The aim of this population-based study was to assess independent prognostic factors in ovarian cancer by analyzing observed and relative survival in a representative Spanish population.
MethodsWe carried out a retrospective, observational, population-registry-based study. Data on 207 patients with ovarian cancer were provided by the Castellon Cancer Registry. Observed and relative survival were described at 1, 3 and 5 years. The effect of prognostic factors on survival was assessed with univariate and multivariate analyses.
ResultsThe median follow-up was 40.8 months (range: 12–108 months). Observed and relative survival rates at 1, 3 and 5 years were 79%, 51%, 33%, and 84%, 58%, 40%, respectively. Age older than 70 years showed worse observed survival in the univariate and multivariate analyses. Only FIGO stage was an independent prognostic factor for observed and relative survival.
ConclusionsSurvival is poor in patients with ovarian cancer. In our population-registry-based study, only age at diagnosis and FIGO stage were independent prognostic factors for observed survival, whereas only FIGO stage could be considered a prognostic factor for relative survival.
El objetivo de este estudio poblacional fue evaluar los factores pronósticos independientes de cáncer de ovario mediante el análisis de la supervivencia observada y relativa en una población española representativa.
MétodosSe realizó un estudio retrospectivo, observacional, y basado en un registro de población. Los datos de 207 pacientes con cáncer de ovario proceden del Registro de Cáncer de Castellón. Se describió la supervivencia observada y relativa a 1, 3 y 5 años. El efecto de los factores pronósticos en la supervivencia se evaluó mediante análisis univariantes y multivariantes.
ResultadosLa mediana de seguimiento fue de 40,8 meses (intervalo: 12-108 meses). Las tasas de supervivencia observada y relativa a 1, 3 y 5 años fueron de 79%, 51% y 33% y de 84%, 58% y 40%, respectivamente. La edad superior a los 70 años mostró la peor supervivencia observada en los análisis univariantes y multivariantes. Sólo el estadio en la clasificación FIGO fue un factor pronóstico independiente de la supervivencia observada y relativa.
ConclusionesLa supervivencia en los pacientes con cáncer de ovario es limitada. En nuestro estudio basado en un registro de población, sólo la edad en el momento del diagnóstico y el estadio de FIGO fueron factores pronósticos independientes para la supervivencia observada, mientras que sólo el estadio de la FIGO se podría considerar un factor pronóstico en la supervivencia relativa.
Ovarian cancer accounts for 5% of all cancers among women and causes more deaths than any other female genital tract cancer. In Spain (Europe) approximately 8/100,000 habitants will develop ovarian cancer. Ovarian cancer is often diagnosed at an advanced stage, and about 70–80% of them will be found at an advanced stage. For this group of patients 5-year-survival is lower than 20%.1
Several prognostic factors for ovarian cancer have been studied and reported in an attempt to increase tumor-free survival.2,3 The most important and constant prognostic factors reported have been International Federation of Gynecology and Obstetrics (FIGO) stage, size of residual tumor after primary surgery and age at diagnosis. Other variables, such as time period, histological type and grade, performance status, preoperative CA 125 levels or other molecular markers seem not to be so predictive.4–6
The main objective of this population registry cancer-based study was to evaluate prognostic factors for ovarian cancer survival in the European Province of Castellon (Spain) during the period 2004–2008.
MethodsThe Province of Castellon is located in the Comunitat Valenciana at the Mediterranean coast in the East of Spain (Europe). The population of Castellon officially reached 604,333 people in 2011, as recorded by the National Statistics Institute of Spain. Although most patients with ovarian cancer are treated in the Castellon University General Hospital – where this study was conducted – there are also 3 public hospitals and 1 private hospital in this province.
According to the Castellon Cancer Registry, follow-up period was since 1 January 2004 to 31 December 2012. The Castellon Cancer Registry Database was used to identify eligible ovarian cancer patients. This population cancer registry is included in the EUROCARE study. Out of a total of 236 patients with ovarian cancer in the database diagnosed between 1 January 2004 and 31 December 2008, there were 207 finally analyzed due to excessive missing data in 29 cases. Patients without histological confirmation and those with borderline cancers or cancers with a low malignant potential were excluded. The incidence rate was adjusted to standard world's population and it is reported as the new diagnosed cases per 100,000 person-year.7
The following available variables were studied: age at diagnosis, FIGO stage,1 histology and grade of differentiation (according to the WHO classification).8 For this study, histological types were grouped in several groups according to a condensed IARC-classification.9 The data analysis was approved by our Ethical and Clinical Investigation Committee.
