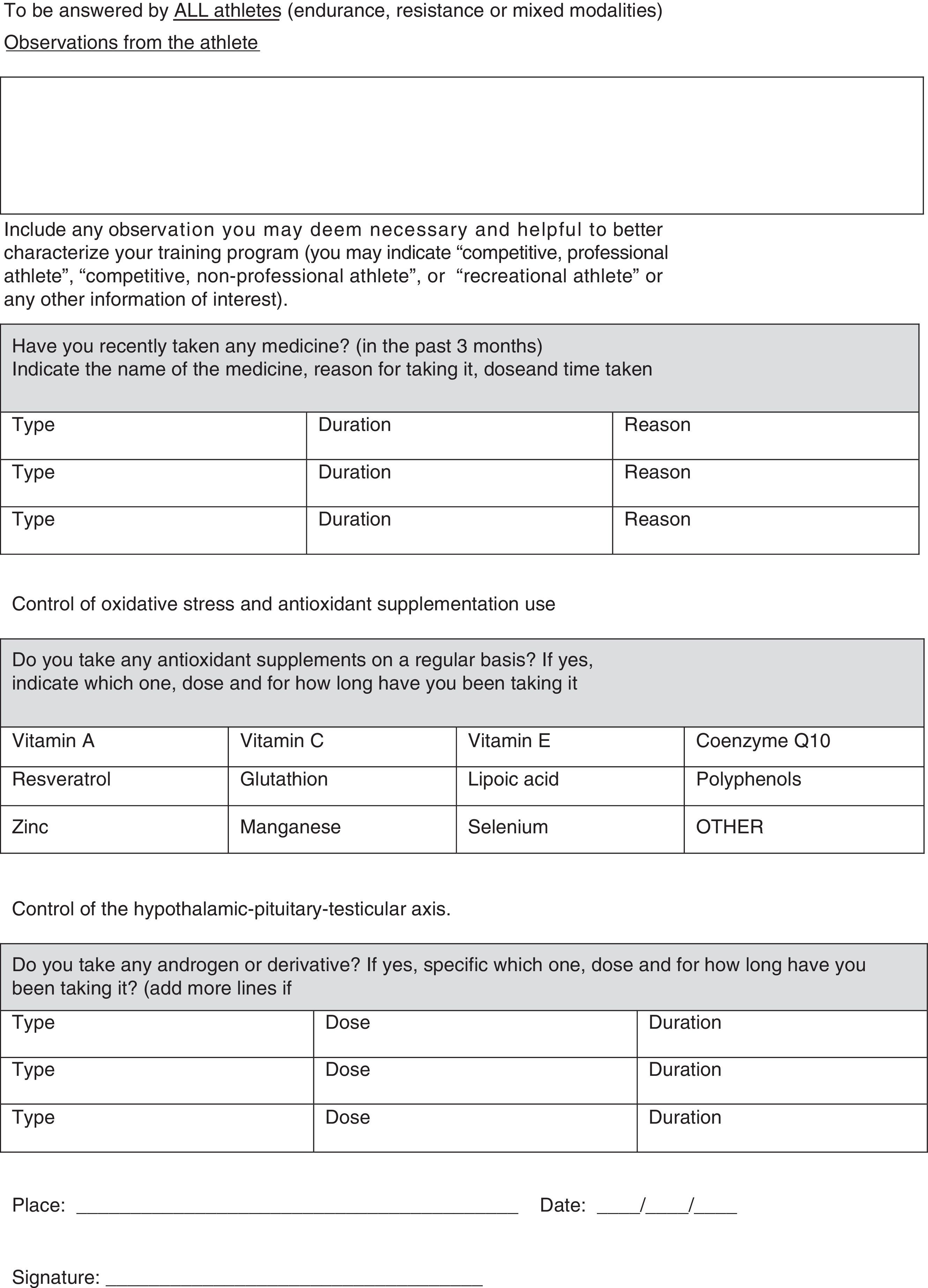
Male athletes in general are subjected to the same causes of infertility as the general population, but sports practice itself may be possibly an additional infertility factor or, at least an aggravating factor for a previously existing fertility condition; on the contrary, being physically active has been hypothesized to favor hormonal and seminological processes and could be beneficial for fertility. In this relationship, the different inherent parameters of physical activity-exercise (training volume, intensity, objective, organization and frequency) are of paramount importance. Therefore, this review discusses both the negative and positive impact of physical exercise on the male reproductive potential. Clear knowledge is lacking on this topic as incongruences exist due to the fact that studies lack standardization in assessment tools or research protocols. So that future studies can reveal more information regarding exercising male fertility, we introduce a unique questionnaire developed with the intent to help standardize future studies on male fertility and exercise.
Los atletas masculinos presentan, generalmente, las mismas causas de infertilidad que la población general; no obstante, la práctica deportiva puede ser, por sí misma, un factor de infertilidad o un factor agravante en el caso de un problema previo de fertilidad; por otra parte, se ha sugerido recientemente que ser físicamente activo puede favorecer el entorno hormonal y seminológico y, por ende, ser beneficioso para la fertilidad. En esta relación, los distintos parámetros inherentes relacionados a la actividad física y al ejercicio (volumen, intensidad, objetivo, organización y frecuencia) son de suprema importancia. Esta revisión versa sobre el impacto, tanto negativo como positivo, del ejercicio físico sobre el potencial reproductivo masculino. No existe un conocimiento consensuado sobre este tópico debido a incongruencias derivadas del hecho de que los estudios carecen de estandarización en las herramientas de evaluación y/o los protocolos de investigación. Con la finalidad de que estudios futuros puedan revelar más información sobre la fertilidad en los hombres que practican ejercicio físico, presentamos un cuestionario único desarrollado con el objetivo de ayudar a estandarizar estudios futuros que versen sobre fertilidad masculina y ejercicio.
