To compare two different types of inserts: ultra-high molecular weight polyethylene (UHMWPE) and cross-linked polyethylene with a quantitative and qualitative study of polyethylene wear particles in synovial fluid 3 years after total knee arthroplasty.
Material and methodsA prospective, randomised, controlled cohort study with blinded evaluation was carried out on 25 patients undergoing staged bilateral total knee replacement, 6 months apart. Knee arthrocentesis was performed on 12 patients 3 years after surgery, and the polyethylene particles were analysed.
ResultsNo significant differences were found in the number of particles generated by the two different types of inserts at 3 years from total knee arthroplasty (3000×: x¯ cross-linked=849.7; x¯ UHMWPE=796.9; p=.63; 20,000×: x¯ cross-linked=66.3; x¯ UHMWPE=73.1; p=.76). Likewise, no differences in the probability of finding elongated (χ2=.19; p=.66) or rounded (χ2=1.44; p=.23) particles in both types of inserts were observed. However, the probability of finding fibrillar particles is 3.08 times greater in UHMWPE.
ConclusionsCross-linked polyethylene does not significantly reduce the generation of polyethylene particles in patients with total knee arthroplasty, 3 years after the surgical procedure.
Comparar dos pares de fricción (metal/polietileno de ultra alto peso molecular [UHMWPE], metal/polietileno de alto entrecruzamiento) mediante análisis cuantitativo y cualitativo de partículas de polietileno en líquido sinovial a los 3 años postintervención en pacientes portadores de prótesis total de rodilla (PTR).
Material y métodosSe llevó a cabo un estudio de cohortes prospectivo, aleatorizado, con evaluación ciega incluyendo 25 pacientes a quienes se intervino de PTR de manera bilateral, con 6 meses de diferencia. A los 3 años postintervención, se realizaron artrocentesis de rodilla a 12 pacientes y se analizaron las partículas de polietileno.
ResultadosNo se hallaron diferencias significativas en el número de partículas generadas por los diferentes insertos de polietileno a los 3 años tras la implantación de una artroplastia total de rodilla (3.000×: x¯ entrecruzado=849,7; x¯ UHMWPE=796,9; p=0,63; 20.000×: x¯ entrecruzado=66,3; x¯ UHMWPE=73,1; p=0,76). Tampoco existen diferencias significativas entre los 2 tipos de inserto, entre la posibilidad de encontrar partículas de forma elongada (χ2=0,19; p=0,66) ni redonda (χ2=1,44; p=0,23). Sin embargo, la probabilidad de encontrar partículas de forma fibrilar es 3,08 veces mayor en el UHMWPE.
ConclusionesEl polietileno altamente entrecruzado no reduce significativamente la generación de partículas de polietileno en aquellos pacientes intervenidos mediante una artroplastia total de rodilla, con muestras a 3 años postintervención.
The biological effects of wear are a limiting factor over the medium to long term for the survival of a total knee arthroplasty.1
Different prosthetic materials have been designed to reduce the production of polyethylene and minimise its biological effects.2
Ultra high molecular weight polyethylene (UHMWPE) is currently the material of choice for the load-supporting surface of total knee arthroplasties. Cross-linked polyethylene emerged as an alternative to UHMWPE based on the hypothesis that it would reduce wear.