Statistical analysisQuantitative variables were defined as median and range. Qualitative variables were described as frequency and percentages. To calculate Expected survival for a similar population without ovarian cancer Ederer's method was used adjusting women's mortality tables by age and year in the Comunitat Valenciana for the period 2004–2008, obtained from the National Statistics Institute of Spain. Observed survival was defined as time between diagnosis and date of death of a patient for any cause and was calculated using Kaplan–Meier actuarial method. The Relative survival is the ratio between Observed survival and Expected survival. A Cox proportional hazard model10 was used for multivariate analysis of Observed survival. Univariate analysis of Observed survival was performed using also this method but including only one variable. According to Dickman,11 a generalized linear model with a Poisson error structure was used for multivariate analysis of Relative survival. This model has been previously used by others.12 Univariate analysis of Relative survival was performed using also this method but including only one variable.
All tests were two-tailed and the level of p<0.05 was considered statistically significant. STATA for Windows, version 12 (StataCorp, College Station, TX) was used to perform the statistical analyses.
ResultsTwo hundred and seven patients were included in the study (Codes C56, C57.0-4 from International Classification of Disease, ICE-10). The median age at diagnosis was 65 years (range: 22–98). Diagnosis was established by means of histological analysis of primary tumor in 161 cases (77.8%), histological analysis of metastases in 7 cases (3.4%), diagnostic imaging in 20 cases (9.6%), cytology in 11 cases (5.3%) and death certificate in 8 patients (3.9%). A total of 186 cases (89.9%) presented as a primary tumor without metastasis, in 8 cases (3.9%) it was a secondary tumor. Median of the total number of tumors was 1 (range 1–3).
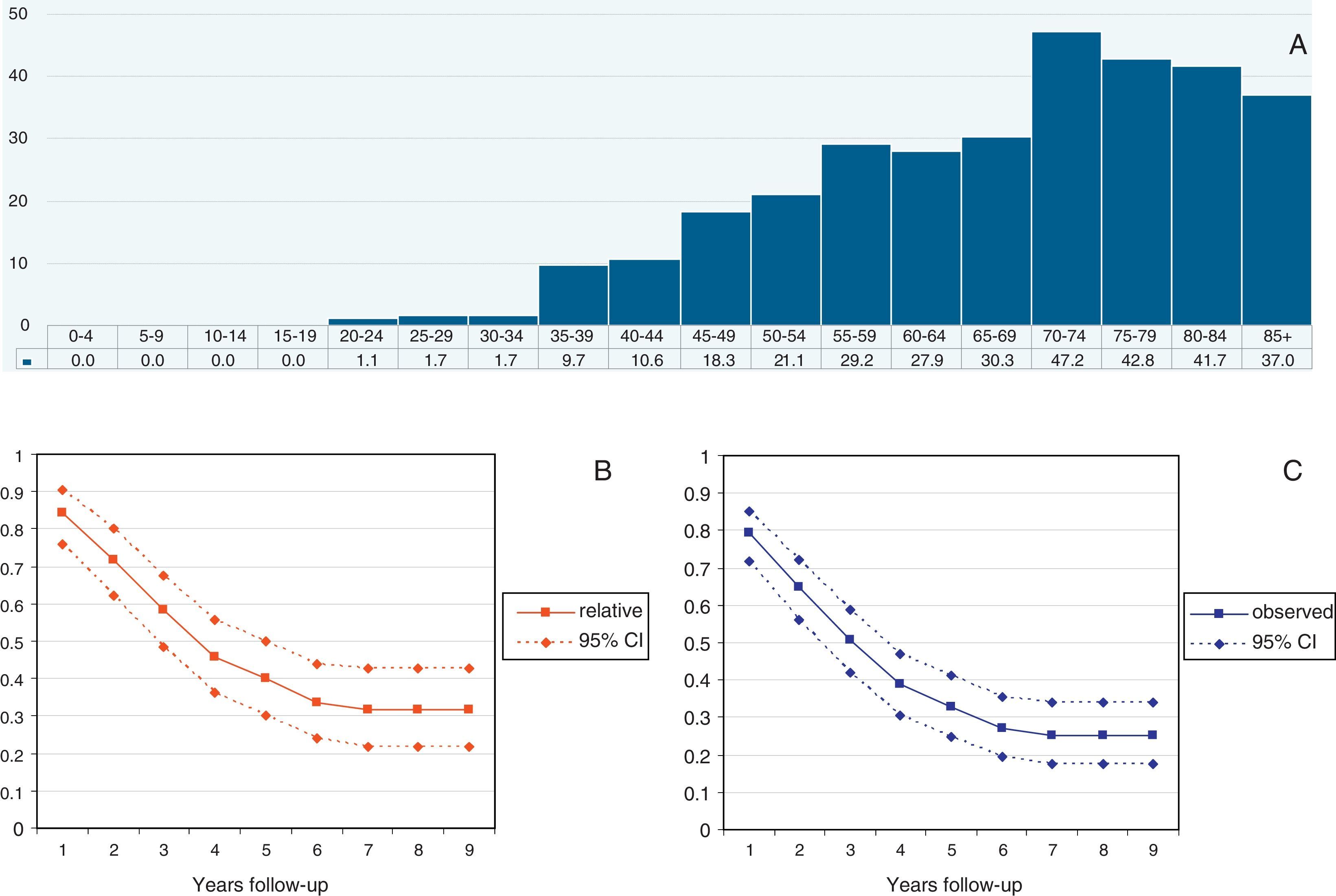
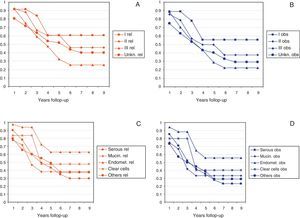
Median follow-up time was 40.8 months (range 12–108 months). A total of 151 patients died during the follow-up (73%). The median overall Observed survival was 49% (range: 21–96%), whereas Relative survival was 56% (range: 26–96%). The incidence of cases by year is detailed in Table 1. Cumulative probability of Observed and Relative survival at 1, 3 and 5 years of follow-up is shown in Table 2. Standardized incidence rate by age and year is shown in Fig. 1A. Observed and Relative survival curves (including 95% confidence interval) are shown in Fig. 1B and C, respectively.
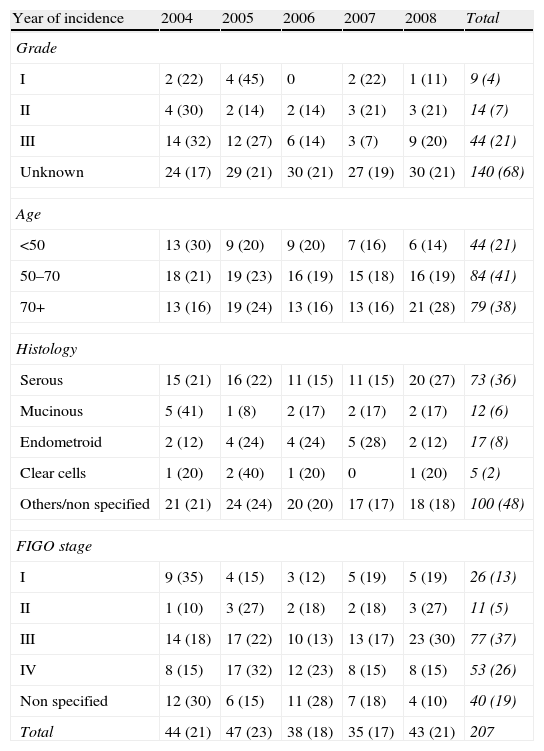
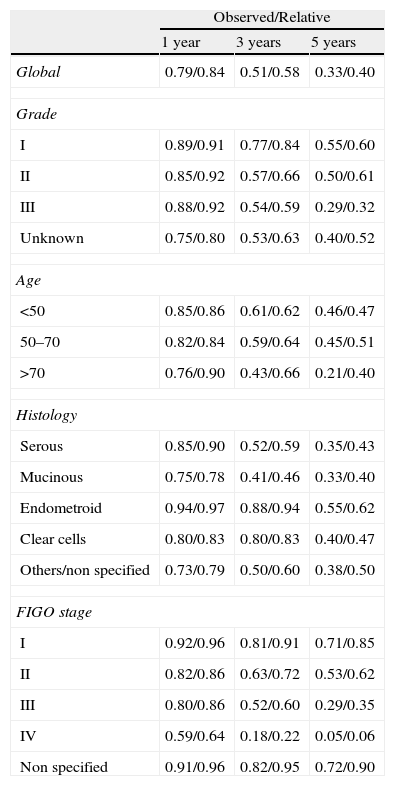
Tumor characteristics according to year of incidence.
