7,8,39 Acid phosphatase has also been implicated in the virulence of bacteria and parasites,1,27 where it plays a major role in survival within phagocytic cells by suppressing the neutrophil superoxide anion production.23,24 Further experimental studies may be useful to clarify the possible importance of these enzymes as virulence factors in the pathogenesis of cryptococcosis.
Conflict of interestThe authors declare no conflict of interest.