Several studies have been performed in recent decades with the aim of finding a way to reduce polymer wear in total knee prostheses (TKP) using cross-linked polyethylene, thereby improving the mechanical properties of the load-supporting material.3
Although the results obtained using cross-linked polyethylene in total hip prostheses have been highly encouraging,4,5 its benefits in comparison with UHMWPE in TKP are still controversial.6–9
Factors such as the number, size and shape of the wear particles of polyethylene seem to be critical in the development of osteolysis. A greater volume and sub-micrometre size of polyethylene wear particles stimulate a greater response by the macrophages.8–11 Wear particles are generated in the joint and disperse in the synovial fluid. Some of them remain in the capsule, while other migrate towards the bone-implant interface, causing osteolysis and aseptic loosening.11
The most significant characteristics of the adverse biological reactions associated with failure of a total arthroplasty are the size, concentration, material and shape of wear particles. The size of the particles is significant as phagocytes play a key role in macrophage activation.8 The critical size range for phagocytosis induction after the activation of the macrophages by wear particles has been estimated to stand at from .2 to 10μm.12
The association between the rate of polyethylene wear and osteolysis or loosening has been described by several authors. Kobayashi et al. found that the morphological characteristics and concentration of polyethylene particles accumulating in the tissues were the most important factors in the pathogenesis of osteolysis.10
ObjectivesThe chief aim of this study is to compare the number and concentration of particles in the synovial fluid following total knee arthroplasty between cross-linked polyethylene and UHMWPE at 3 years after the operation.
The secondary aim here is to compare the morphology of the particles isolated from the synovial fluid in both types of polyethylene 3 years after implantation.
Materials and methodsA prospective and randomised study of cohorts was undertaken, with blind evaluation. The protocol was approved by the local Ethics Committee and the participants signed their informed consent for inclusion in the study at the moment of the operation and for the extraction of synovial fluid 3 years after the operation.
25 patients operated for bilateral TKP were included, with a 6 month time gap between operations. An UHMWPE (Sulene®) prosthesis was implanted in one knee and a cross-linked polyethylene (Durasul®) prosthesis was implanted in the other.13 The implant characteristics are described in Table 1. The Natural Knee prosthesis design (Zimmer, Warsaw, IN, USA) was implanted in all of the knees.
Characteristics of the 2 types of polyethylene inserts used.
| Type | UHMWPE Sulene (Zimmer) | Cross-linked Durasul (Zimmer) |
|---|---|---|
| Material | GUR 1020 | GUR 1050 |
| Manufacturing method | Moulded sheets | Moulded sheets |
| Dose (KGray) | 25–40 | 95 |
| Pre-irradiation temperature (°C) | Ambient temperature | 125 |
| Post-irradiation fusion | Not applicable | 150°C during 2h |
| Sterilisation method | Nitrogen Gamma | Ethylene oxide |
| Storage method | Nitrogen gas | Vacuum |
All of the surgical operations were performed by the same surgeon. The first knee to be implanted was the one that caused the greatest discomfort for the patient. The type of implant used in the first operation was decided by means of randomisation.
3 years after surgery arthrocentesis of the synovial fluid was performed by the same surgeon under completely sterile conditions.
The inclusion criteria for this study covered patients aged from 65 to 80 years old with primary grade 3 or 4 arthrosis, according to Ahlbäck's classification. The exclusion criteria were diagnosis of gonarthrosis due to inflammatory disease or osteonecrosis of the femoral condyle.
12 patients were analysed at follow-up after 3 years.
One patient rejected an operation for total arthroplasty of the second knee due to family problems. One patient was excluded due to medical complications. Polyethylene particles could not be isolated in 6 samples due to failure of organic material digestion, an excess of contaminated particles or a technical fault during filtration. Five patients did not attend the postoperative check at 3 years, so that arthrocentesis could not be performed.
The final sample included 91.6% of women and 8.4% men, with an average of 70.9 years old (SE=4.2).
Isolation techniqueThe standards of the American Society for Testing and Materials (ASTM) were followed. These consider filtration to be the most effective method for obtaining particles,14 and the samples were isolated and characterised.
