Cats are frequent carriers of Microsporum canis and veterinary students are at high risk of exposure and acquisition of the organism a la infección.
ObjectivesAn outbreak of zoonotic ringworm carried by a litter of stray cats is described. Four veterinary students, four dogs, and six cats living in five separate locations were affected. All had direct or indirect contact with the infected kitten litter. We tried to identify the causal dermatophyte.
MethodsConventional and mycological culture methods were used.
ResultsMicroscopic features of scrapings and hairs treated with 20% KOH strongly suggested a M. canis etiology, and a diagnosis of ringworm was empirically supported by successful treatment of humans and animals. Nevertheless, cultures failed to show the expected morphology.
ConclusionsCulture features of our strain are compared with those described by other authors for dysgonic M. canis strains. Epidemiological features are also discussed.
Los gatos son frecuentemente portadores de Microsporum canis. Los estudiantes de veterinaria están especialmente expuestos a la infección.
ObjetivosSe describe un brote de tiña zoonótica difundido por una camada de gatos callejeros. Cuatro estudiantes de veterinaria, cuatro perros y seis gatos de cinco localizaciones diferentes se vieron afectados. Todos tuvieron contacto directo o indirecto con la camada de gatitos infectados. Se intenta identificar el dermatofito causal.
MétodosSe utilizan los procedimientos micológicos morfológicos y de cultivo convencionales.
ResultadosLos hallazgos microscópicos en pelo y raspados cutáneos aclarados en KOH al 20% sugirieron fuertemente una etiología por M. canis, y el diagnóstico de tiña fue apoyado empíricamente por el éxito en el tratamiento de humanos y animales. Sin embargo, los cultivos no mostraron la morfología esperada.
ConclusionesLos caracteres del cultivo de nuestra cepa son comparados con los descritos por otros autores en cepas disgónicas de M. canis. Las características epidemiológicas son discutidas también.
Many veterinary students are quite prone to adopt pets. Kittens are a favourite because they can be easily kept in student flats, and litters are offered for adoption all year around.
Cats are known carriers of Microsporum canis, and since the authors have witnessed some previous episodes of zoonotic infection in students related to stray kittens, the students are advised every year during the lectures about ringworm about the potential risks.
M. canis produces a wide range of clinical manifestations, from asymptomatic carriage to acute inflammatory kerion lesions; however microscopic examination of KOH mounts frequently shows sinuous, well branched, septate, slender hyphae and chains of arthrospores, as well as the ectothrix invasion of hairs.
Isolates are also easily recognized by their young radiate colonies, the dense cottony aerial mycelium, the early production of a bright yellow pigment, and the heavy formation of large, thick walled, echinulate, spindle shaped macroconidia.
Nevertheless, these characteristic culture features are not always observed. Dysgonic strains have been described in Spain4,5,7 and elsewhere,1–3,6,9 and produce the same in vivo manifestations but lack most of these growth characters in culture. Moreover, these strains seem to have an increased infectivity for animal and human hosts.1,6,8
Patients and methodsDiagnostic procedures: Human and animal patients were all diagnosed in the Infectious Pathology Diagnostic Service of the Clinical Veterinary Hospital (CVH) at the University of Extremadura (Cáceres, Spain). Samples were taken of skin lesions by direct scraping and scotch taping, processed for microscopic examination in 20% KOH and Lactophenol–Cotton Blue mounts, and inoculated onto Sabouraud dextrose agar (SDA) as well as SDA with chloramphenicol and actidione. Cultures were incubated at 30°C and observed each week up to 3 months. Cultures demonstrating growth were subcultured every 2 weeks.
