The aim of this study was to evaluate the use of phase-contrast MR imaging to diagnose normal pressure hydrocephalus (NPH) and differentiate it from other neurological disorders with similar clinical symptoms.
MethodsThe study included 108 subjects, of whom 61 were healthy controls and 47, patients; in the patient group, 19 had cerebrovascular disease (CVD) and 28 had NPH. All patients underwent a phase-contrast MRI study and several CSF flow and velocity parameters were measured at the aqueduct of Sylvius. Discriminant analyses were performed to evaluate the classification capacity of both individual parameters and the combination of different parameters.
ResultsMaximum diastolic velocity, mean flow, and stroke volume showed statistically significant differences that could be used to distinguish between NPH and CVD patients (P<.001). Stroke volume and mean flow showed no false positive results and successful classification rates of 86% and 79%, respectively. No other parameters or combination produced better results.
ConclusionsPhase-contrast MR imaging is a useful tool for the early diagnosis of patients with NPH. CSF flow quantitative parameters, along with morphological features in a conventional MR study, enable us to differentiate between NPH and CVD patients.
El objetivo de este estudio es valorar si la RM en contraste de fase es una herramienta útil en el diagnóstico de la hidrocefalia a presión normal (HPN), así como su diferenciación con otras afecciones neurológicas muy similares clínicamente.
MétodosSe incluyó a un total de 108 sujetos, de los cuales 61 eran sujetos sanos control, y 47 pacientes; 19 de ellos fueron clasificados en el grupo de pacientes con enfermedad cerebrovascular isquémica (ECI) y 28 pacientes dentro del grupo de HPN. A todos los pacientes se les realizó una RM en contraste de fase con cuantificación de parámetros de flujo y velocidad de LCR en el acueducto de Silvio. Se evaluó la capacidad de clasificación de los parámetros individualmente y combinándolos mediante análisis discriminantes.
ResultadosLos parámetros de velocidad máxima diastólica, flujo promedio y volumen por ciclo mostraron diferencias estadísticamente significativas para separar a los pacientes con HPN y con ECI (p<0,001). El volumen por ciclo y el flujo promedio no presentaron falsos positivos, con tasas de acierto del 86% y 79%, respectivamente. El resto de parámetros y la combinación de todos ellos no mejoraron los resultados.
ConclusionesLa RM en contraste de fase es una herramienta muy útil para el diagnóstico precoz de los pacientes con HPN. La cuantificación de parámetros de flujo de LCR junto con la valoración del estudio morfológico de la RM convencional permite diferenciar a los pacientes con HPN de los pacientes con ECI.
The most widely accepted theory regarding the pathophysiological mechanism behind normal pressure hydrocephalus (NPH), also known as symptomatic hydrocephalus, is a change in the CSF reabsorption mechanisms.1,2 We should be aware that the purpose of ventricular shunting is not to reduce intraventricular pressure, but rather to decrease the CSF pulse pressure.
During systole, the entry of arterial blood into the normal brain creates an increase in intracranial volume which is compensated by the displacement of CSF through the spinal canal. This in turn gives rise to CSF flow that is craniocaudal and centrifugal to venous sinus flow. During diastole, the exit of blood reduces intracranial volume and CSF flows in the opposite direction. Displacement of the fluid depends on the relative intracranial elasticity.
Patients with NPH display decreased intracranial elasticity, and the force of systole cannot be transmitted centrifugally in the same way. As a result, it moves centripetally, thereby compressing lateral ventricles and increasing CSF flow through the aqueduct of Sylvius.3–6
NPH is characterised by the clinical signs of dementia, gait instability, and urinary incontinence (Hakim's triad).7 Although this is the most typical form of presentation, some series have shown that up to 30% of patients only present one of these symptoms. Diagnosing NPH is difficult in many cases, given that other neurological diseases may also present these frequently non-specific symptoms.8,9 In most cases, the initial manifestation is gait instability, which may also be associated with discrete cognitive changes.10 These changes are predominantly subcortical, and it is often difficult to identify clinical differences that distinguish the entity from other types of dementia, such as vascular or parkinsonian dementias.11,12
Classic radiological diagnosis of NPH is based on the exaggerated dilation of the ventricular system compared to the subarachnoid space; this can be viewed using a computed tomography (CT) study. The decrease in subarachnoid space tends to be more pronounced at the convexity. Nevertheless, if no other signs are considered, this sign is highly non-specific; it may appear in either secondary communicating hydrocephalus or cerebral atrophy.