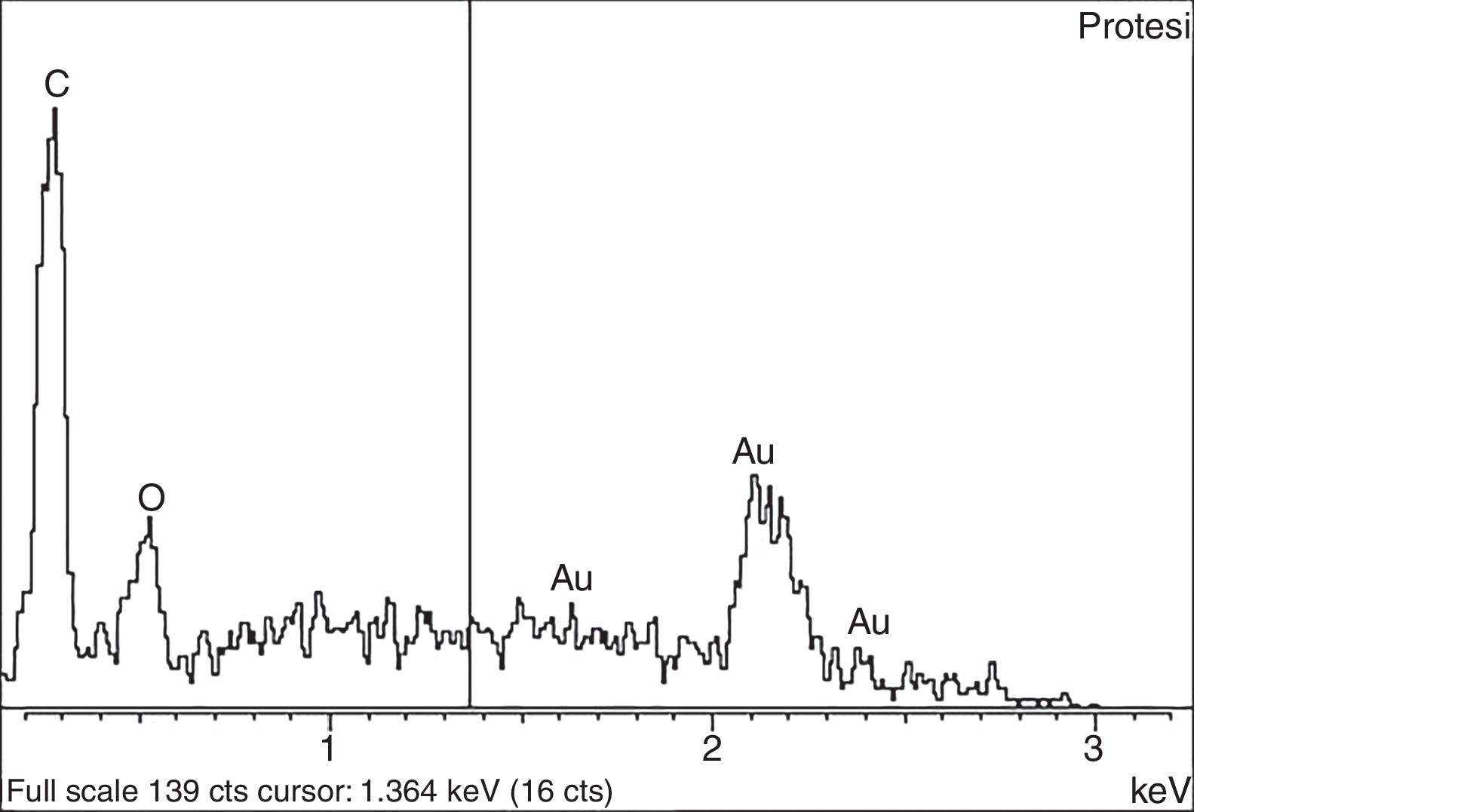
The extracted samples were kept at −20°C in a sterile recipient until they were processed. Proteins, lipids and other organic elements were duly eliminated from the samples, after which they were examined using a scanning electron microscope. Microscope findings were confirmed by analysis and spectrometry (Fig. 1). The polyethylene particles were identified, characterised and counted using a technique previously validated by Ries et al.15 This technique was modified in some ways, as their study was in vitro conditions so that a shorter digestion time with hydrochloric acid (HCl) was required, together with dilution in methanol.