Atletas do sexo masculino geralmente têm as mesmas causas de infertilidade que a população em geral; contudo, o desporto em si pode ser um fator de infertilidade ou um fator agravante no caso de problema de fertilidade anterior. Além disso, recentemente, tem sido sugerido para ser fisicamente ativo pois pode promover melhoras no ambiente seminológico e hormonal e, portanto, ser benéfico para a fertilidade. Neste sentido, os diversos parâmetros inerentes relacionados à atividade física e exercício físico (volume, intensidade, finalidade, organização e frequência) são de suma importância. Este artigo trata do impacto, tanto positivo como negativo, do exercício físico sobre o potencial reprodutivo masculino. Não há conhecimento consensual sobre este tema, por causa de inconsistências decorrentes do fato de que os estudos carecem de padronização em ferramentas de avaliação e/ou protocolos de pesquisa. A fim de que estudos futuros possam revelar mais informações sobre a fertilidade em homens que praticam exercício, apresentamos um questionário desenvolvido para ajudar a padronizar os estudos futuros que lidam com a fertilidade masculina e o exercício.
Physical activity programs improve health and the quality of life, but excessive or exhaustive exercise exposure can lead to negative side effects, with one of the most recently recognized being possible fertility issues in both females and males when training load is elevated and intense, though this is not universally recognized. Physical activity (exercise) could exert beneficial or detrimental effects depending on the several inherent exercise regimen parameters: type, intensity, volume, objective, or organization. Regrettably, there is still lack of consensus on these effects.1 Extensive and important work has been done assessing female athletes in this regard but this reviewed is delimited to males only.
In males where results seem more controversial, it has been observed that prolonged intensive exercise (and training) may lead to adverse effects on physiological systems, particularly, the reproductive system and fertility with alterations in reproductive hormone levels,2–12 atrophy of the testicular germinal epithelium and adverse effects on spermatogenesis,13–15 changes in semen parameters including abnormal sperm morphology,2,3,7,10,11,16 and reduced sperm motility.16
Nevertheless, there are still many discrepancies in studies regarding the effect of exercise and physical activity on male fertility. Therefore, the aim of the present article is to review the impact of physical exercise on reproductive performance and fertility as well as to introduce a unique questionnaire that may help standardize future studies on male fertility and exercise.
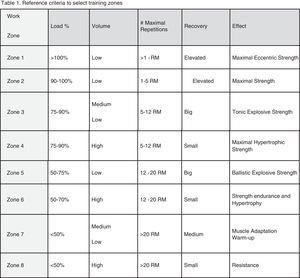
Exercise male reproductive performance and fertilityHormonal alterations – endurance trainingNegative effectWhile there is no clear consensus, acute and chronic exercise is associated with suppressed endocrine functions at the hypothalamic and testicular levels; specifically, suppression of both gonadotropin-releasing hormone (GnRH) and serum testosterone.17 Taking testosterone (T) as a major regulator of the hypothalamic-pituitary-testicular (HPT) axis, available studies can be included into three categories: those that do not show changes in either T (free [fT] or total [tT] T) or any of the other HPT hormones,18–20 those that show no changes in testosterone (fT or tT) but show changes for the other HPT hormones,7,21,22 and those that show changes in fT or tT but there could or could not be changes, for the other HPT hormones.2,3,10,23–28
Some studies may reflect training stimuli that were not sufficient enough to alter the axis (low or moderate performance level, or low volume used in the study), which could be plausible explanations for studies not detecting changes in T but detecting changes for other hormones (Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH)). Among works detecting changes in T the most habitual finding is decrease in T without changes in FSH or LH.2,3,10,22,27 Research has focused primarily on distance runners, but studies have been conducted with other endurance sports. For example cyclists, were findings are controversial. One study reported no differences in hormonal profiles of cyclists.16 Other studies have revealed decreased T values in professional cyclists, during one specific race (Tour of Spain),29,30 researcher found cyclists coming into competition with the greatest training volume had lower T levels than those of other comparative cyclists. This finding is supported by other studies.5,31 Likewise, when trained and untrained subjects performing four hours of road bicycling at the highest possible level were compared, it was observed that FSH levels were higher in the trained subjects, suggestive of a compensatory hypogonadism as result of intensive chronic training or Sertoli cell dysfunction.32
Hypogonadal states have been observed as a result of chronic ultra-endurance-training when running or cycling 10–20 or more hours per week.33 Triathletes, normally undergoing high volume in the three sports modalities composing this discipline, showed, when compared to cyclists and recreationally active men, significantly lower levels of estradiol and T.12 However, not all previous studies have observed such differences.16
Rowing has also assessed the effect of training on HPT. Heavy training seems to result in decreased free Testosterone Cortisol ratio (T/C) when compared to basal levels; though decreases greater than 30% have been reported, but never drop below the threshold value indicating overstrain of 0.35×10−3.34 Urhausen et al.35 observed that T, T/sex hormone – binding globulin (SHBG), and T/C decreased during a 7-week period of training, stopping with recovery of only one week and with two rowers showing normalized values within several weeks of training discontinuation. A 2-week period of high-training volume significantly decreased T 30min after finishing a long-distance rowing test; such observed changes indicate decreased adaptive ability.36
Such decreased adaptivity was also observed in another study as evidenced by reduced basal fT and fT/C ratio as well as an altered T response to an exercise challenge after a heavy training period of three weeks.37 Similarly Vinther et al.38 found that values, albeit within normal range, were in the lower part of the normal spectrum. Therefore, as reported by some, T and cortisol (C) are altered as a result of changes in training volume, and the values of such hormones appear related to weekly training volume.39
Not only does the volume of training induce hormonal alterations, but in fact, the intensity of the exercise may also exert an influence on hormonal profile alterations. In this regard, Safarinejad and co-workers,10 compared moderate-intensity exercise (60% Maximal Oxygen Consumption (VO2max)) to high-intensity exercise (80% VO2max) for a period of 12 weeks and reported alterations in hormones (returning to normal during recovery) with a decrease in T and blunted response to a Gonadotropin Releasing Hormone (GnRH) stimulation challenge in both groups but alterations being more marked in the high intensity group.
