Several autoimmune disorders have been associated with a variety of hematopoietic malignancies, particularly lympho-proliferative disorders. Multiple myeloma (MM) is one of the most common hematologic malignancies and has been described in the context of a variety of autoimmune conditions. Due to their diversity and rarity, the clinical features of autoimmune conditions associated with MM have not been elucidated and the pathogenesis remains unclear. In this report, we describe two cases of autoimmune conditions in the setting of MM and review the current literature.
Varios trastornos autoinmunes se han asociado a una variedad de neoplasias malignas hematopoyéticas, particularmente trastornos linfoproliferativos. El mieloma múltiple (MM) es una de las neoplasias malignas hematológicas más comunes y ha sido descrito en el contexto de una variedad de condiciones autoinmunes. Debido a su diversidad y rareza, las características clínicas de las condiciones autoinmunes asociadas con el MM no han sido aclaradas y la patogénesis sigue siendo poco clara. En este artículo se describen dos casos de condiciones autoinmunes en el marco del MM y se realiza una revisión de la literatura actual.
The relationship between autoimmune diseases and myeloma was initially observed in the 1960's.1–4 Multiple retrospective and cohort studies have shown that the incidence of lymphoproliferative diseases is greater in patients carrying autoimmune disorders. Multiple myeloma (MM), a plasma cell neoplasia and the second most common lymphoproliferative disorder, has been associated with a wide spectrum of autoimmune related conditions. 5–8A retrospective cohort study of more than 4000 white and black male veterans postulated that various types of immune-mediated conditions such as pernicious anemia, systemic sclerosis and systemic lupus erythematosus were related to MM development.9
Herein we report on two patients with MM presenting with autoimmune conditions rarely described in conjunction with MM (angioedema and myasthenia gravis) as autoimmune disorders associated with MM and summarize the current literature regarding autoimmune manifestations of MM.
Patient and MethodsThis review is based on information from the Medline/PubMed and Scopus database using combinations of the keywords multiple myeloma, auto antibodies, myasthenia gravis, rheumatoid arthritis, systemic lupus erythematous, urticaria, paraneoplastic and autoimmunity. We included reports that were published in English, Spanish and French and specifically described autoimmune disorders associated with MM. Forty-four articles met our selection criteria.
Patient 1A 70 year old male was seen for initial consultation in 2013 for history of free lambda chain monoclonal gammopathy as well as focal episodes of angioedema. He initially developed intermittent episodes of facial swelling involving different parts of his face in 2008. He was noted to have proteinuria during one of his angioedema episodes and was referred to a hematologist. Over the next few years, the attacks involved also his forehead, cheek, tongue and sometimes the bottoms of the hands and feet. These episodes occurred without any antecedent triggers or associated factors and were relieved with Benadryl. Involvement of the upper respiratory or gastrointestinal tracts was never observed.
A full workup for multiple myeloma (MM) was then performed, and he was noted to have immune globulins within normal limits except for IgG of 648mg/dl, free lambda elevated to 27.85mg/dl with a decreased kappa lambda ratio to 0.02 and serum and urine immunofixation revealing free lambda light chains. The percentage of plasma cells was 15% by bone marrow examination with immunohistochemistry demonstrating lambda restriction and a normal karyotype with an extra copy of 1q25 in 75% of plasma cells.
At that time the diagnosis of lambda light chain multiple myeloma ISS I was confirmed without any evidence of anemia, bone disease, hypercalcemia or impaired renal function. Nonetheless, the question remained of whether the episodes of focal angioedema may be related to an acquired C1q inhibitor antibody related to multiple myeloma. Further testing noted normal levels of C1 esterase inhibitor, C3 complement, C4 complement and C1q complement. Although there was no direct evidence that could confirm the association, it has not been effectively ruled out either. Patient has not received antimyeloma therapy and angioedema episodes have been controlled symptomatically.