The preparation technique involved different processes which were performed in a sterile chamber. In each case 37% HCl was added (J.T Baker) for digestion with magnetic agitation of the different solutions at ambient temperature and at 50°C. Subsequently the solution was diluted with methanol (CH3OH at 99.8%, Merck) and it was then filtered through a .05μm polycarbonate filter contained in a test tube.
Once the sample was in the .05μm thick polycarbonate filter (Whatman International Ltd, Maidstone, UK), this was adhered to a Petri plate and coated by Polaron E5000 cathodic pulverisation at from 3 to 12kV with a gold layer about 30nm thick.
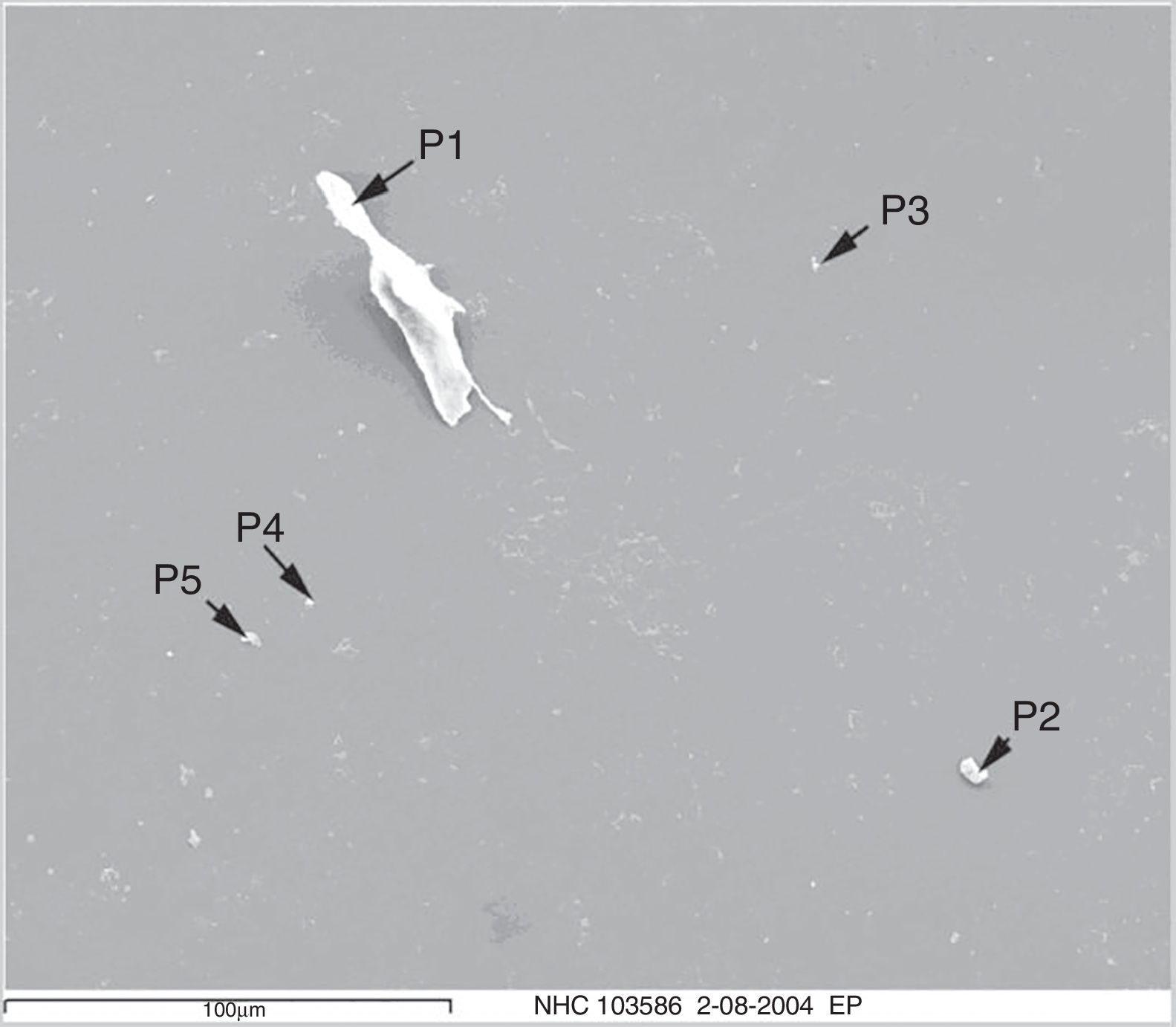
After fixing, the sample was examined using a Quanta 200 (FEI, Co.) scanning electron microscope. 40 fields in different magnifications were identified for each sample: 20 fields at 3000× (Fig. 2) and 20 fields at 20,000× (Fig. 3). These magnifications were selected to achieve a more appropriate resolution of different sizes.
