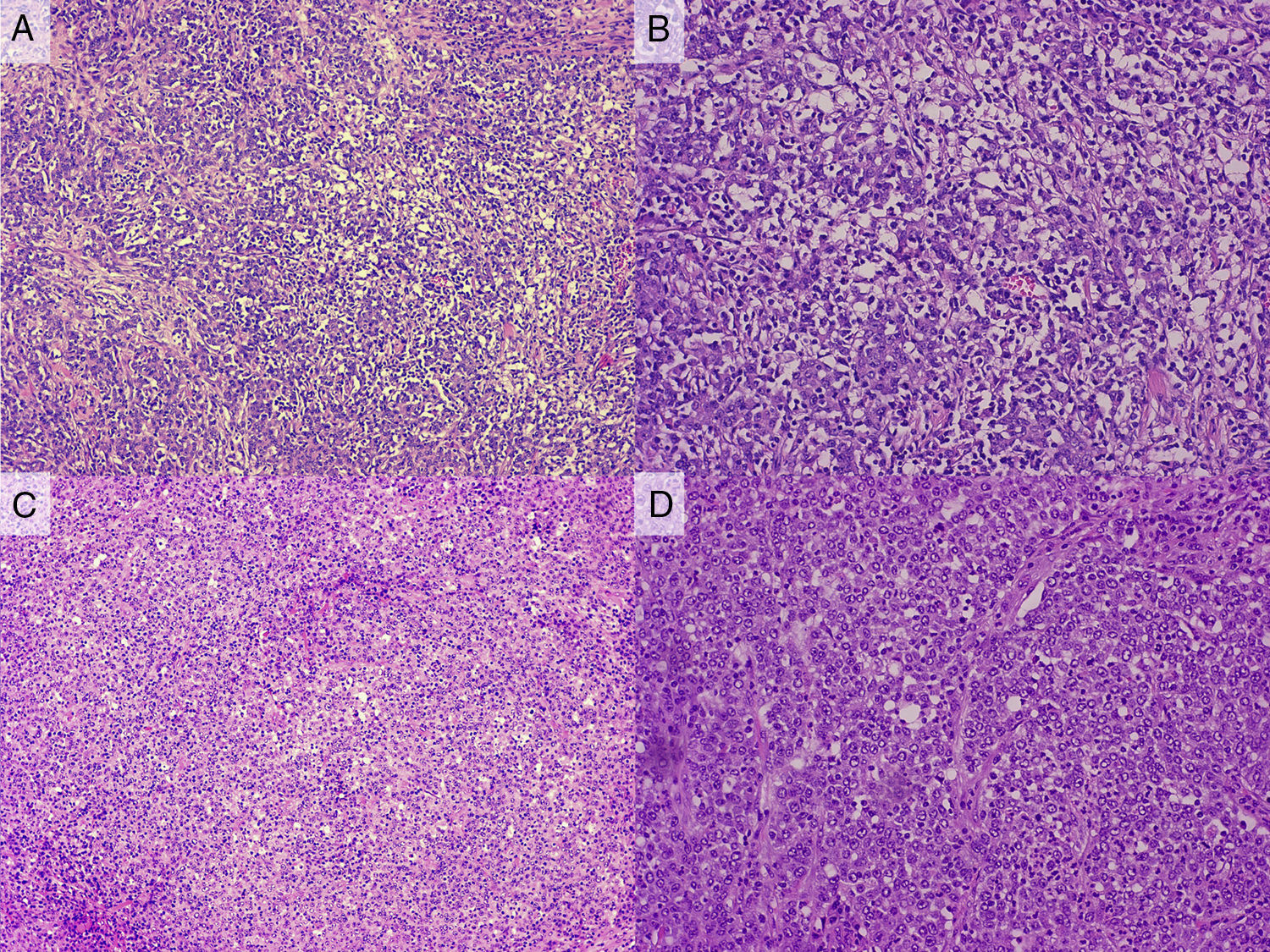
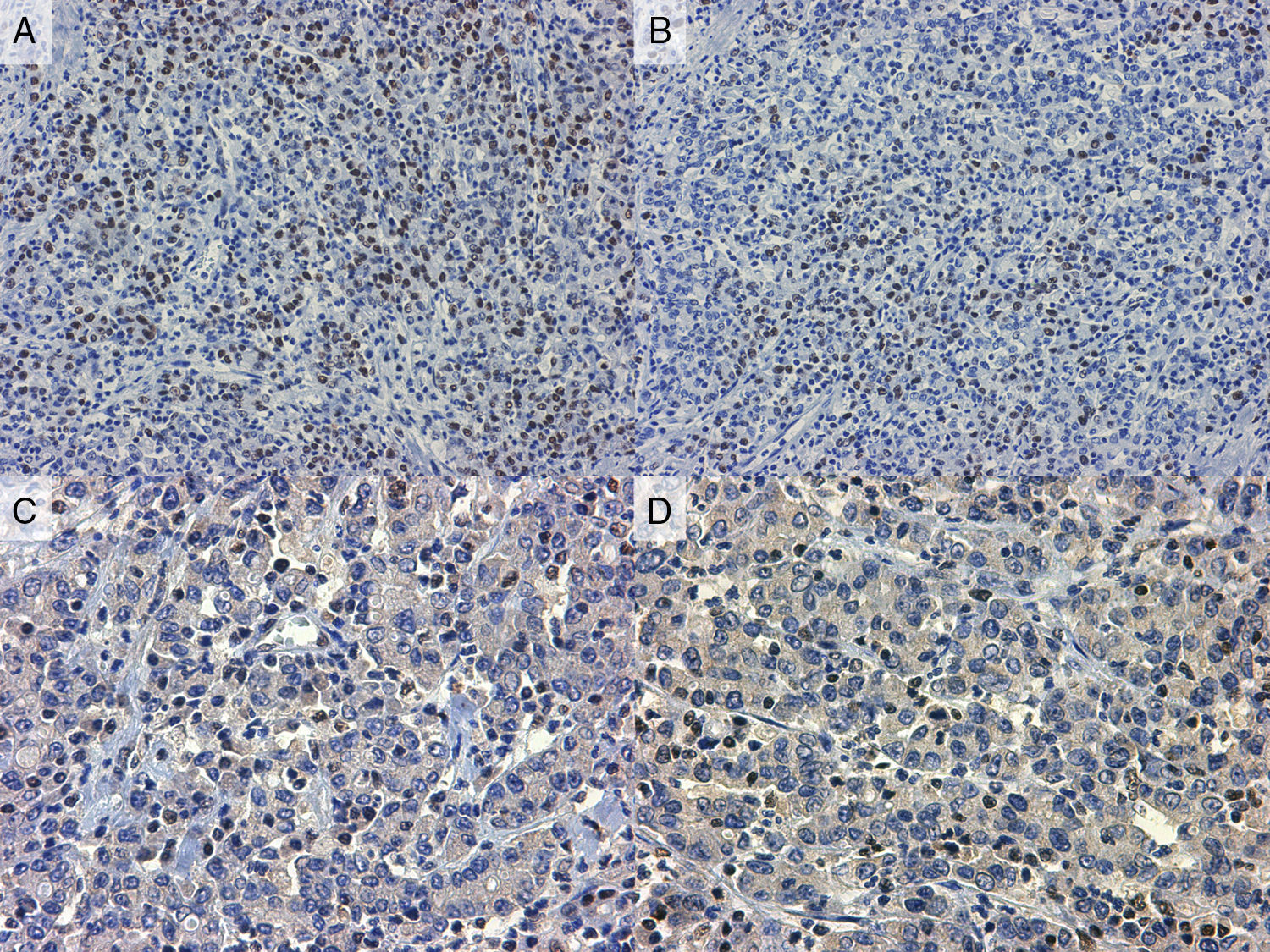
Lymphoepithelioma like carcinoma (LELC) is a well-known neoplastic lesion that mainly involves the stomach and which has been linked to Epstein Barr virus infection. There are exceptional cases of intestinal involvement by LELC, with 7 reported cases to date.
We report a new case of LELC affecting the right colon and review the literature on this rare disorder, with special emphasis on pathogenesis, molecular features and differential diagnosis.
El carcinoma tipo linfoepitelioma es una entidad conocida que suele afectar al estómago y se ha relacionado con infección por el virus de Epstein-Barr. La afectación intestinal es mucho más infrecuente, y en la literatura en inglés se han publicado solo 7 casos hasta la fecha.
Describimos un caso de carcinoma tipo linfoepitelioma que afectó al colon derecho y realizamos una revisión de la literatura con especial énfasis en su patogenia, características moleculares y diagnóstico diferencial.
Artículo
Comprando el artículo el PDF del mismo podrá ser descargado
Precio 19,34 €
Comprar ahora