Furthermore, our research group has shown that maximal intensity exercise imposed on physically active subjects during a short period of time results in alterations in FSH, LH, prolactin (PRL), dehydroepiandrosterone and C values but no significant changes in T, progesterone and estradiol; such alterations returned to pre-training values after three days during the recovery period.7
In soccer, Grandi and Celani40 have observed that the LH response to a GnRH-Thyrotropin Releasing Hormone (TRH) challenge test was less in the professional players as compared to non-professional players; while no significant hormone changes were observed during the training season, PRL basal levels were increased after a strenuous soccer game. In basketball, an initial transient increase in T was observed to later on decrease, along with a decrease in T/C, from mid-season on.41
The evidence discussed above points to the hormonal response to exercise being affected by both by intensity and volume of training. Although the complexity in hormone feedback mechanisms and the disparity in results in the available literature demands that a consensus in study design and protocols needs to be reached. The heterogeneity observed in different studies with regards to, but not limited, the type of athlete (sedentary vs. amateur vs. professional), the type of intervention (observational vs. interventional) should be minimized.
Positive effectThough studies analyzing long term response are scarce, imposed training loads seem to elicit an acute response with an initial increase in androgenic hormones, but when programs are sustained, often surpassing the habitual training levels of the athletes, there may be a decrease in basal levels of these hormones.42–45 The loaded used, time of application and type of athletes are key factors for the long-term effects of training. Frequently, exercising subjects present with higher T basal levels that sedentary subjects.46,47 Intervention with moderate intensity aerobic exercise resulted in increased serum dihydrotestosterone (DHT),48 in fT49 and SHBG48,49 in sedentary subjects. Also, Vaamonde and colleagues50 have recently reported improved values for FSH, LH, T and the T/C ratio when comparing sedentary subjects to physically active subjects.
Hormonal alterations – resistance trainingResistance exercise elicits acute post-exercise hormonal responses whereas prolonged, long-term training has an effect or basal or resting concentrations.51-53 Hormonal responses after a resistance training session, depend on the load, the used muscle mass and the impact that the session may impose on the organism. Though not to be discussed here we must be aware that use and abuse of anabolic androgenic steroids may be common feature in resistance training athletes and that these compounds may alter the HPT axis and inhibit endogenous T production, which may be reversible or not depending especially on dose and time of use.
Negative effectWhile an increase in the resting levels of T is common feature of chronic response to strength training,5 when resistance training becomes excessive, with the athlete coming close to overtraining or with persistent fatigue, T levels may even decrease from baseline.54
Yet not fully elucidated such response may involve amino acid deficit and central nervous system alterations,2 alterations in neurotransmitters or increase in T-inhibiting hormones, and aging processes.54
Positive effectFor male athletic performance, it may be beneficial to alter the levels of circulating anabolic hormones and the anabolic-catabolic ratio as testosterone, among others, is directly involved in muscle adaptation to exercise.
Generally speaking, albeit discrepancies exist, including theories that changes in hormones are not mechanistic, but a result of hemoconcentration or decreased hepatic clearance, adequately applied resistance training provokes an increase in tT and fT levels after one training session,51,55 with magnitude of changes depending on different factors such as load (intensity,56 volume,6 recovery,57 amount of muscle mass involved, athlete's age,58 experience and performance level.58 The magnitude of the load used will determine the time that T levels are elevated after training.6
Reproductive function – endurance trainingNegative effectThough controversy, as in hormonal studies, there is evidence that long-term exhaustive exercise seems to negatively affect sperm quality and reproductive potential.11
One study reports that even up to 10% of the assessed runners exhibit severe oligospermia, yet other authors report non-existing or merely subclinical alterations due to exercise training.2,19,23,59 Increase in “round cells” has also been reported2 indicating a possible infectious and/or inflammatory environment. Arce and colleagues2 were able to, retrospectively establish a volume threshold of 100km/wk for semen alterations to occur, as they found alterations in sperm density, motility, morphology and in vitro sperm penetration of standard cervical mucus in endurance-trained runners when compared to resistance athletes or sedentary subjects. Similarly, Safarinejad et al.10 observed a negative effect of training on sperm parameters, though reverting during recovery, in high-intensity training athletes when compared to moderate-intensity ones. Conversely, Hall and co-workers60 could not find an influence on sperm count, morphology, or motility after a period of six weeks of gradually intensified training followed by two weeks of detraining.