The mixed technique of digestion with HCl and dilution with methanol enables us to obtain a clear sample that is free of organic particles, for optimum observation using the scanning electron microscope.
Statistical analysisThe descriptive statistical data included the number of polyethylene particles and their morphology.
To compare the number of particles depending on the type of polyethylene, non-parametric dependent data variance analysis was performed.
Analysis of the morphology of the isolated particles was carried out according to the repeated measurements model for binary data (GEE), which is an appropriate and general approach for the analysis of different types of correlated data, particularly if the results are binary.
The level of statistical significance was set at 5% bilaterally, and analysis was undertaken using version 9.1 of the SAS and version 23 of the SPSS statistical packages.
ResultsThe average volume of synovial fluid obtained was 6.85ml (SD=6.93ml). There were no significant differences in this respect between UHMWPE (x¯ = 8.68ml; SD=9.11ml) and cross-linked polyethylene (x¯ = 5.03; SD=3.17ml) (p=.175).
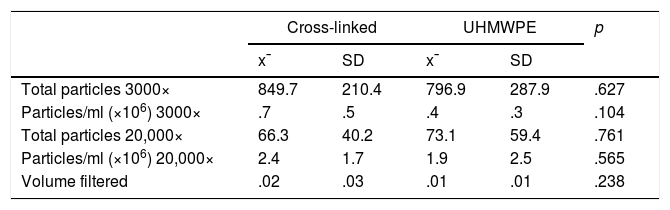
Table 2 shows the number and concentration of polyethylene particles found in both polyethylene inserts at magnifications of 3000× and 20,000×. No significant differences were found between the number of particles generated by the different polyethylene inserts at magnifications of 3000× or 20,000×.
Number and concentration of polyethylene particles found in both polyethylene inserts at 3000× and 20,000× magnifications, 3 years after implantation.
| Cross-linked | UHMWPE | p | |||
|---|---|---|---|---|---|
| x¯ | SD | x¯ | SD | ||
| Total particles 3000× | 849.7 | 210.4 | 796.9 | 287.9 | .627 |
| Particles/ml (×106) 3000× | .7 | .5 | .4 | .3 | .104 |
| Total particles 20,000× | 66.3 | 40.2 | 73.1 | 59.4 | .761 |
| Particles/ml (×106) 20,000× | 2.4 | 1.7 | 1.9 | 2.5 | .565 |
| Volume filtered | .02 | .03 | .01 | .01 | .238 |
Particle size varied considerably, from less than 1μm to 50μm.
Particle morphology was described according to the type of insert. The corresponding data are shown in Table 3. There were no statistically significant differences between both types of insert in terms of the possibility of finding elongated particles (χ2=.19; p=.66) or rounded ones (χ2=1.44; p=.23). Nevertheless, significant differences were found in terms of the probability of finding fibroid particles (χ2=4.64; p=.03), according to the coefficients estimated in the GEE model.