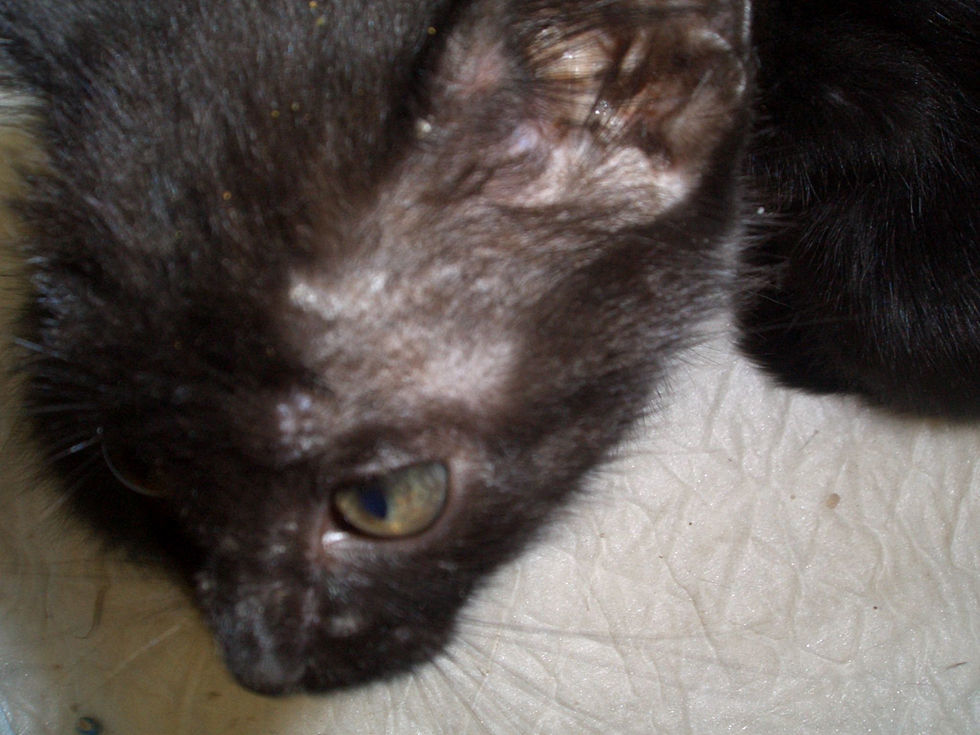
Case reports: In September 2007 an orphaned litter of 5 stray kittens was rescued by an animal protection association. The animals were undernourished with dirty fur on arrival (Fig. 1). They were nursed for 3 weeks under the care of the CVH, and then offered for adoption. The kittens were subsequently adopted by students from two flats (A and B).
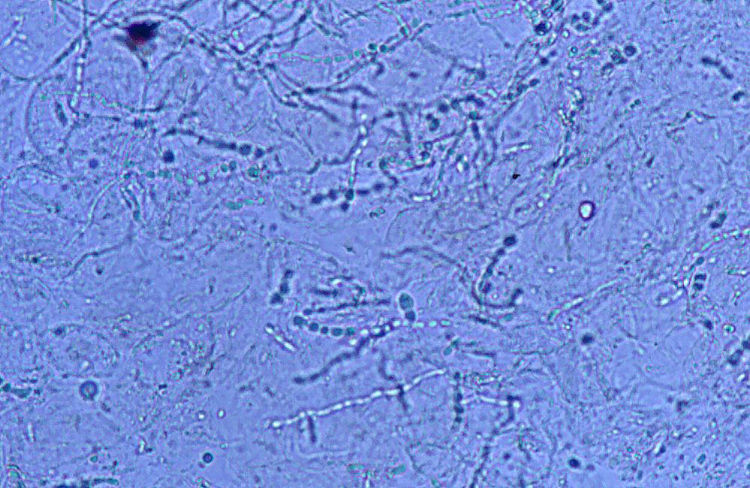
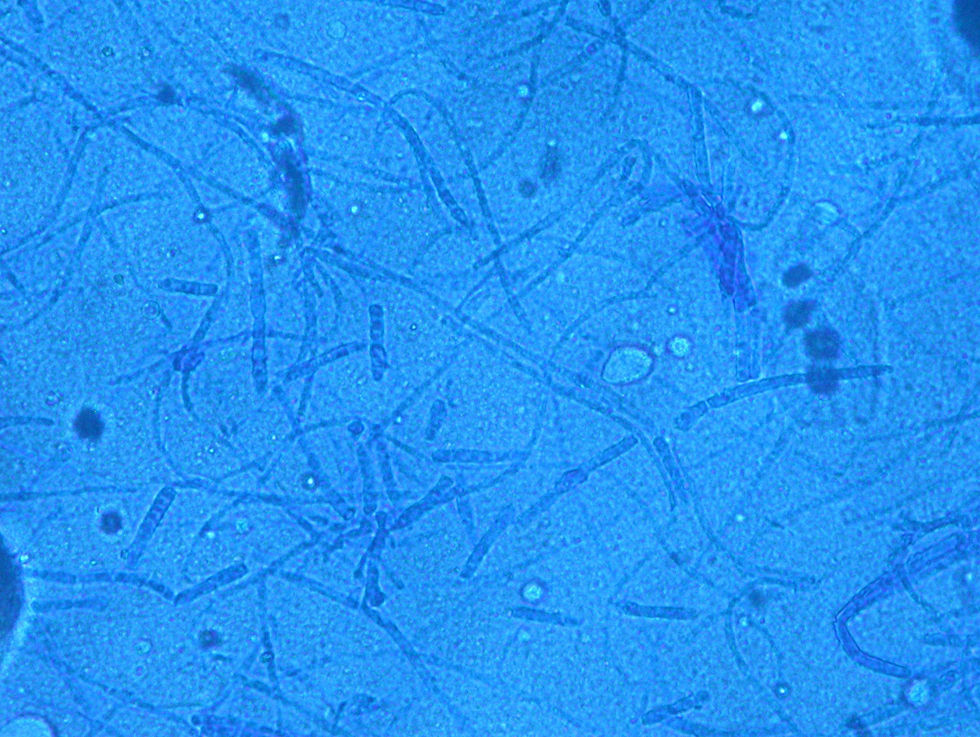
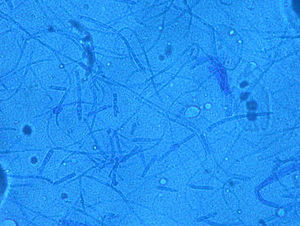
Case 1: The dog of one CVH interns developed alopecic annular pruriginous lesions on the medial tarsus of right hind leg while the kittens were still being nursed in the Hospital. The dog did not visit the CVH, and did not have any direct contact with the kittens. The owner herself had frequent contact with the cats, taking routine precautions when nursing them, but did not demonstrate any lesion herself. Hairs and skin scrapings of dog's lesions were treated with 20% KOH and examined at 400×, showing abundant well ramified hyphae with arthrospore chains (Fig. 2). The samples were inoculated on SDA plates and incubated at 30°C. The dog's dermatitis was diagnosed as ringworm and treated accordingly with 6 enilconazole baths (0.2% in tap water, one every 4 days), with complete recovery in 1 month. Restriction of movement, hygienic measures, and hypochlorite disinfection were implemented in the dog's environment. No recurrences were observed and the dog's owner did not develop infection.