Patient 2A 53 year old African American male presented with a 30 year history of myasthenia gravis; he initially experienced upper body weakness, ptosis, diplopia, loss of peripheral vision and was treated with pyridostigmine. He was assessed in 2011 for a progressive hemoglobin drop from 14.7 to 13 with normal levels of serum iron, B12, folic acid and elevated total protein. Further workup revealed a paraprotein peak in the blood (IgG of 3786mg/dl with an IgG Kappa peak of 2.65mg/dl), bone marrow with 29% plasma cells, and no high risk cytogenetic abnormalities with the exception of deletion of ETV6 stain in 8% of the cells. At that time the diagnosis of asymptomatic IgG Kappa multiple myeloma ISS II (normal albumin and B2 3.7) was confirmed without any evidence of anemia, bone disease, hypercalcemia or impaired renal function.
Therefore he continued with close observation; during his nine month follow up he had recurrence of his old symptoms of myasthenia gravis (only right eye ptosis) and was treated with pyridostigmine by his neurologist. Two months later, he demonstrated progression of the disease with an increasing paraprotein peak, kappa free light chain of 67.21mg/dl, kappa:lambda ratio of 93.35 as well as progressive cytopenias. He started to have symptoms— in particular, fatigue and mild back pain. CT scans revealed punctate lytic lesions at the vertebral bone and nondisplaced fractures in the fourth and fifth ribs. A repeat bone marrow biopsy showed 30% plasma cells and complex cytogenetic abnormalities including p11 deletion,p12 addition, and additional copies of chromosomes 3, 5, 9 and 15. MLL gene loss was seen in 35% of the cells.