| Year of incidence | 2004 | 2005 | 2006 | 2007 | 2008 | Total |
| Grade | ||||||
| I | 2 (22) | 4 (45) | 0 | 2 (22) | 1 (11) | 9 (4) |
| II | 4 (30) | 2 (14) | 2 (14) | 3 (21) | 3 (21) | 14 (7) |
| III | 14 (32) | 12 (27) | 6 (14) | 3 (7) | 9 (20) | 44 (21) |
| Unknown | 24 (17) | 29 (21) | 30 (21) | 27 (19) | 30 (21) | 140 (68) |
| Age | ||||||
| <50 | 13 (30) | 9 (20) | 9 (20) | 7 (16) | 6 (14) | 44 (21) |
| 50–70 | 18 (21) | 19 (23) | 16 (19) | 15 (18) | 16 (19) | 84 (41) |
| 70+ | 13 (16) | 19 (24) | 13 (16) | 13 (16) | 21 (28) | 79 (38) |
| Histology | ||||||
| Serous | 15 (21) | 16 (22) | 11 (15) | 11 (15) | 20 (27) | 73 (36) |
| Mucinous | 5 (41) | 1 (8) | 2 (17) | 2 (17) | 2 (17) | 12 (6) |
| Endometroid | 2 (12) | 4 (24) | 4 (24) | 5 (28) | 2 (12) | 17 (8) |
| Clear cells | 1 (20) | 2 (40) | 1 (20) | 0 | 1 (20) | 5 (2) |
| Others/non specified | 21 (21) | 24 (24) | 20 (20) | 17 (17) | 18 (18) | 100 (48) |
| FIGO stage | ||||||
| I | 9 (35) | 4 (15) | 3 (12) | 5 (19) | 5 (19) | 26 (13) |
| II | 1 (10) | 3 (27) | 2 (18) | 2 (18) | 3 (27) | 11 (5) |
| III | 14 (18) | 17 (22) | 10 (13) | 13 (17) | 23 (30) | 77 (37) |
| IV | 8 (15) | 17 (32) | 12 (23) | 8 (15) | 8 (15) | 53 (26) |
| Non specified | 12 (30) | 6 (15) | 11 (28) | 7 (18) | 4 (10) | 40 (19) |
| Total | 44 (21) | 47 (23) | 38 (18) | 35 (17) | 43 (21) | 207 |
Frequencies (rounded %).
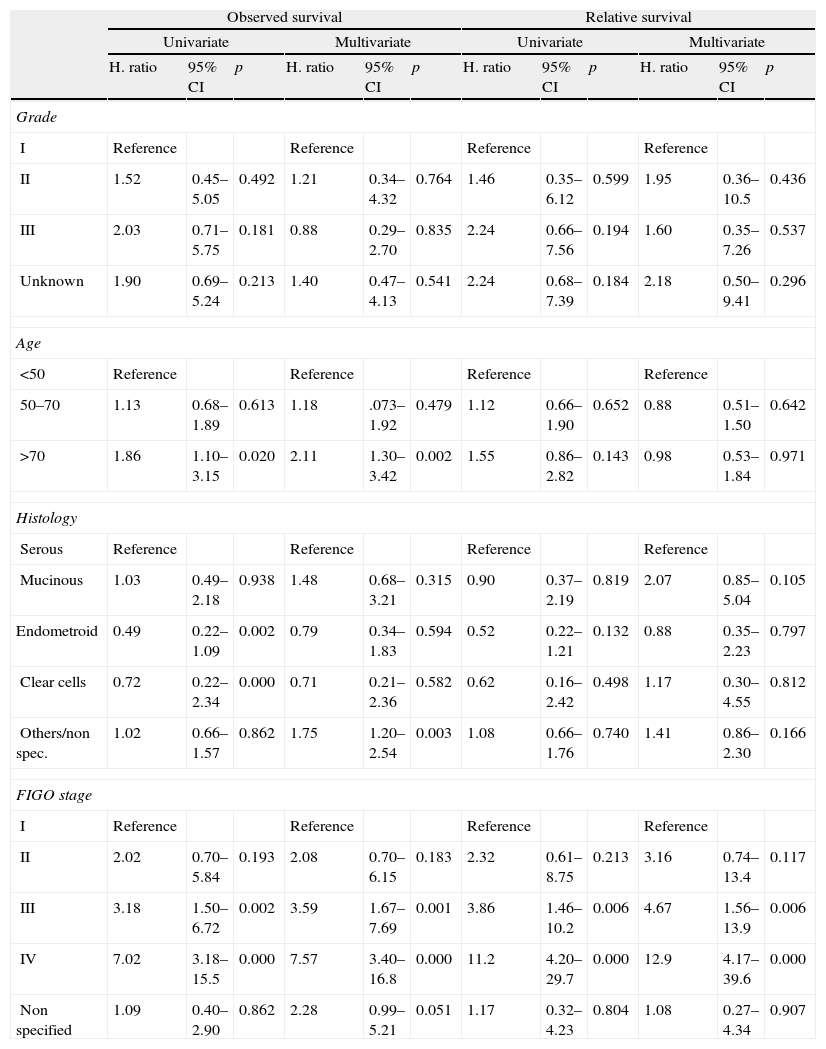
Cumulated probability of survival: Observed and Relative survival by years of follow-up.