Magnetic resonance (MR) imaging can be used to observe other characteristic, although not diagnostic, signs of NPH. The first is the increase of flow void in the third ventricle; this occurs due to increased CSF flow velocity and appears as marked signal hypointensity in the T2 sequences. Periventricular and deep white matter hyperintensities, also typically observed in T2 sequences, are associated with microcirculatory changes,13 but these changes are also non-specific. While these changes appear more frequently in patients with NPH,14,15 they may also be observed in other patients with cognitive impairment of vascular origin, and even in 30% to 80% of the healthy elderly population.13
Other important methods for diagnosing patients with NPH included measuring CSF opening pressure,16 measuring CSF outflow resistance,17 and dynamic studies such as the tap test.18 The tap test involves extracting 40 or 50mL CSF by lumbar puncture, and while its positive predictive value is very high (up to 90%), its sensitivity is quite low (26–61%). The saline infusion test including a measurement of CSF outflow resistance is more sensitive (57–95%) and has a high positive predictive value (75–92%).19 In conclusion, wide ranges of results have been reported for these techniques, all of which are invasive.
NPH is the only form of dementia that can be treated with ventricular shunting. However, there is no consensus on which patients should undergo surgery; although diagnostic guidelines have been published,20 there are no set parameters that allow doctors to diagnose NPH precisely.21,22 According to some studies, the only evidence pointing to a diagnosis of NPH is good response to ventricular shunting.23 However, we know that the response also depends on the surgical technique and postoperative complications, which arise in up to 28% of cases.24,25 Therefore, the response to ventricular shunting cannot be considered a reproducible test result.
In this context, phase contrast-MRI (PC-MRI) offers new possibilities for diagnosing NPH by allowing doctors to calculate quantitative CSF flow parameters throughout the cardiac cycle.25 Some studies employing this non-invasive technique have demonstrated the presence of hyperdynamic flow in the aqueduct of Sylvius in patients with NPH.3,4,6,24–26
The purpose of this study was to evaluate whether PC-MRI can be used to differentiate between patients with NPH and those with ischaemic cardiovascular disease (ICD), since both groups may present the same conventional MRI findings.
Patients and methodsPatientsWe performed a PC-MRI study on a total of 124 patients over a period of 5 years (from January 2005 to December 2011). A total of 108 were finally included in the study (42 men, 66 women) and their ages ranged from 20 to 91 years (56±21 years). All patients gave their consent for their images to be included anonymously in this study. No specific authorisation was required by the Ethics Committee since the MRI study was performed as part of normal clinical practice.
Subjects were classified as healthy, NPH patients, and ICD patients based on the criteria listed below:
- 1.
Healthy subjects (61 individuals): 43 men and 18 women, mean age 46 years. Subjects selected for the control group were volunteers who had been referred for an MRI study due to headache or vertigo. They presented no neurological changes (such as ataxia or dementia) and no incontinence. These subjects had never experienced either intracranial hypertension or head trauma.
- 2.
Patients diagnosed with NPH (28 patients): 23 men and 5 women, mean age 71 years. The following inclusion criteria were applied:
- -
Ventricular enlargement >0.3 on Evans index (measured as the ratio of the distance between the frontal horns to the transverse diameter of the inner table of the skull), plus gradual onset gait instability.
- -
Ventricular enlargement and mild cognitive impairment.
- -
Ventricular enlargement and urinary incontinence.
- -
No cardiovascular risk factors, diabetes, hypertension, or atherosclerosis.
- -
No history of head trauma, intracranial hypertension, or subarachnoid haemorrhage.
- -
Having undergone ventricular shunting and displaying clinical improvement at least 6 months after surgery.
- -
- 3.