High-level athletes have been typically training for many years, making it difficult to establish a potential harmful training threshold (volume and/or intensity) as they normally start training at pre- or peri-pubertal years. Nevertheless, high volume cycle training seems to correlate with sperm morphology anomalies.61 In fact, in triathletes, a cycling volume of 300km/wk correlates with serious degree of morphology abnormalities and possible leading to fertility impairment. Also, ultra-endurance athletes when compared to other athletes show significantly different values for the various sperm parameters with morphology showing the greatest difference, with clinical relevance (<5% normal forms).11
In animal models decreased number of cells from the spermatogenic lineage at different stages of development has been observed with training,14,15 some of these studies also revealed decreased levels of serum hormones, enzymes and antioxidant agents.14,15 Vaamonde et al.62 have reported exercise-related alterations in sperm which may be prevented with antioxidant agents. Vaamonde et al.63 also recently reported that, similarly to sperm morphology, cycling volume positively correlates to sperm deoxycribonucleic acid (DNA) fragmentation, also observing high correlation between training volume, sperm DNA fragmentation, percentage of morphological abnormalities and TUNEL(+) cells (unpublished data Vaamonde).
Regarding reproductive tract abnormalities, it has been reported that a soccer player exhibited testicular mal-development;64 as the athlete had cryptorchidy it is hard to establish a relationship to sports practice, however, with greater training load all other symptoms worsened. In this regard, continuous strenuous exercise has also been linked to erectile dysfunction or impotence65 as tiredness and fatigue may lead to reduced libido, especially in bicycling exercise64,66 due to microtrauma and compression on the genital area. In rats, decreased testicular, epididymal, prostatic, and seminal vesicles somatic indices have been reported as a result of exercise.15
Positive effectFar less studied is the positive effect of exercise or physical activity on seminal parameters. Vaamonde et al.50 reported improved semen parameters in physically active men when compared to sedentary people, such findings being supported by hormonal differences. Other studies with endurance-trained men report that average semen parameters values are usually within normal limits16 or show subclinical alterations.2,3,19 In this regard, significant alterations, even if subclinical, have been reported to require a certain “training volume-threshold” (∼100km/wk) before occurring.3 Also in animals similar results have been reported, not only on sperm parameters but also on testicular health.67,68
Training load affects oxidative stress; as such, superoxide dismutase (SOD) capacity has been reported to be increased in elite athletes69 which can affect the testicular environment. Physically active men, in comparison to higher level athletes, have been shown to have higher levels of seminal antioxidant compounds and lower levels of seminal reactive oxygen species (ROS), oxidative stress and sperm DNA fragmentation.70 Erectile dysfunction has also been reported to be less common in physically active subjects (non-athletes) than in sedentary; moreover, this condition has been seen to improve as a result of unhealthy lifestyle modification like becoming physically active (minimum energy expenditure of 200kcal/day).71
Also in animal models it has been observed that exercise reduces markers of oxidative stress and increases antioxidant-related enzymes,68 decreasing age-related damage, especially if exercise practice begins earlier in life,72 and also decreasing DNA damage.73 Moreover, testicular atrophy, inflammation and degeneration, related to aging, is decreased in animals submitted to exercise.68
Reproductive function – resistance trainingNegative effectResistance training is many times intimately relate to androgenic anabolic steroid (AAS) use and regrettably little is known, if any on sperm quality as a result of resistance training without steroid use. It has been observed that the HPT axis and its hormones are altered by supraphysiologic levels of exogenous AAS and that these changes may lead to semen alterations like azoospermia, oligospermia, teratozoospermia (mainly head and mid-piece), and oftentimes testicular atrophy74,75 as evidenced by alterations in germinal epithelium, Leydig cells, among others.76
Positive effectNo clear evidence exists of a positive effect of resistance training on semen parameters. Arce et al.2 has observed, when comparing endurance and resistance athletes, that the latter did not manifest negative alterations as seen in endurance athlete's semen characteristics so this could be indicative that, at least, this type of training is not substantially detrimental to semen quality.
Mixed modalitiesMany team sports, such as soccer, basketball and rugby, among others, because of their inherent characteristics must be considered as mixed modalities as their training is not purely endurance or resistance. Athletes participating in such sports may also be at risk for altered hormone and semen values. Exercise may be especially deleterious in case of existing pathologies like varicocele77 and the pathology may be especially aggravated in times of intensified training or competition.64 Sperm motility has also been observed to be negatively altered in some soccer players.40
Scientific evidence seems to support the existence of a minimum level of volume, the so-called volume-threshold, for detrimental effects to take place, either hormonal or seminological.2,3,8 As Hackney et al.8,33 highlight, alterations may well represent the accumulative effect, more than the acute response, of years of training load. Competition or post-competition periods, as well as periods of intensified training seem to be particularly deleterious. Some of the latest research has shown that, training intensity, and not only volume, is greatly important in this equation as well, as Vaamonde et al.78 point out with even correlation to sperm DNA damage and alteration is oxidative stress-related parameters. Nevertheless, high-load exercise seems to exert negative impact on male fertility potential, modulated by duration, volume, intensity and type of modalities, though most of the observed alterations are subclinical and revert upon ceasing or decreasing practice.