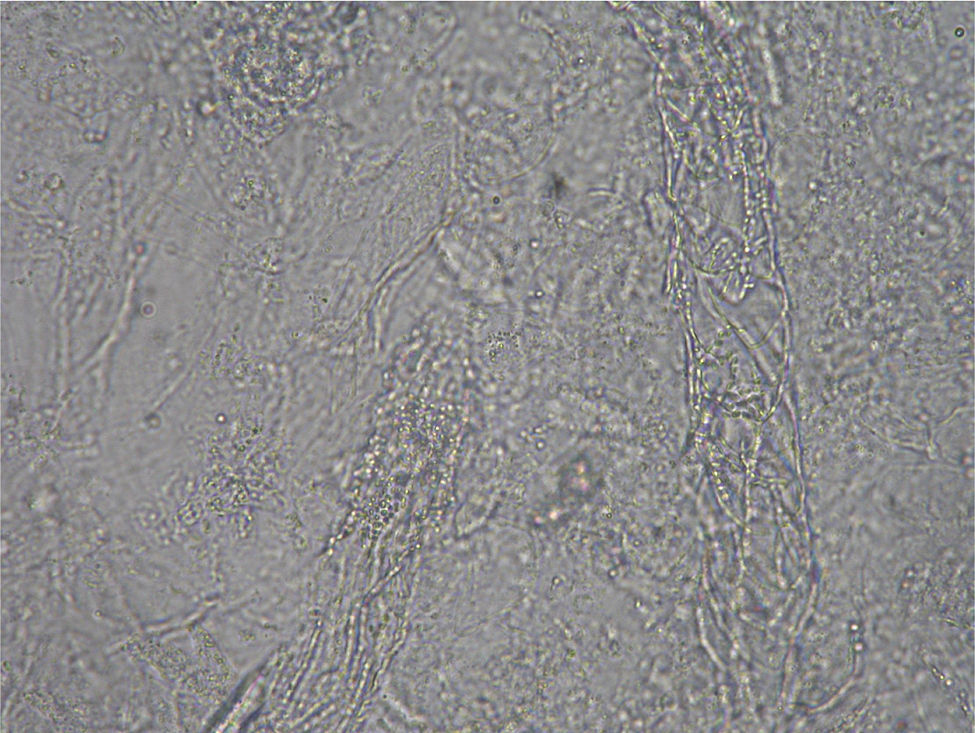
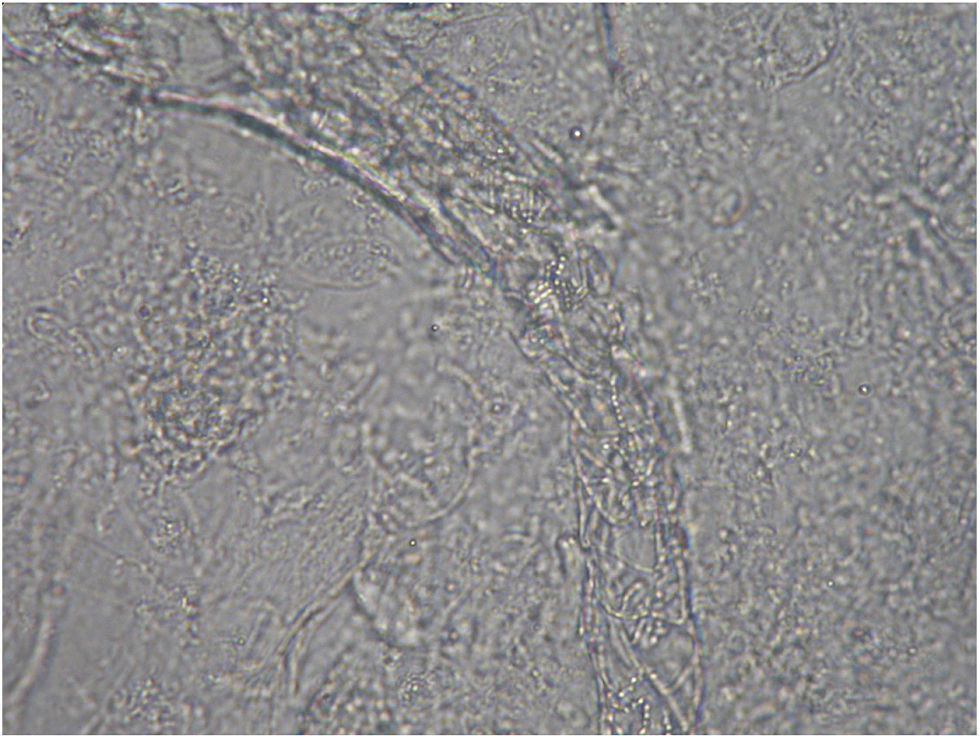
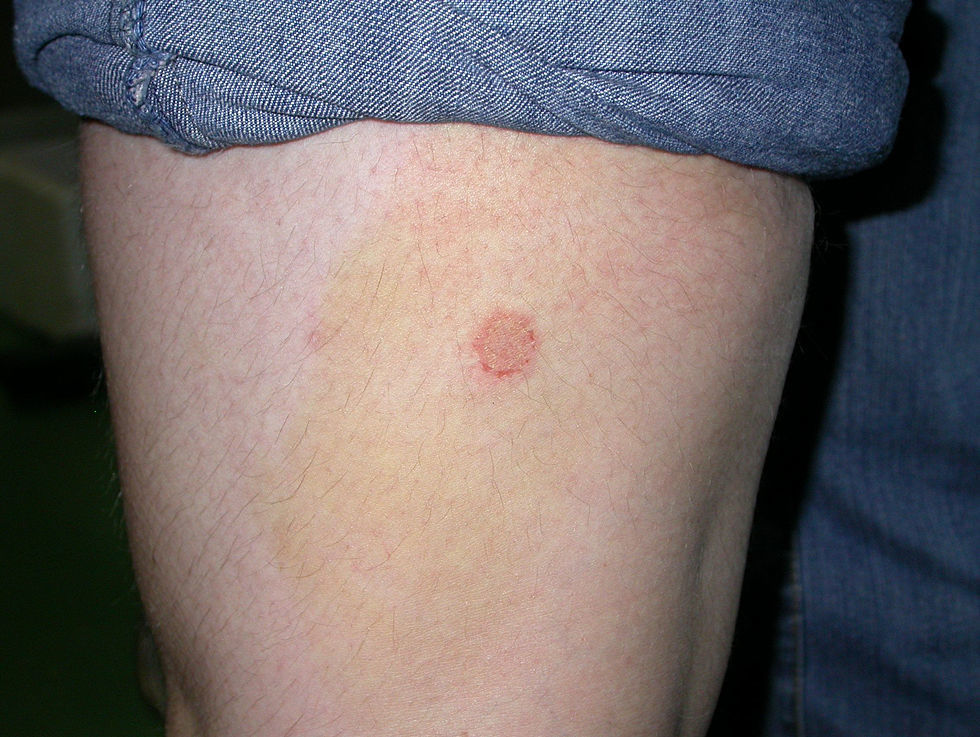
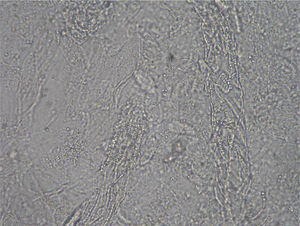
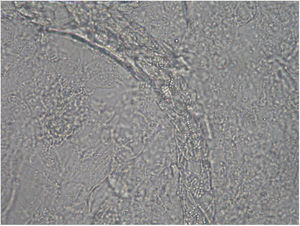
Case 2: Two weeks after their arrival, the flat A-kittens showed alopecic patches in face and limbs (Fig. 3) and were carried to our Diagnostic Service. Hairs and skin scrapings were taken and processed. Huge amounts of slender ramified hyphae, small arthrospores in chains, and hair sheath ectothrix colonization (Fig. 4) were observed at 400×. The dermatitis was diagnosed as ringworm. Samples were also inoculated on SDA plates. Two days later, three of the four flat A-student mates, one adult cat, and one dog showed annular pruriginous lesions on the face, thorax, forearms, and legs. Lesions in all cases showed abundant hyphae, arthrospore chains, and a hair sheath morphology identical to that observed in the kittens (Fig. 5). Scrapings and hair were inoculated on SDA plates. Our findings and a diagnosis of ringworm were reported and the students were immediately referred to a dermatologist for treatment. The affected cats were treated with oral griseofulvin (40mg/kg) and local povidone iodine. Lesions cleared in 1 month. The dog received enilconazole baths twice a week and lesions healed in about 3 weeks. Hygienic measures and hypochlorite disinfection were implemented, but movement of animals was not restricted. Treatment of human cases with oral (250mg/day) and topic (1% cream) terbinafine was effective in about a month, but the adult cat's owner had two recurrences, one in February and another in May.