Patients with ICD (19 patients): 10 men and 9 women, mean age 70 years. Patients presented recent onset, progressive moderate cognitive impairment and at least one of the following:
- -
Increase in white matter signal intensity of more than 25% in T2 MRI sequences due to leukoaraiosis and presence of atherosclerosis at other locations.
- -
Presence of cardiovascular risk factors (hypertension, diabetes, or tobacco use).
- -
We excluded 16 patients after performing the MRI and measuring flow due to the reasons listed below. Patients with obstructive hydrocephalus (2), specifically patients with stenosis of the aqueduct of Sylvius with supratentorial hydrocephalus and an absent or diminished aqueduct and PC-MRI showing lack of flow signal in the aqueduct: patients diagnosed with type 1 Chiari malformation (1); patients with a history of subarachnoid haemorrhage and suspected communicating reduced absorption hydrocephalus (1); patients with intra-axial tumours (1); patients with a parietal cortical infarct (1); patients with less than 6 months of clinical follow-up (8); patients with continuous, persistent arrhythmias (1); patients younger than 20 years of age (1).
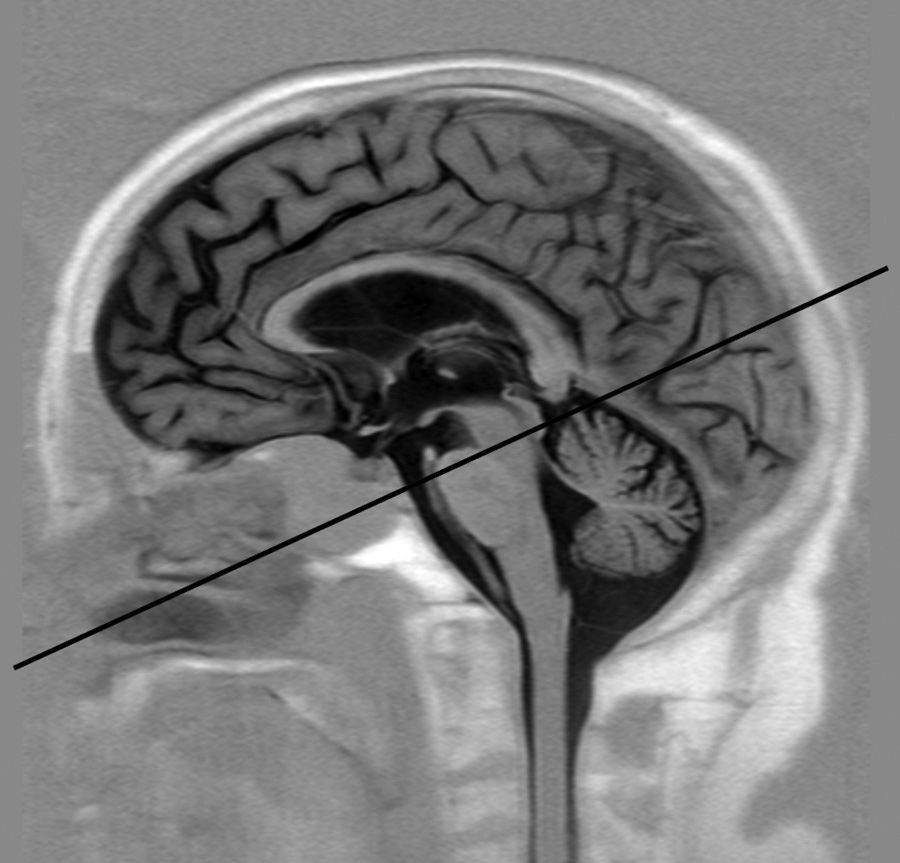
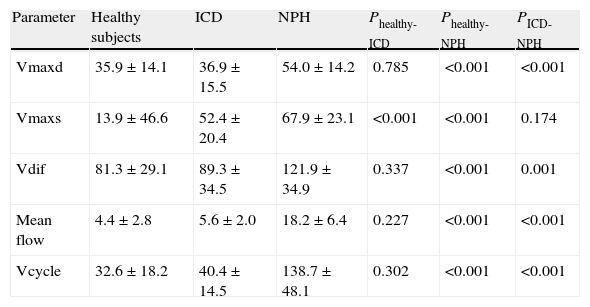
Obtaining imagesThe PC-MRI study was performed using an oblique plane transversal to the aqueduct of Sylvius (Fig. 1). We used a 3T MRI unit (Archieva r3.2, Philips, The Netherlands) with the following imaging parameters: TR=16ms, TE=8ms, angle of inclination=10°, 25 images/cycle, acquisition matrix=324×324, slice thickness=5mm, pixel size=0.33×0.33mm, encoding velocity=15cm/s, retrospective cardiac synchronisation. Caudocranial velocities were encoded as positive values, while craniocaudal velocities were codified as negative values. To prevent the variations in flow associated with the circadian rhythm, all imaging studies were carried out in the same time window (between 11.00 and 18.00).27
The MR study also included transversal T2-weighted spin-echo sequences, coronal FLAIR turbo spin echo sequences, and sagittal T1 weighted turbo spin echo sequences with initial inversion recovery.