Number of particles of each type and percentage according to polyethylene type, 3 years after implantation.
| 3 years | ||
|---|---|---|
| Elongated | Cross-linked (No.=12) | 6 (50.0%) |
| UHMWPE (No.=12) | 8 (66.7%) | |
| Total (No.=24) | 14 (58.3%) | |
| Rounded | Cross-linked (No.=12) | 12 (100.0%) |
| UHMWPE (No.=12) | 11 (91.7%) | |
| Total (No.=24) | 23 (95.8%) | |
| Fibroid | Cross-linked (No.=12) | 6 (50.0%) |
| UHMWPE (No.=12) | 12 (100.0%) | |
| Total (No.=24) | 18 (75.0%) |
In connection with particle morphology, rounded ones are more prevalent at 3 years in cross-linked polyethylene, while fibroid ones are more prevalent in UHMWPE.
The statistical significance of fibroid morphology derives from the fact that the probability of fibroid particle shape occurring is 3.08 times greater in high molecular weight polyethylene than it is in cross-linked polyethylene (OR=3.08 [CI 95%: 1.11–8.59]).
DiscussionThe main finding of our study is that cross-linked polyethylene does not significantly reduce the generation of polyethylene particles in patients operated for total knee arthroplasty in samples taken 3 years after implantation.
The characteristics of the polyethylene particles (their number, size and shape) have been described as critical factors in the appearance of osteolysis.8 When there are higher volumes of particles with the presence of sub-micrometre particles as well as elongated ones, it is more probable that there will be macrophage stimulation and activation.11 The biological effects of polyethylene do not solely depend on the total volume of wear and the number of particles generated, as they are also influenced by the proportion of biologically active particles.16
Wear isolation techniquesDifferent methods of taking tissue samples have been described in the literature. The original method used by Campbell was the first to be used in the 1990s, and he and his co-workers were the pioneers in using sodium hydroxide (NaOH) to isolate polyethylene particles.17 Many of the protocols now published are based on Campbell's method.
However, in our study we used the combined technique of digestion with HCl and dilution with methanol. This was presented in the Sixth World Biomaterials Congress, and it has been used by several authors, including Michael D. Ries and Marcus L. Scott.15,18 This procedure makes it possible to obtain a clear sample that is free of organic particles.19
The results obtained in our study agree with the findings of Niedzwiecki et al.,20 who also used a 37% solution of HCl. They describe how serum proteins are effectively eliminated. In their study they obtained polyethylene particles comparable in size and shape with those isolated using NaOH.14
Other studies have used potassium hydroxide (KOH), concentrated nitric acid (HNO3), papain or other enzymes such as collagenase dissolved in a phosphate reagent. Baxter et al.21 carried out an exhaustive review of the periprosthesis tissue digestion methods used by different authors. To date none of these methods has been identified as the one of choice, and more studies are needed to establish the degree to which these different approaches are effective.
Our results show that our technique for identifying and quantifying polyethylene particles in vivo in the synovial fluid from patients with total knee arthroplasty is both precise and reproducible. This technique makes it possible to study the in vivo behaviour of prosthesis models made of different materials and therefore with different friction levels.
Results at 3 yearsIn a recent in vivo study, Hinarejos et al.7 carried out a prospective randomised clinical trial using the NaOH protocol in synovial fluid from 34 patients with the same design of prosthesis. 50% of the patients were implanted with UHMWPE and the other 50% received cross-linked polyethylene with sequential tempering. They performed an arthrocentesis one year after surgery and found no statistically significant differences between the 2 groups in the reduction of the concentration, size or shape of the polyethylene particles. They also found a high degree of variability in the concentration of particles in both groups, and they suggest that the rate of wear of the polyethylene is determined by a range of factors which are more important than the type of polyethylene used.