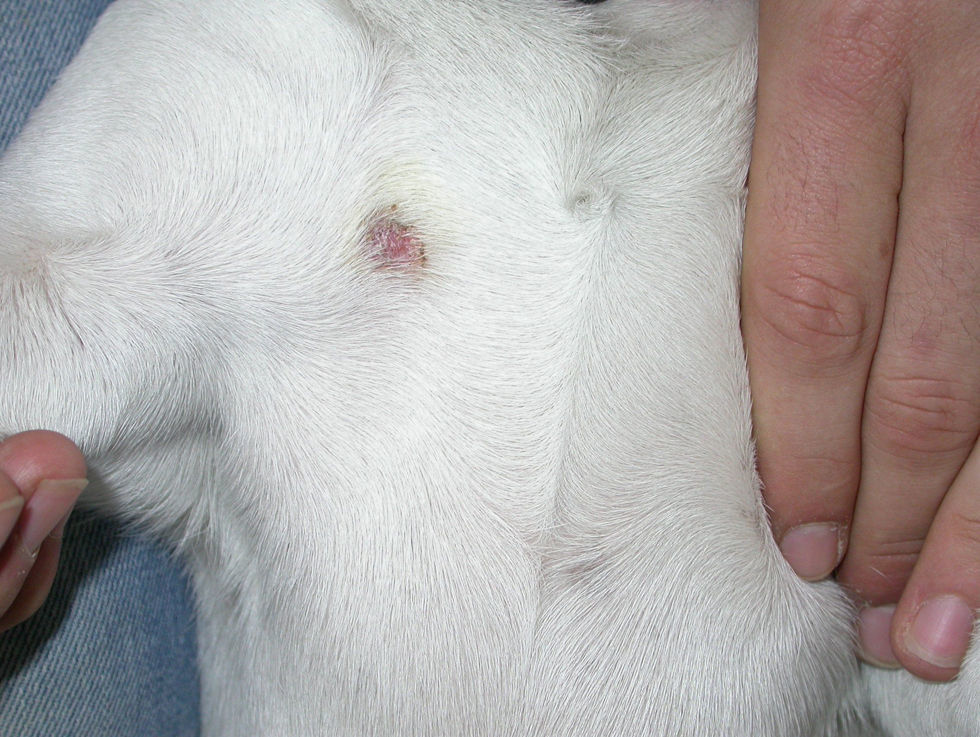
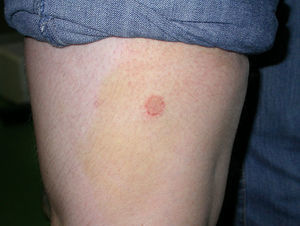
Case 3: One of the affected kittens was adopted by another student, who lived alone (flat B). The kitten showed small ventral and inguinal alopecic patches, and when the owner noticed that the litter was infected with ringworm she began to treat the kitten with griseofulvin 25mg/kg. No other measures were adopted. Twenty days later she observed annular non-pruriginous lesions on her own legs (Fig. 6) and came to our Diagnostic Service. The samples obtained by scotch taping were treated with Lactophenol–Cotton Blue, and looked at 400× under a microscope—several small sized, well ramified hyphae, and arthrospore chains were observed. Scrapings were inoculated on SDA plates. A diagnosis of ringworm was established and the owner of the kitten was referred to the dermatologist. Restriction, hygienic, and disinfection measures were then implemented. Oral terbinafine 250mg/day treatment for the owner and griseofulvin 40mg/kg oral treatment for the kitten were effective within 1 month with no recurrences.
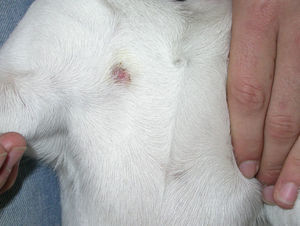
Case 4: One student from the other flat (C) visited flat A on one occasion with her dog. The dog developed one single annular thoracic lesion, which was fairly inflammatory and pruriginous (Fig. 7). Specimens were obtained and analysed as usual, and showed the same display of hyphae and ectothrix arthrospores as the other animal cases. Once diagnosed as ringworm, a treatment with enilconazole 0.2% baths twice a week was implemented, being effective in 3 weeks. Scraping samples were cultivated on SDA plates. Restriction of movements and hygienic and disinfection measures were taken. Neither the dog's owner nor her mates or their dogs were affected.