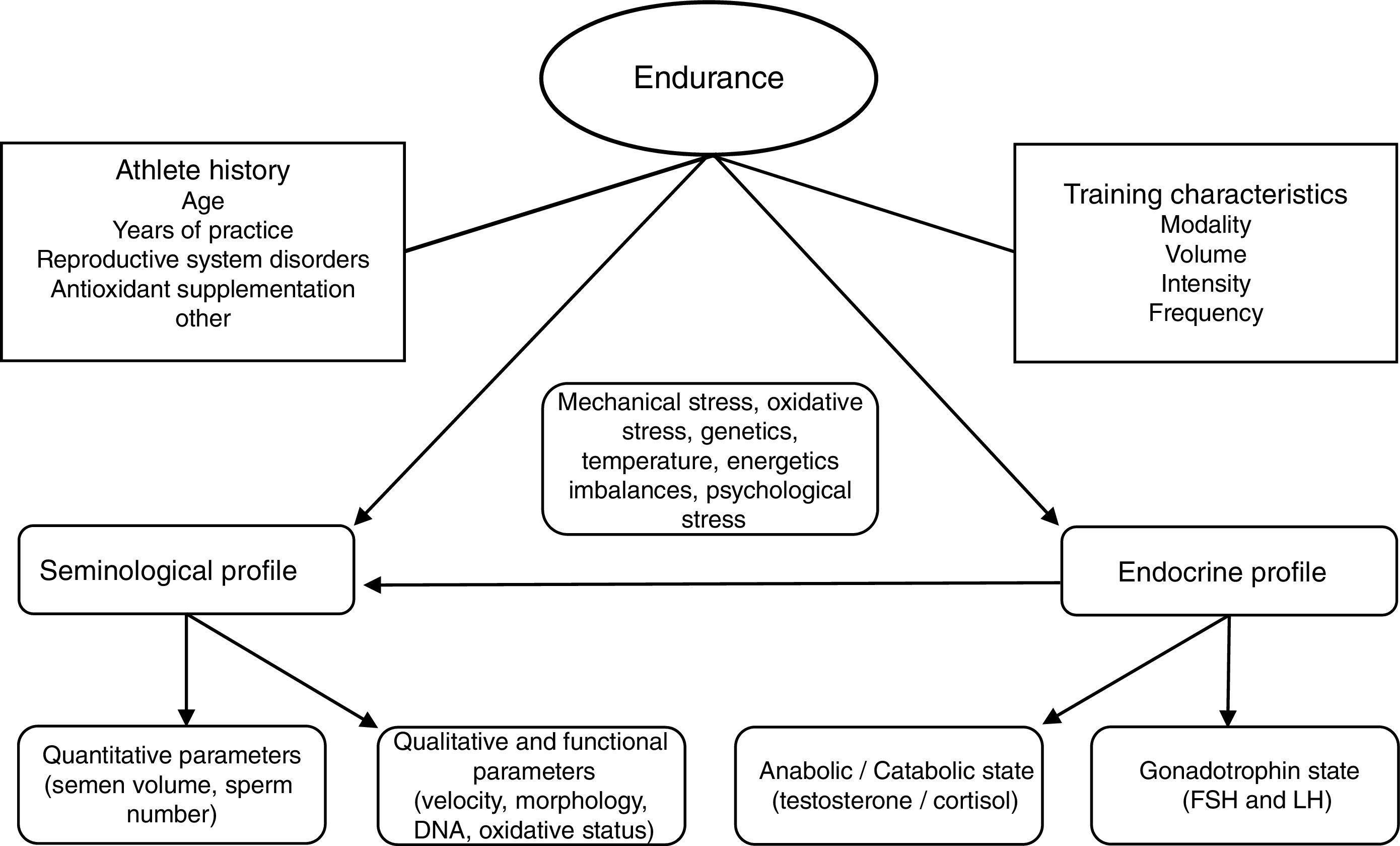
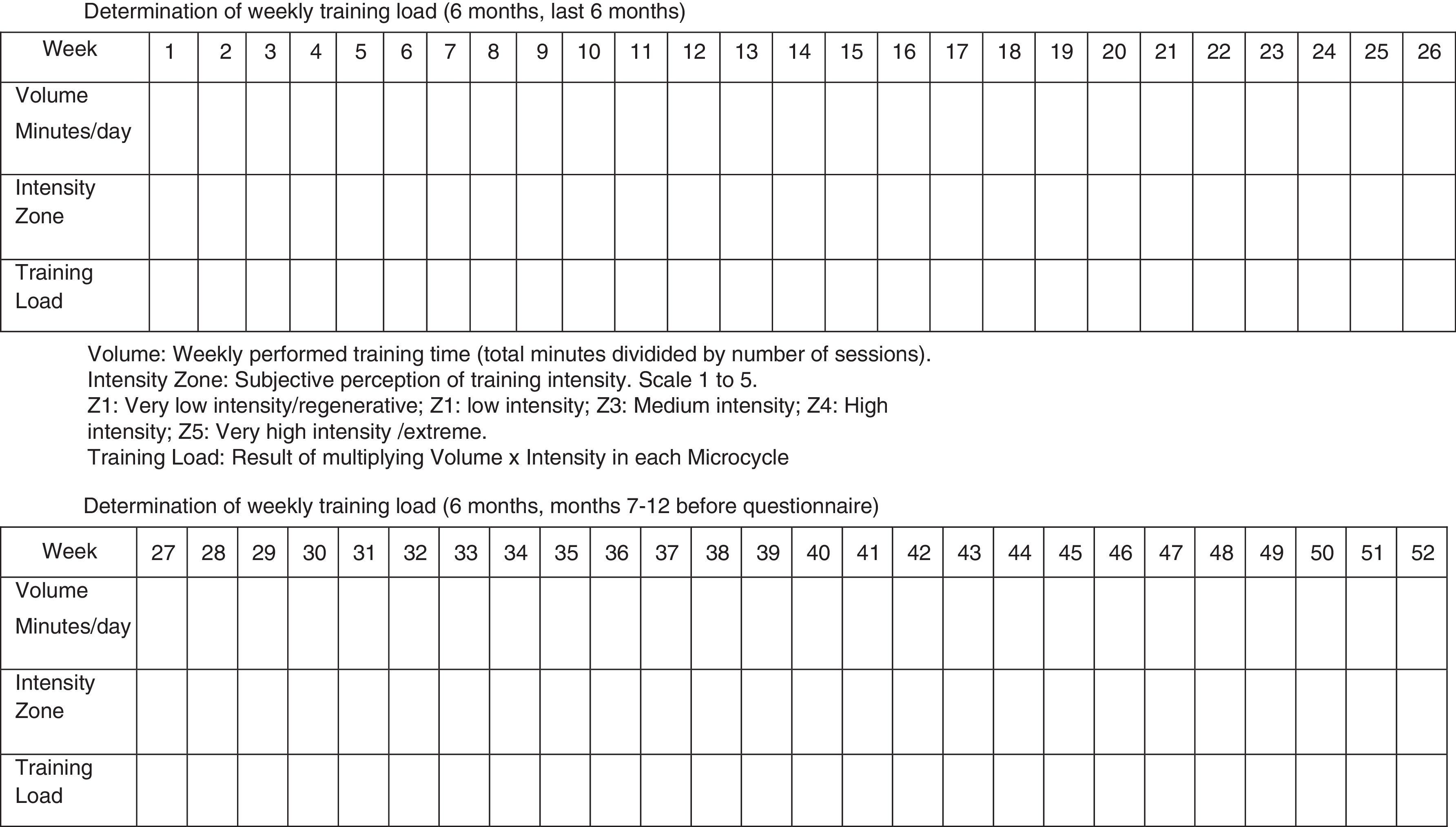
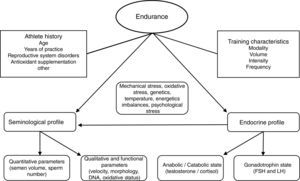
We believe many other inherent parameters of exercise training (frequency, recovery, clothing, environmental temperature, degree of muscular-skeletal damage, infections and/or inflammations) may play in to affecting reproductive function and looking at just intensity or volume is too delimiting to research designs. The final influence-effect, as in other physiological systems, will depend on the subject's own characteristics and how well his adaptive systems are prepared for the challenge training imposes (see Fig. 1). It is important that