| Observed/Relative | |||
| 1 year | 3 years | 5 years | |
| Global | 0.79/0.84 | 0.51/0.58 | 0.33/0.40 |
| Grade | |||
| I | 0.89/0.91 | 0.77/0.84 | 0.55/0.60 |
| II | 0.85/0.92 | 0.57/0.66 | 0.50/0.61 |
| III | 0.88/0.92 | 0.54/0.59 | 0.29/0.32 |
| Unknown | 0.75/0.80 | 0.53/0.63 | 0.40/0.52 |
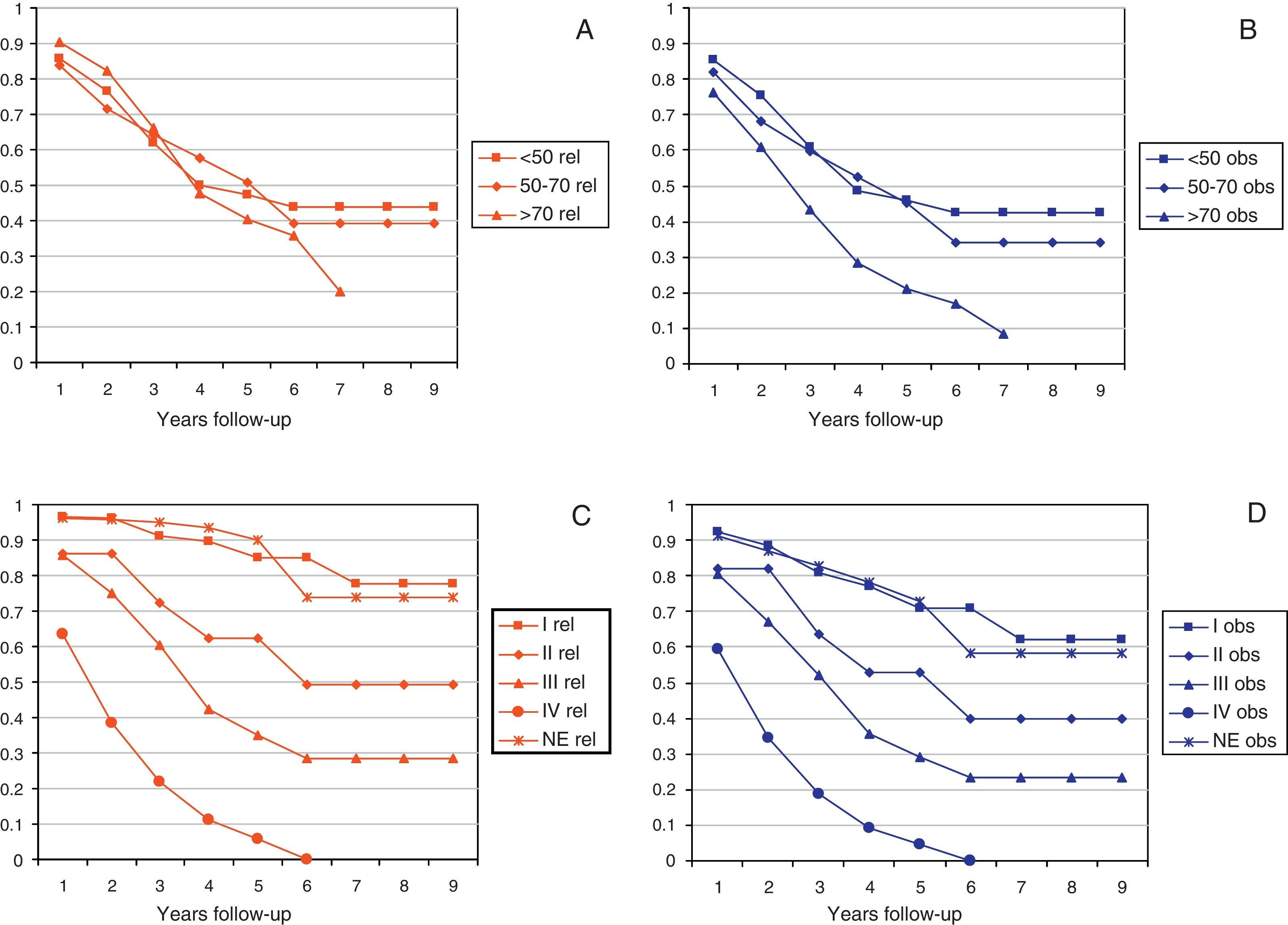
| Age | |||
| <50 | 0.85/0.86 | 0.61/0.62 | 0.46/0.47 |
| 50–70 | 0.82/0.84 | 0.59/0.64 | 0.45/0.51 |
| >70 | 0.76/0.90 | 0.43/0.66 | 0.21/0.40 |
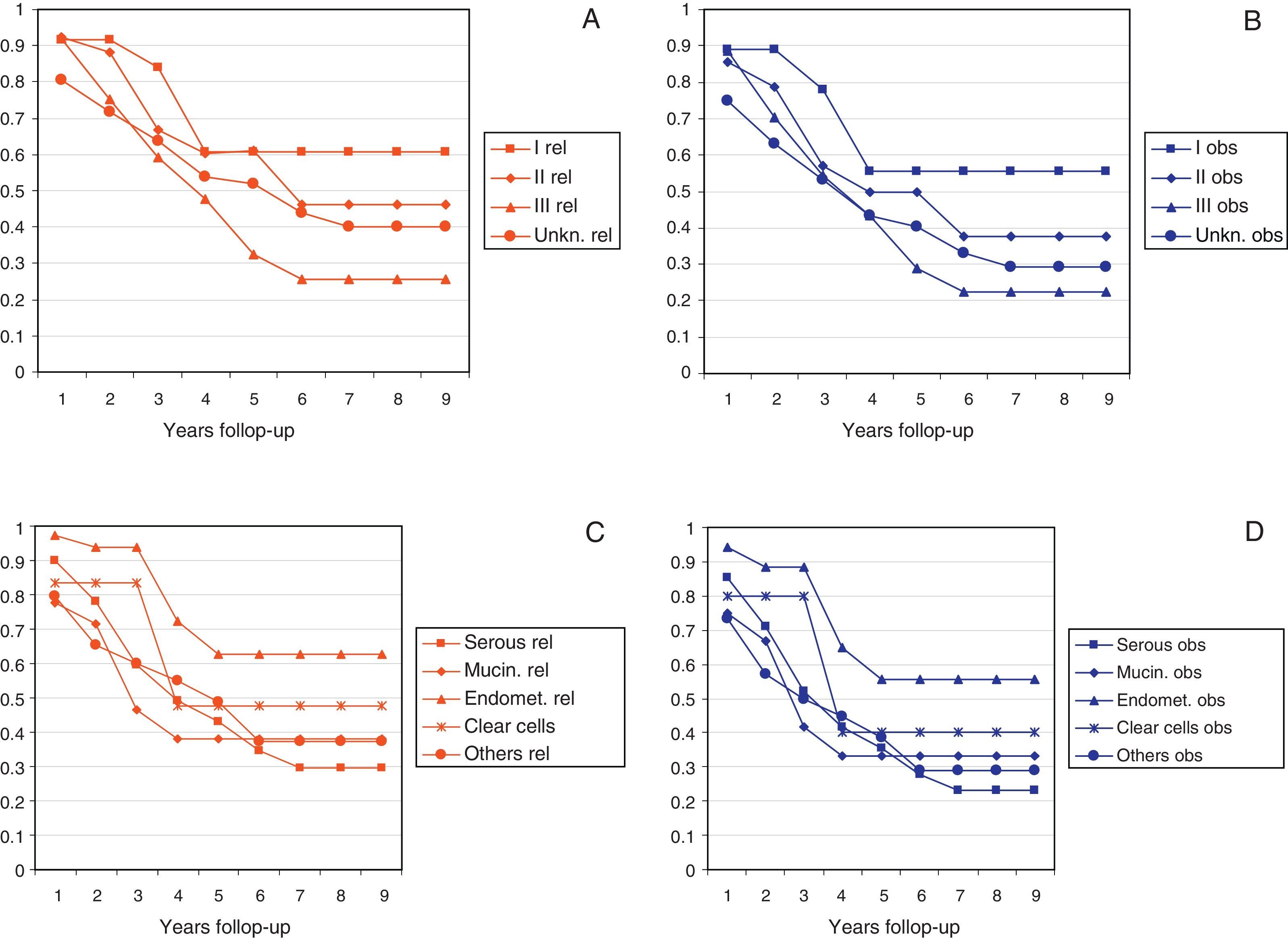
| Histology | |||
| Serous | 0.85/0.90 | 0.52/0.59 | 0.35/0.43 |
| Mucinous | 0.75/0.78 | 0.41/0.46 | 0.33/0.40 |
| Endometroid | 0.94/0.97 | 0.88/0.94 | 0.55/0.62 |
| Clear cells | 0.80/0.83 | 0.80/0.83 | 0.40/0.47 |
| Others/non specified | 0.73/0.79 | 0.50/0.60 | 0.38/0.50 |
| FIGO stage | |||
| I | 0.92/0.96 | 0.81/0.91 | 0.71/0.85 |
| II | 0.82/0.86 | 0.63/0.72 | 0.53/0.62 |
| III | 0.80/0.86 | 0.52/0.60 | 0.29/0.35 |
| IV | 0.59/0.64 | 0.18/0.22 | 0.05/0.06 |
| Non specified | 0.91/0.96 | 0.82/0.95 | 0.72/0.90 |
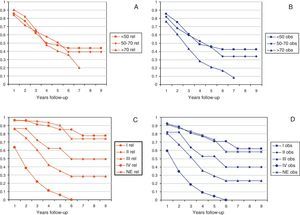
Figs. 2 and 3 show Observed and Relative survival curves according to available variables. As seen in these figures, the groups with a worse prognosis were age over 70 years, histological grade III, serous tumors and stage IV. On univariate analysis for Observed survival (Table 3), age over 70 years showed statistically significant differences compared with patients under 50 years. Statistically significant differences were also seen for patients in stage III and IV comparing with stage I. On multivariate analysis for Observed survival, age at diagnosis and stage showed both statistically significant differences. On multivariate analysis for Relative survival, the excess of mortality was approximately five times higher among stage III and twelve times higher among patients with stage IV comparing with stage I.