The patient received 4 cycles of bortezomib, lenalidomide, and dexamethasone (RVD)10 with suboptimal response. He then underwent stem cell mobilization with bortezomib, dexamethasone, thalidomide, platinum, adriamycin, cyclophosphamide and etoposide (VDTPACE) for stem cell mobilization and collection.11 Subsequently undergoing an autologous stem cell transplantation with a high dose melphalan based conditioning regimen and achieved a very good partial response (VGPR).
His symptoms of myasthenia gravis recovered completely off all treatment, noticing less fatigue and muscular weakness compared to before his transplant. He discontinued pyridostigmine and denies any other symptoms related to this disease.
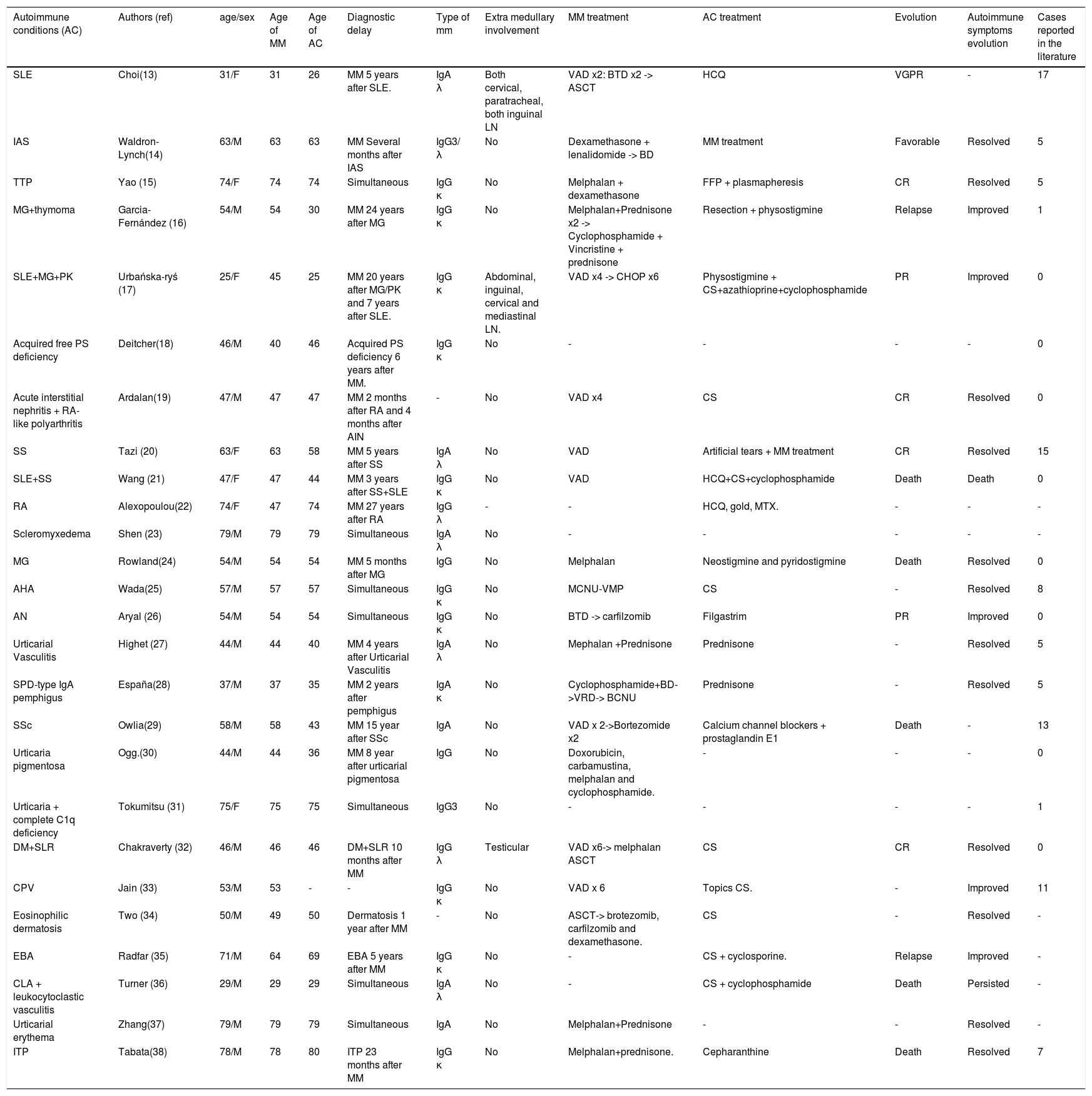
DiscussionMultiple myeloma is a B cell malignancy characterized by clonal expansion of malignant plasma cells in bone marrow. Although the etiology is poorly understood, there is some evidence for immune dysregulation or sustained immune stimulation in the pathogenesis of this disease.12 The concurrence of autoimmuned conditions and MM is rare and has not been fully investigated. We herein describe the course of two patients with MM who presented with two different autoimmune conditions: myasthenia gravis and angioedema. To date, more than 25 different autoimmune conditions related to MM have been reported in the literature, which are summarized in Table 1.13–38 In the review of reported cases, the mean age of diagnosis of MM and the autoimmune condition was 55 and 54 years old, respectively. One third of the cases had the diagnosis of their autoimmune condition before the onset of MM, and 40 percent (10 of 25 cases) the diagnosis were made simultaneously. Among the autoimmune conditions related to MM, SLE was the most common with 17 reported cases, followed by SS and SSc with 15 and 13 respectively. The autoimmune symptoms resolved or improved with MM treatment in the majority of cases, as in the case of patient 2.