Quantitative analysisQuantitative analysis of PC-MRI scans included 4 steps: (a) segmentation of the aqueduct of Sylvius; (b) extraction of CSF flow-vs-time curves; (c) aliasing correction, and (d) calculating quantitative CSF parameters.
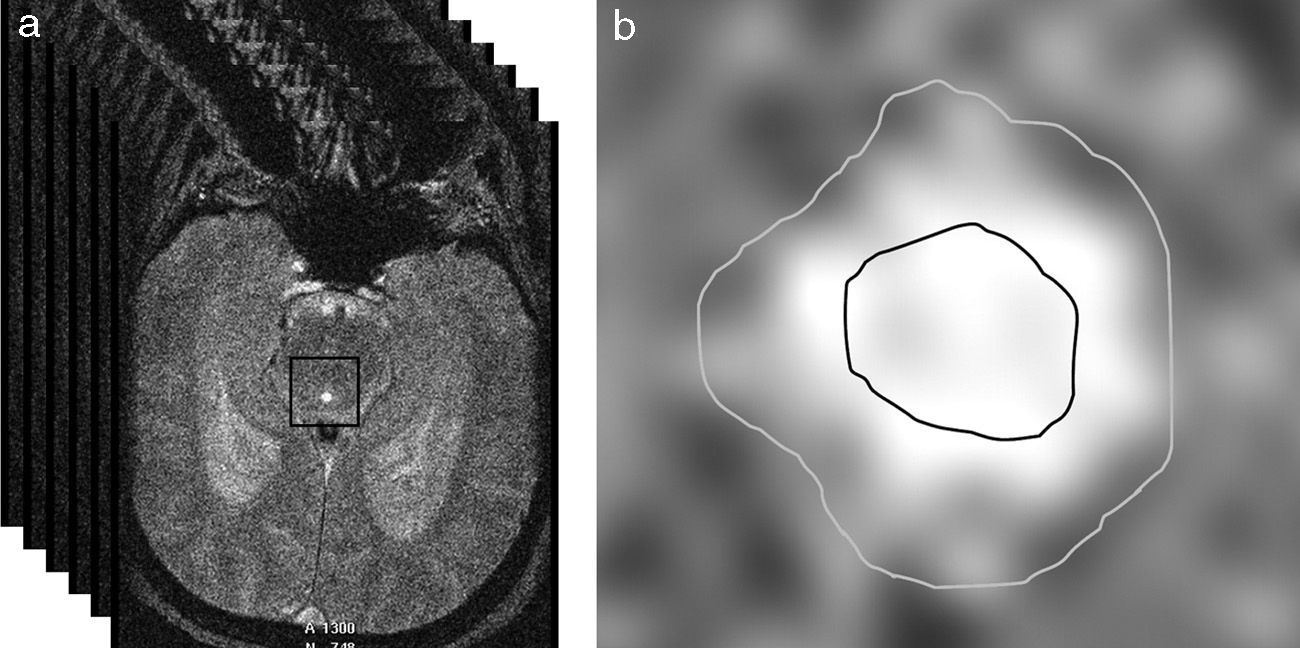
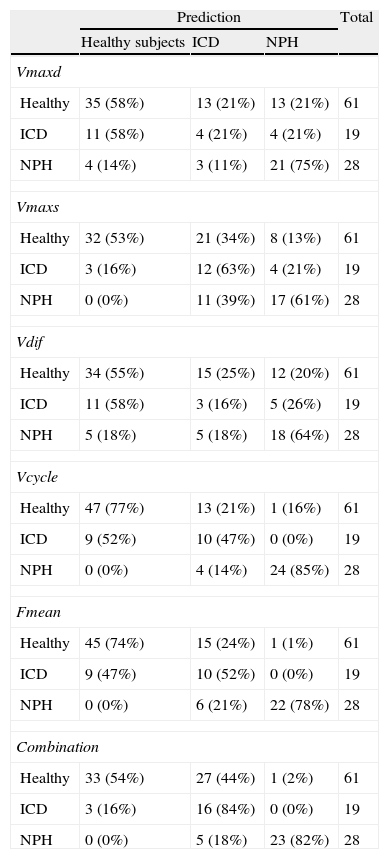
The aqueduct was segmented by manually selecting a point within it and automatically applying a threshold method. In essence, this method converts an image in greyscale into a binary image with 2 possible values: 0 or 1 (black or white), depending on whether the pixel intensity is higher or lower than the established threshold.28 Segmentation gives rise to 2 regions of interest (ROI): one area internal to the aqueduct which lets us calculate CSF velocity, and an area including the entire aqueduct and used to obtain flow values (Fig. 2). Two ROIs have to be used so that laminar flow effects (due to flow being faster in the centre of the aqueduct than near its walls) do not contaminate flow velocity measurements.29