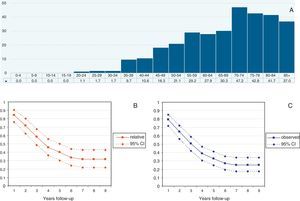
Observed and Relative survival: univariate and multivariate analyses.
| Observed survival | Relative survival | |||||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| H. ratio | 95% CI | p | H. ratio | 95% CI | p | H. ratio | 95% CI | p | H. ratio | 95% CI | p | |
| Grade | ||||||||||||
| I | Reference | Reference | Reference | Reference | ||||||||
| II | 1.52 | 0.45–5.05 | 0.492 | 1.21 | 0.34–4.32 | 0.764 | 1.46 | 0.35–6.12 | 0.599 | 1.95 | 0.36–10.5 | 0.436 |
| III | 2.03 | 0.71–5.75 | 0.181 | 0.88 | 0.29–2.70 | 0.835 | 2.24 | 0.66–7.56 | 0.194 | 1.60 | 0.35–7.26 | 0.537 |
| Unknown | 1.90 | 0.69–5.24 | 0.213 | 1.40 | 0.47–4.13 | 0.541 | 2.24 | 0.68–7.39 | 0.184 | 2.18 | 0.50–9.41 | 0.296 |
| Age | ||||||||||||
| <50 | Reference | Reference | Reference | Reference | ||||||||
| 50–70 | 1.13 | 0.68–1.89 | 0.613 | 1.18 | .073–1.92 | 0.479 | 1.12 | 0.66–1.90 | 0.652 | 0.88 | 0.51–1.50 | 0.642 |
| >70 | 1.86 | 1.10–3.15 | 0.020 | 2.11 | 1.30–3.42 | 0.002 | 1.55 | 0.86–2.82 | 0.143 | 0.98 | 0.53–1.84 | 0.971 |
| Histology | ||||||||||||
| Serous | Reference | Reference | Reference | Reference | ||||||||
| Mucinous | 1.03 | 0.49–2.18 | 0.938 | 1.48 | 0.68–3.21 | 0.315 | 0.90 | 0.37–2.19 | 0.819 | 2.07 | 0.85–5.04 | 0.105 |
| Endometroid | 0.49 | 0.22–1.09 | 0.002 | 0.79 | 0.34–1.83 | 0.594 | 0.52 | 0.22–1.21 | 0.132 | 0.88 | 0.35–2.23 | 0.797 |
| Clear cells | 0.72 | 0.22–2.34 | 0.000 | 0.71 | 0.21–2.36 | 0.582 | 0.62 | 0.16–2.42 | 0.498 | 1.17 | 0.30–4.55 | 0.812 |
| Others/non spec. | 1.02 | 0.66–1.57 | 0.862 | 1.75 | 1.20–2.54 | 0.003 | 1.08 | 0.66–1.76 | 0.740 | 1.41 | 0.86–2.30 | 0.166 |
| FIGO stage | ||||||||||||
| I | Reference | Reference | Reference | Reference | ||||||||
| II | 2.02 | 0.70–5.84 | 0.193 | 2.08 | 0.70–6.15 | 0.183 | 2.32 | 0.61–8.75 | 0.213 | 3.16 | 0.74–13.4 | 0.117 |
| III | 3.18 | 1.50–6.72 | 0.002 | 3.59 | 1.67–7.69 | 0.001 | 3.86 | 1.46–10.2 | 0.006 | 4.67 | 1.56–13.9 | 0.006 |
| IV | 7.02 | 3.18–15.5 | 0.000 | 7.57 | 3.40–16.8 | 0.000 | 11.2 | 4.20–29.7 | 0.000 | 12.9 | 4.17–39.6 | 0.000 |
| Non specified | 1.09 | 0.40–2.90 | 0.862 | 2.28 | 0.99–5.21 | 0.051 | 1.17 | 0.32–4.23 | 0.804 | 1.08 | 0.27–4.34 | 0.907 |
This study has included all the cases of invasive ovarian cancer diagnosed in the Spanish Province of Castellon during 2004–2008. The incidence rate of ovarian cancer in our province can be considered as intermedium-low when it is compared with other population cancer registries, in our country or internationally.13,14
Survival analysis showed that both Observed and Relative survival progressively decreases at 3 and 5 years of follow-up. Observed survival was 51% and 33%, whereas relative survival was 58% and 40%, respectively. These data indicate similar survival than the rest of Spain (relative 5-year survival 39%), similar to data reported in EUROCARE from other countries in Europe (relative 5-year survival 41%) and a little below the United States of America.15,16
Age at diagnosis has been repeatedly reported as an independent risk factor for ovarian cancer.3,17–22 In fact, patients over 70 years in our study showed almost two times greater chance of death than patients under 50 years. It is probable, that comorbidities (heart problems, hypertension, diabetes, etc.) in this group of older patients affect the prognosis for two main reasons. On the one hand, it is possible that an aggressive treatment cannot be withstood by weak patients, affecting directly their survival. On the other hand, it is possible that treatments applied in these patients were given in a less aggressive or uncompleted way, what could also affect their potential benefit. Nevertheless, in our study, Relative survival, having into account the Spanish characteristics, demonstrated that age at diagnosis is not an independent risk factor. In consequence, it could be assumed that tumor aggressiveness is similar in any age of diagnosis.