Summary of clinical characteristics in 26 reported cases of MM associated with autoimmune conditions (AC).
| Autoimmune conditions (AC) | Authors (ref) | age/sex | Age of MM | Age of AC | Diagnostic delay | Type of mm | Extra medullary involvement | MM treatment | AC treatment | Evolution | Autoimmune symptoms evolution | Cases reported in the literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLE | Choi(13) | 31/F | 31 | 26 | MM 5 years after SLE. | IgA λ | Both cervical, paratracheal, both inguinal LN | VAD x2: BTD x2 -> ASCT | HCQ | VGPR | - | 17 |
| IAS | Waldron-Lynch(14) | 63/M | 63 | 63 | MM Several months after IAS | IgG3/ λ | No | Dexamethasone + lenalidomide -> BD | MM treatment | Favorable | Resolved | 5 |
| TTP | Yao (15) | 74/F | 74 | 74 | Simultaneous | IgG κ | No | Melphalan + dexamethasone | FFP + plasmapheresis | CR | Resolved | 5 |
| MG+thymoma | Garcia-Fernández (16) | 54/M | 54 | 30 | MM 24 years after MG | IgG κ | No | Melphalan+Prednisone x2 -> Cyclophosphamide + Vincristine + prednisone | Resection + physostigmine | Relapse | Improved | 1 |
| SLE+MG+PK | Urbańska-ryś (17) | 25/F | 45 | 25 | MM 20 years after MG/PK and 7 years after SLE. | IgG κ | Abdominal, inguinal, cervical and mediastinal LN. | VAD x4 -> CHOP x6 | Physostigmine + CS+azathioprine+cyclophosphamide | PR | Improved | 0 |
| Acquired free PS deficiency | Deitcher(18) | 46/M | 40 | 46 | Acquired PS deficiency 6 years after MM. | IgG κ | No | - | - | - | - | 0 |
| Acute interstitial nephritis + RA-like polyarthritis | Ardalan(19) | 47/M | 47 | 47 | MM 2 months after RA and 4 months after AIN | - | No | VAD x4 | CS | CR | Resolved | 0 |
| SS | Tazi (20) | 63/F | 63 | 58 | MM 5 years after SS | IgA λ | No | VAD | Artificial tears + MM treatment | CR | Resolved | 15 |
| SLE+SS | Wang (21) | 47/F | 47 | 44 | MM 3 years after SS+SLE | IgG κ | No | VAD | HCQ+CS+cyclophosphamide | Death | Death | 0 |
| RA | Alexopoulou(22) | 74/F | 47 | 74 | MM 27 years after RA | IgG λ | - | - | HCQ, gold, MTX. | - | - | - |
| Scleromyxedema | Shen (23) | 79/M | 79 | 79 | Simultaneous | IgA λ | No | - | - | - | - | - |
| MG | Rowland(24) | 54/M | 54 | 54 | MM 5 months after MG | IgG | No | Melphalan | Neostigmine and pyridostigmine | Death | Resolved | 0 |
| AHA | Wada(25) | 57/M | 57 | 57 | Simultaneous | IgG κ | No | MCNU-VMP | CS | - | Resolved | 8 |
| AN | Aryal (26) | 54/M | 54 | 54 | Simultaneous | IgG κ | No | BTD -> carfilzomib | Filgastrim | PR | Improved | 0 |
| Urticarial Vasculitis | Highet (27) | 44/M | 44 | 40 | MM 4 years after Urticarial Vasculitis | IgA λ | No | Mephalan +Prednisone | Prednisone | - | Resolved | 5 |
| SPD-type IgA pemphigus | España(28) | 37/M | 37 | 35 | MM 2 years after pemphigus | IgA κ | No | Cyclophosphamide+BD->VRD-> BCNU | Prednisone | - | Resolved | 5 |
| SSc | Owlia(29) | 58/M | 58 | 43 | MM 15 year after SSc | IgA | No | VAD x 2->Bortezomide x2 | Calcium channel blockers + prostaglandin E1 | Death | - | 13 |
| Urticaria pigmentosa | Ogg.(30) | 44/M | 44 | 36 | MM 8 year after urticarial pigmentosa | IgG | No | Doxorubicin, carbamustina, melphalan and cyclophosphamide. | - | - | - | 0 |
| Urticaria + complete C1q deficiency | Tokumitsu (31) | 75/F | 75 | 75 | Simultaneous | IgG3 | No | - | - | - | - | 1 |