(a) Automatic selection of a square matrix around the aqueduct of Sylvius beginning at a point marked manually inside the aqueduct. Intensity thresholds are applied within this matrix to obtain segmentation. (b) Segmentation of the aqueduct of Sylvius by applying thresholds. The inner area of interest was used to measure only velocities, whereas the exterior area included the entire aqueduct and was used to measure flow.
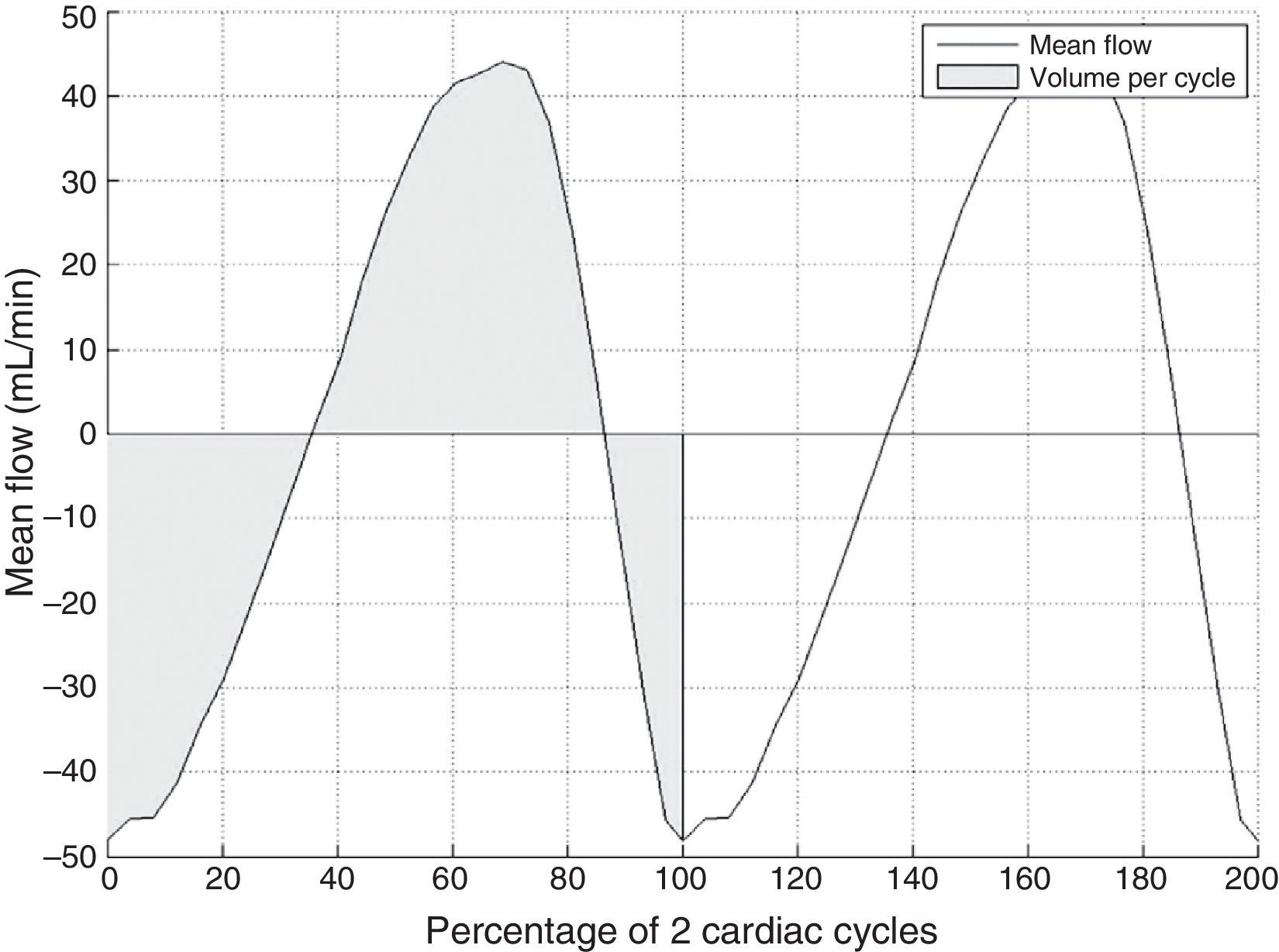
Next, flow-vs-time curves are extracted by propagating the 2 ROIs to the rest of the 25 images in the MRI sequence that were acquired throughout the cardiac cycle. This curve may display aliasing, that is, pixels whose velocity values have been encoded erroneously because flow velocity at that point was higher than the range specified in the MR sequence (15cm/s in this study). The effect of aliasing on the time curve is seen as very abrupt changes which can be detected and corrected automatically.28
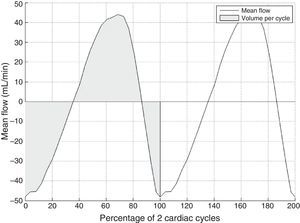
Lastly, the method delivers a flow-vs-time curve without aliasing (Fig. 3) that can be used to obtain several different quantitative parameters:
- -
Maximum diastolic velocity.
- -
Maximum systolic velocity.
- -
Velocity amplitude, obtained as the difference between the maximum diastolic and systolic velocities.
- -
Flow at each pixel, calculated for every time point by multiplying pixel area by its velocity.
- -
Mean flow, calculated as the average flow at all pixels in the aqueduct at each time point.
- -
Volume per cycle, calculated as the absolute value of the average area below the curve during a cardiac cycle.
We performed ANOVA tests with a post hoc test to study the differences between healthy subjects, patients with ICD, and patients with NPH. Homogeneity of variance was studied using the Levene test. A post hoc Newman–Keuls test was used for homoscedastic cases. Otherwise, we applied the Tamhane T2 test. Values of P<.05 were considered statistically significant. We also completed a discriminant analysis to study whether any linear combination of the calculated quantitative parameters was more effective for classifying patients. All analyses were performed using SPSS (version 13.0, IBM, USA).