When the grade of differentiation of the tumor was analyzed, not statistically significant differences were found. These results are in agreement with those of other authors and could be explained by a known inter-observer variability when establishing the degree of differentiation of a tumour.23,24
Serous tumors have been reported to have a worse prognosis that other epithelial tumours.13,25 However, our study did not reach statically significance in analysis for histological type. Only for Observed survival in univariate analysis some differences were found. It is possible, that the relatively low number of cases could explain these paradoxical results.
As it is well known, ovarian cancer is diagnosed frequently at an advanced stage. In fact, near 60% of the patients in our population were diagnosed in FIGO stage III and IV, whereas only a minority of these tumors (18%) was diagnosed in an early stage. For early ovarian cancer, Observed survival was similar to those reported for other authors. Nevertheless, for advanced stages, the survival of our population was worse than in other western countries. For FIGO stage IV, 5-year survival was 0.05%, that is, close to zero. As a consequence, FIGO stage was confirmed in our study as the main important independent risk factor for survival, both in univariate and multivariate analyses. This results are also in concordance with those reported by other authors.26,27 Early diagnosis is one of the main objectives of population cancer care. Moreover, it is possible that a significant increase on population survival reflects an earlier diagnosis. There is not an effective population screening program in ovarian cancer, but it is known that many patients may benefit from ultrasound diagnosis and tumor markers levels, such as CA 125, in order to anticipate diagnosis. Consequently, this early diagnosis could improve survival for a very important group of patients.28 Although our population-based study reflects that only 18% of patients are diagnosed in an early stage, we believe that this percentage will be increased in the following years for two main reasons. First, access to the health system is becoming increasingly frequent, and second new technologies such as image tests and laparoscopic approaches to abdominal cavity may diagnose cancers in earlier stages.
Another important prognostic factor in ovarian cancer is residual tumor after cytoreductive surgery. In fact, among patients undergoing operative intervention for ovarian cancer, the proportion of patients undergoing complete cytoreductive surgery is independently associated with overall survival time.29 Unfortunately, our study was not able to determine this relationship, since this variable was not available from our population registry.
It is important to emphasize that only population-based studies can demonstrate independent prognostic factors that can affect the entire population. Therefore, this is the only reliable way to analyze which is the impact of these risk factors on survival. One of the main strengths of our study is that this population-registry cancer study includes data from several institutions in our province, and not from a single institution. As a consequence, it reflects our reality better than studies conducted in very specialized institutions. Moreover, in contrast to clinical studies, in which patients are selected, population-based studies are based upon a heterogeneous group of patients and can be used to determine prognostic factors with low risk of selection bias. As a consequence, it is highly likely that our findings reflect what really happened in Spain during the period study 2004–2008.
ConclusionPatients with ovarian cancer present a poor survival. In our population registry-based study, only age at diagnosis and FIGO stage were independent prognostic factors for observed survival, whereas only FIGO stage can be considered a prognostic factor for relative survival.
Conflict of interest statementThe authors declare that there are no conflicts of interest.
Ethical responsibilitiesProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.