| DM+SLR | Chakraverty (32) | 46/M | 46 | 46 | DM+SLR 10 months after MM | IgG λ | Testicular | VAD x6-> melphalan ASCT | CS | CR | Resolved | 0 |
| CPV | Jain (33) | 53/M | 53 | - | - | IgG κ | No | VAD x 6 | Topics CS. | - | Improved | 11 |
| Eosinophilic dermatosis | Two (34) | 50/M | 49 | 50 | Dermatosis 1 year after MM | - | No | ASCT-> brotezomib, carfilzomib and dexamethasone. | CS | - | Resolved | - |
| EBA | Radfar (35) | 71/M | 64 | 69 | EBA 5 years after MM | IgG κ | No | - | CS + cyclosporine. | Relapse | Improved | - |
| CLA + leukocytoclastic vasculitis | Turner (36) | 29/M | 29 | 29 | Simultaneous | IgA λ | No | - | CS + cyclophosphamide | Death | Persisted | - |
| Urticarial erythema | Zhang(37) | 79/M | 79 | 79 | Simultaneous | IgA | No | Melphalan+Prednisone | - | - | Resolved | - |
| ITP | Tabata(38) | 78/M | 78 | 80 | ITP 23 months after MM | IgG κ | No | Melphalan+prednisone. | Cepharanthine | Death | Resolved | 7 |
Abbreviations: AC Autoimmune condition, AHA Autoimmune Hemolytic Anemia, AN Autoimmune neutropenia, AIN Acute interstitial nephritis, ASCT autologous stem cell transplantation, AvWD Acquired Von Willebrand disease, BCNU Melphalan, Cyclophosphamide, Adriamycin and Carmustine, BD Bortezomib and Dexamethasone, BTD Bortezomib, Thalidomide, and Dexamethasone, CLA Cutis laxa acquisita, CPV Cutaneous paraneoplastic vasculitis, CR Complete remission, CS Corticosteroids, DM Dermatomyositis, EBA Epidermolysis bullosa acquisita, F Female, FFP Fresh frozen plasma, HCQ Hydroxychloroquine, IAS Insulin autoimmune syndrome, ITP Immune thrombocytopenic purpura, LN Lymph node, MCNU-VMP Ranimustine, Vindesine, Melphalan and Prednisolone, MG Myasthenia gravis, MM Multiple myeloma, M Male, PK Palmoplantar keratoderma, PR Partial response, PS Protein S, SLE Systemic lupus erythematosus, SLR Sarcoid-like reaction, SPD Sucorneal pustular dermatosis, SS Sjögren syndrome, SSc Systemic sclerosis, RA Rheumatoid Arthritis, TTP Thrombotic thrombocytopenic purpura, VAD Vincristine, Doxorubicin and Dexamethasone, VRD Bortezomib, Lenalidomide, Dexamethasone, VGPR Very good partial response.
B-cell hyperactivity, considered a trademark of autoimmune conditions, favors the escape of abnormal cell clones from normal regulatory mechanisms.13 Therefore, it has been suggested that immune related conditions can be associated with an elevated MM risk.12,21 In the case of SLE, the mouse lupus model showed an increased prevalence of MM, and more than 30% of the specimens showed monoclonal gammopathy.13 It has been recently reported that plasma cell growth factors such as B lymphocyte stimulator (BLyS) are elevated in patients with SLE.
Several theories have been proposed to explain the coexistence of MM and an immune condition. One hypothesis suggests that a spontaneous autoreactive clone of B cells undergoes physiological maturation and produces monoclonal auto antibodies.14,39
The association of Myasthenia Gravis and MM was first reported by Rowland et al24 who diagnosed the plasma cell dyscrasia in a patient five months after the diagnosis of MG. Because the protein abnormality was considered to be possibly related to the myasthenic syndrome, therapy for MM was instituted and caused a remission of myasthenic symptoms. In this regard, our reported case (patient 2) is similar because treatment of MM resulted in resolution of MG.