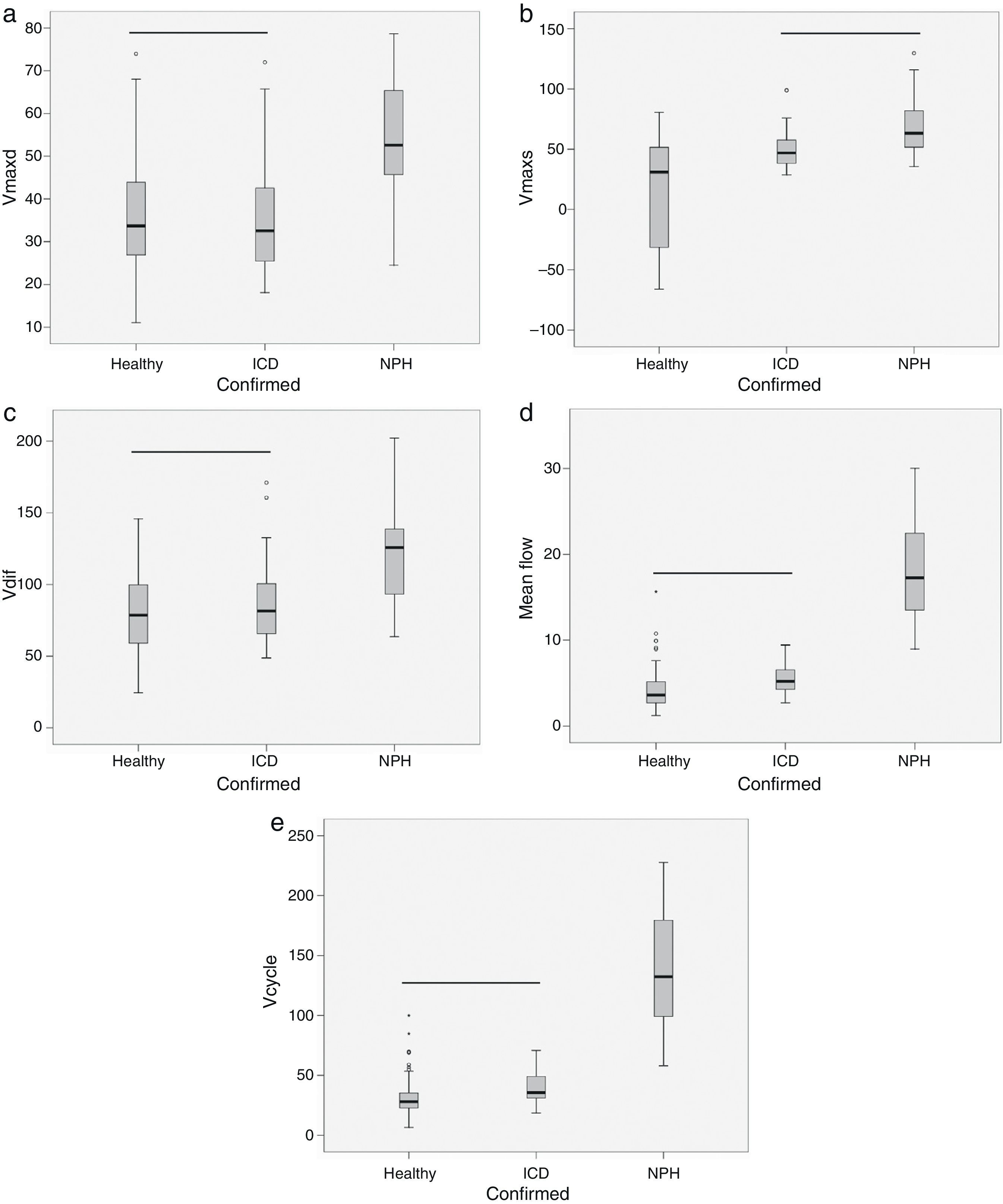
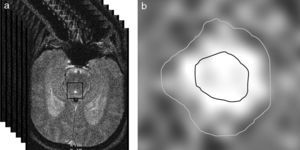
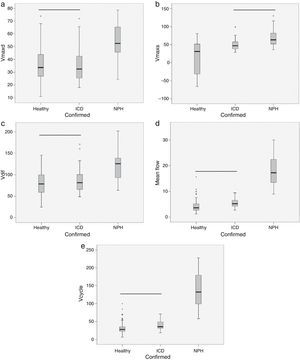
ResultsThe volume per cycle, mean flow, maximum diastolic pressure, and velocity amplitude showed statistically significant differences between patients with ICD and those with NPH (Table 1). We should point out that maximum systolic pressure could not be used to distinguish between groups, but that this variable was significantly different between patients with ICD and healthy subjects (Table 1). Fig. 4 displays the case distribution and the different degrees of intragroup overlap for each of the variables.