well designed studies with standardized assessment tools regarding testing, exercise conditions and subject characteristics (fertility especially) be performed; this especially holds true for mixed modalities sports, probably the most widely practiced and the least studied in this context at this time.
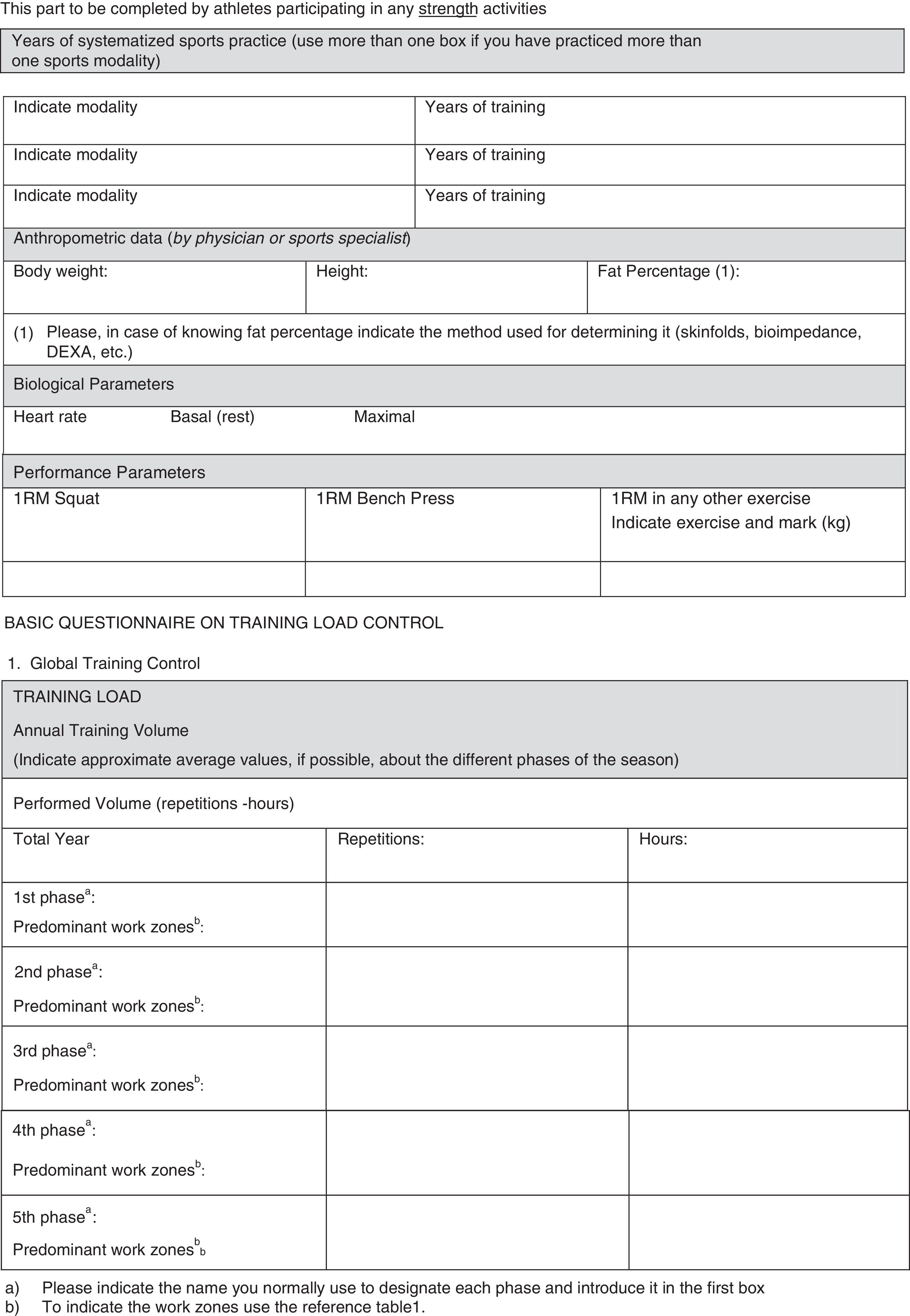
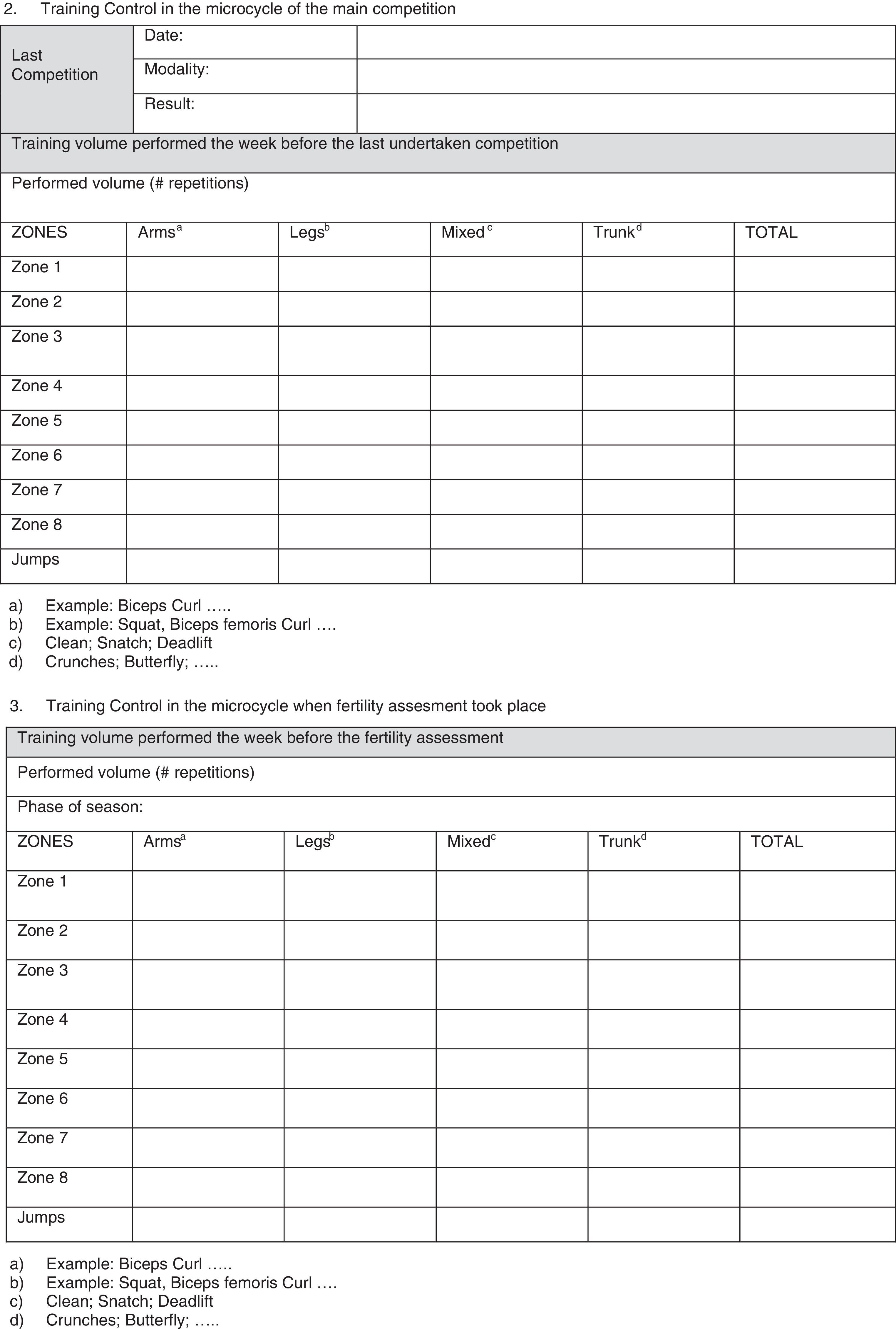
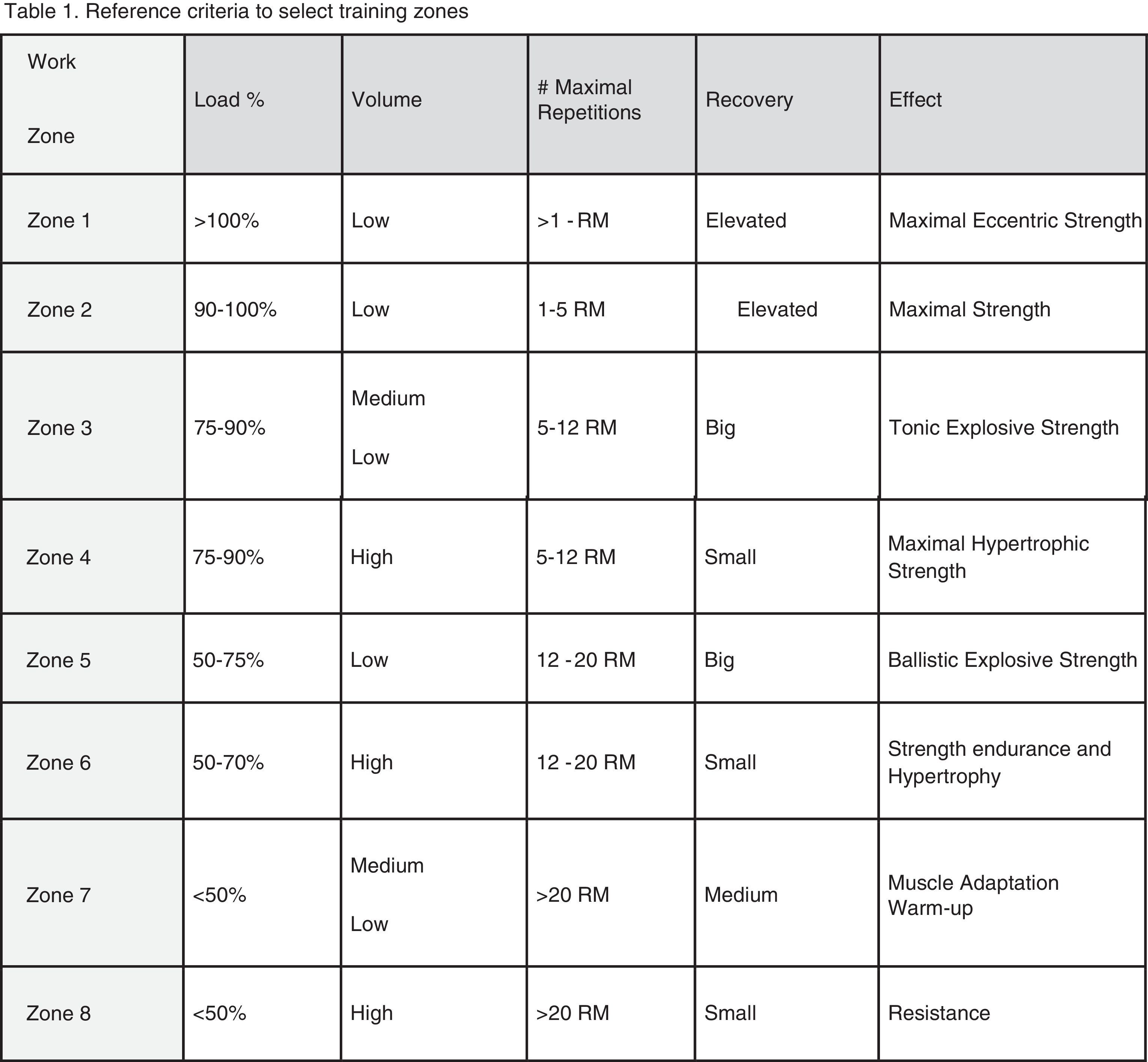
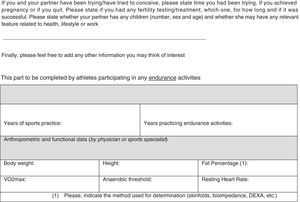
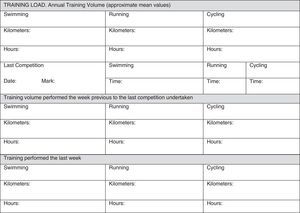
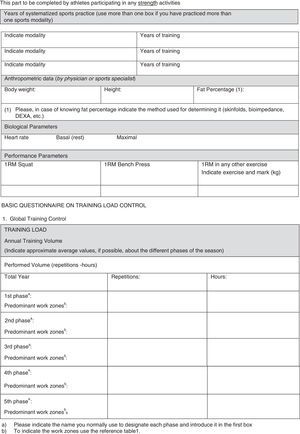
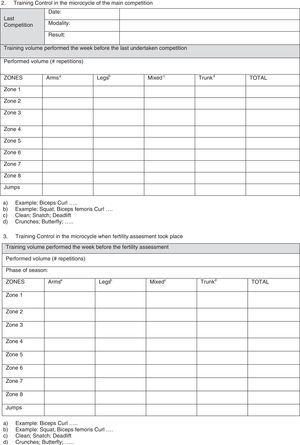
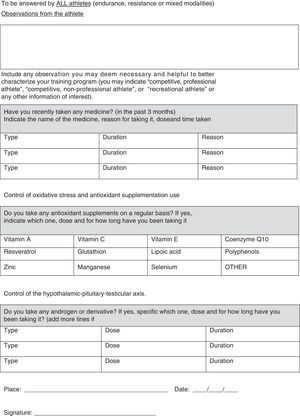
In our opinion, one critical aspect necessary in this research is a more encompassing questionnaire that addresses key issues regarding exercise practice and fertility status. To this end, we have developed and included with this review a new developed instrument based upon our collective experience, to use by researchers wanting to study exercise and fertility more extensively (see Appendix 1). It is important to note that this questionnaire, if used in research, be used with the subjects’ informed consent and Ethics Committee approval.
In conclusion, awareness must be raised that exercise must be considered as a potential cause for urological and andrological male fertility problems. As such, exercise training should be assessments in males. Also, more research is needed to reflect the positive effect of moderate and low-level exercise on reproductive potential in order to make safe recommendation in lifestyle practices to help couples in achieving pregnancy.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
All professionals using this questionnaire must be aware that, along with the questionnaire, participants from any study need to sign an informed consent about the study/assessment and ethical guidelines must be followed. Moreover, no data or biological samples from the participants must be used without the participants’ knowledge and approval.