Minoda et al.6 analysed in vivo synovial fluid using the NaOH protocol and found no statistically significant differences between both types of implant in a clinical and radiological evaluation after 2 years.
Other authors such as Wolfarth et al.22 analysed the size and morphology of particles in synovial fluid extracted from the tissue around the prosthesis in 4 patients, using Campbell's method. They concluded that the morphology and size do not differ between the 2 groups; although the concentration of particles is higher in the tissue as the synovial fluid is filtered continuously, it is representative of the concentration in the latter.
On the contrary, Iwakiri et al.23 using Campbell's method reported differences in the polyethylene particles that had separated from cross-linked polyethylene, as they were less numerous, smaller and more rounded.
Recently, Minoda et al.9 published a study on synovial fluid one year after surgery on 16 knees. They compared conventional polyethylene with cross-linked sequentially tempered polyethylene. Their results show that there are no significant differences between the 2 groups in terms of the size and shape of the particles. Nevertheless, they found significant differences in the reduction in the number of particles in patients with cross-linked sequentially tempered polyethylene.
Comparison of the studies by Hinarejos et al.7 and Minoda et al.9 suggests that the design of prosthetic joint surfaces may influence the in vivo generation of polyethylene particles,11,24–26 so that this would influence the long-term development of osteolysis. This fact would explain the differences between our study and others which included different prosthesis designs.
In vitro studies by other authors such as Muratoglu et al.27 show that the wear of cross-linked polyethylene is 80–90% lower than that of UHMWPE. Fisher et al.28 found that polyethylene particles smaller than 1μm were 6 times more active than particles larger than 1μm for a constant volume of wear.
Our results at 3 years differ from those of Muratoglu et al.27 and Fisher et al.28 This may be due to the different ambient conditions used, as the model used by Muratoglu and Fisher is in vitro, while our tests were carried out in vivo. The number of particles obtained in vivo is clearly lower than those obtained by in vitro tests.8
One of the advantages of our study is that all of the surgical operations were performed by the same team, using the same procedure, prosthesis design and implanted materials. The polyethylene insert was the only difference between both groups. This fact makes it possible to control the external variations associated with the procedure. On the other hand, we try to control the effect of intrinsic patient factors, such as their weight, level of physical activity and prosthesis size.
Another strong point of this study is that we use a filter with .05μm pores when processing the synovial fluid. Although the particles that cause the most wear are from .1 to 1μm29 in size, a small filter may have increased the retention of the particles that cause osteolysis.
One of the limitations of our study is the sample size. Although the majority of studies contain a similar or lower number of cases, it would be advisable to increase the number of patients studied. Additionally, we do not know how loss of some of the sample in the 3-year follow-up may have influenced the results.
Another limitation of this study is the follow-up time. Although the majority of studies cover only 2 years after the operation, we believe it is necessary to increase follow-up time to observe the effects of polyethylene wear over time.
As Paxton et al.30 pointed out, it would be prudent to consider whether the increased cost of implants with cross-linked polyethylene and the lack of empirical evidence for their supposed clinical advantages justify their use in TKP. Nevertheless, the favourable results obtained in hip prostheses6,30,31 should encourage more long term studies (over 10 or 15 years after implantation) to evaluate the possible benefits of cross-linked polyethylene in TKP.
We are able to conclude that this study shows no advantages deriving from the use of cross-linked polyethylene in comparison with UHMWPE at 3 years after the operation.
Level of evidenceLevel of evidence III.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed are in agreement with the ethical norms of the relevant human experimentation committee, the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work governing the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in this paper. This document is held by the corresponding author.
Conflicts of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Lasurt-Bachs S, Torner P, Maculé F, Prats E, Menéndez-García F, Ríos-Guillermo J, et al. El polietileno de alto entrecruzamiento no reduce el desgaste en la artroplastia total de rodilla. Rev Esp Cir Ortop Traumatol. 2018;62:197–203.