Case 5: Another student's dog (Flat D) that interacted at the Faculty with the flat A-dogs prior to their confinement developed small alopecic annular lesions on the thorax and the left foreleg. Scrapings and hair samples were processed as usual, showing the same morphology and distribution of hyphae and arthrospores as in all the other cases. Diagnosed as ringworm, the samples were inoculated on SDA and enilconazole 0.2% baths were implemented once weekly, and the dog was cured in 1 month. Restriction, hygienic, and disinfection measures were adopted. The owner was not affected.
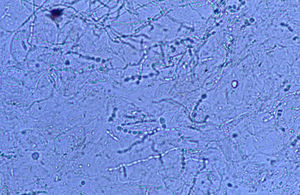
Culture resultsColonial growth was visible after 2 weeks of incubation. M. canis was not recognized in plain SDA or in the actidione- or chloramphenic-supplemented plates. The young colonies (about 18 days) appeared grayish, were fully submerged with a raised darker center, and displayed thick distorted hyphae radially arranged. When mature (40 days of incubation) they acquired a dull khaki colour with a very short aerial mycelium. No trace of bright yellow pigment was visible on the reverse, and neither typical macroconidia nor microconidia were observed. Only some scarce, multicellular, thick walled structures (Fig. 8) roughly similar to chlamydospore chains or misshapen macroconidia were observed in 100-day cultures. Colonial features of the cultures were fairly unstable and pleomorphic, reverting to mycelia sterilia after about the second or third subculture on SDA.
DiscussionThe disappointing lack of a recognizable dermatophyte in obvious cases of ringworm lead us to change the batch of culture medium three times, and made us suspect a dysgonic strain of M. canis strain, as these strains often present with features similar to those seen in our isolates. The colonial morphology with submerged radial growth, velvety aerial mycelium, and a raised center agreed with the descriptions of Sánchez et al.7 and Pereiro Jr et al.,5 who also found wide, inverse branching hyphae, also described by English2; only the radial disposition of mycelium suggested the regular M. canis morphology. In contrast with other author reports,3–5 our strain never produced yellow pigment, and only greyish and dim khaki colours were visible on both sides of the plates. All the authors describe poor conidiation with scarce, anomalous, and irregularly shaped macroconidia. Our thick walled structures were especially similar to those described in Refs. 1, 4, 5. The morphology of our cultures was far less stable than that described in Ref. 7, and never reverted to typical M. canis morphology as documented in Refs. 2, 6, 9, nor did they show features compatible with Microsporum distortum as seen in the Brasch strains.1 A striking feature of this outbreak was the highly contagious nature of the strain, a feature also noted in Refs. 1, 6 for their strains. In our case, six cats, four dogs, and at least four individuals were infected or reinfected in five separate locations. Direct contact was proven in most cases, but in some the carriages of arthrospores by the owners’ clothes or footwear seem the most probable way of infection, and in two cases only one actual contact is known between infected and healthy animals. Environmental persistence of fungus is also an important consideration. The only case of reinfection was in flat A, the focus of the infection. Despite aggressive and repeated hygienic and disinfection measures, reinfection occurred on two occasions, 5 and 8 months after the initial infection. Summerbell8 points out that the lack of some growth characters favouring the saprophytic survival of dermatophytes on soil keratin, as macro and microconidia and pigment production, may be an adaptation to the parasitism on the living animal hair keratin, and be accompanied by greater infectivity, by means of enhanced resistance of artrhrospores in the environment as well as by an increase of infective ability of the mycelium fragments. The dsygonic mutation of M. canis could be an example of a trend in this direction.
To all affected and unaffected veterinary students, who kindly answered our epidemiological enquiries.