The incidence of monoclonal gammopathy developing during the course RA has been estimated to be 1-2%.22 Moreover, Srinivasulu et al.40 reported the case of two patients who presented with inflammatory arthritis as the initial manifestation of MM. Reza-Ardalan et al. studied the similarity in the cytokine milieu of MM and RA. They showed that in the context of MM, an immunologic reaction directed against light chain molecules and tumoral antigens deposited within the synovium could result in RA-like polyarthritis.19 Another biologically plausible association between autoimmune thyroid disease (ATD) and MM has been reported regarding the cytokine milieu. IL-6 in particular appears to contribute to the growth of myeloma cells and has a role in the initiation and perpetuation of ATD.41
In a prospective study, less than 4% of AHA cases were due to MM, and only eight cases of AHA at MM presentation have been reported in the literature.40,42 Wada et al. 25 demonstrated that the antibody binding to erythrocytes was similar to myeloma M protein.
Among the paraneoplastic syndromes and cutaneous manifestations of MM, angioedema is exceptional (only 4 cases in the literature).43,44 Angioedema with acquired C1 esterase inhibitor (C1-INH) deficiency is a rare disorder usually identified as acquired angioedema (AAE). It was first described in 1972 by Caldwell et al and is characterized by episodes of bradykinin-mediated angioedema without urticarial.43,45,46 In the acquired form, the deficiency results from markedly increased catabolism of C1 inhibitor due to its consumption by the neoplastic lymphatic tissues (AAE type 1) or by autoantibody mediated inactivation (AAE type 2).46,47 Geha et al.48 presented a patient with IgA MM and acquired C1-inhibitor deficiency who had circulating antiidiotypic antibodies that reacted with the M component. This interaction appeared to cause increased consumption of C1- inhibitor. There is evidence that the M components detected frequently correspond to the C1-inhibitor auto antibodies. Castelli et al.42,44 study group has shown that patients with MGUS and C1-inhibitor auto-antibodies frequently have the same heavy and light chain histotypes. This suggests that a single B cell clonal disorder with different potential clinical evolutions underlies all AAE.49
In correlation with patient 1, it has been documented that C1- INH antigen values can be normal due to elevated amounts of cleaved inactive C1-INH in plasma or normal function levels between angioedema attacks. C1q reduction confirms the diagnosis of AAE, but a minority of patients can present with normal C1q. In this case, the presence of autoantibodies to C1- INH can be investigated. Limits to these procedures are the inadequate standardization of C1- INH functional measurements and the availability to look for anti-C1-INH autoantibodies.49,50
In summary, autoimmunity appears to have an association with multiple myeloma. We reviewed several cases of autoimmune conditions related to multiple myeloma documented in the literature. The heterogeneous set of conditions, diversity among presentations and severity explains the lack of epidemiological data and clinical features. In the majority of cases, the diagnoses were made simultaneously; SLE was the most common AD associated with MM. Myeloma treatment in general improved the autoimmune symptoms. As the pathogenesis is still not clear, different hypotheses have emerged although none proven. Based on only a few studies, it appears that autoimmunity favors the escape of abnormal clones of B cells from regulatory mechanisms leading to emergence of neoplastic B cell clones. On the other hand, other data suggests a spontaneous transformation of an autoreactive clone of B cells that later on undergoes physiological maturation and production of autoantibodies, therefore, explaining the autoimmune manifestations.
This association needs further clinical studies in order to provide an important foundation in understanding the physiology of B cell in MM. In addition, larger retrospective studies could favor the discernment of risk factors, prognosis and essential epidemiologic data in the setting of autoimmune conditions related to myeloma.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicto de interesesThe authors have no conflicts of interest to declare.
Dr. Avila and Dr. Giralt performed the research, designed the research study and wrote the paper. We considered the ethical responsibilities dictated by Revista Colombiana de Cancerología and we do not have any conflict of interest to declare.