Mean±typical deviation and statistical significance of quantitative parameters of CSF in healthy subjects, patients with ICD, and patients with NPH.
| Parameter | Healthy subjects | ICD | NPH | Phealthy-ICD | Phealthy-NPH | PICD-NPH |
| Vmaxd | 35.9±14.1 | 36.9±15.5 | 54.0±14.2 | 0.785 | <0.001 | <0.001 |
| Vmaxs | 13.9±46.6 | 52.4±20.4 | 67.9±23.1 | <0.001 | <0.001 | 0.174 |
| Vdif | 81.3±29.1 | 89.3±34.5 | 121.9±34.9 | 0.337 | <0.001 | 0.001 |
| Mean flow | 4.4±2.8 | 5.6±2.0 | 18.2±6.4 | 0.227 | <0.001 | <0.001 |
| Vcycle | 32.6±18.2 | 40.4±14.5 | 138.7±48.1 | 0.302 | <0.001 | <0.001 |
Units: Vmaxd, Vmaxs, Vdif in mm/s; mean flow in mL/min; Vcycle in μl.
ICD: ischaemic cerebrovascular disease; Fmean: mean flow; NPH: normal pressure hydrocephalus; Vcycle: volume per cycle; Vdif: velocity amplitude; Vmaxd: maximum diastolic velocity; Vmaxs: maximum systolic velocity.
Box-and-whisker plots showing the median (central black line), 25th and 75th percentiles (area of the box) and maximum and minimum values for maximum diastolic velocity (Vmaxd) (a), maximum systolic velocity (Vmaxs) (b), velocity amplitude (Vdif) (c), mean flow (d) and volume per cycle (Vcycle) (e). Measurements were taken in healthy subjects, patients with ischaemic cerebrovascular disease (ICD) and patients with normal pressure hydrocephalus (NPH). The horizontal black line indicates that there are no statistically significant differences between groups. Units: Vmaxd, Vmaxs, Vdif in mm/s; mean flow in mL/min; Vcycle in μl.
The classification obtained using discriminant analysis showed that volume per cycle and mean flow had the lowest error rates, 14% and 21% respectively, for classifying ICD and NPH (4 patients with NPH were classified as having ICD using volume per cycle; 6 patients with NPH were classified as ICD using mean flow) (Table 2). Both parameters were able to correctly identify all patients with ICD. The next best results for classifying patients corresponded to maximum diastolic velocity, with an error rate of 32% (4 patients with ICD were classified as having NPH and 3 patients with NPH were classified as having ICD). Combining quantitative parameters in a discriminant function did not improve individual classification results. It should be noted that no false positives were associated with either volume per cycle or mean flow. Volume per cycle is the parameter with the greatest diagnostic utility.
Results from patient classification using cross-validation of the discriminant analysis for each study parameter and parameter combinations.
| Prediction | Total | |||
| Healthy subjects | ICD | NPH | ||
| Vmaxd | ||||
| Healthy | 35 (58%) | 13 (21%) | 13 (21%) | 61 |
| ICD | 11 (58%) | 4 (21%) | 4 (21%) | 19 |
| NPH | 4 (14%) | 3 (11%) | 21 (75%) | 28 |
| Vmaxs | ||||
| Healthy | 32 (53%) | 21 (34%) | 8 (13%) | 61 |
| ICD | 3 (16%) | 12 (63%) | 4 (21%) | 19 |
| NPH | 0 (0%) | 11 (39%) | 17 (61%) | 28 |
| Vdif | ||||
| Healthy | 34 (55%) | 15 (25%) | 12 (20%) | 61 |
| ICD | 11 (58%) | 3 (16%) | 5 (26%) | 19 |
| NPH | 5 (18%) | 5 (18%) | 18 (64%) | 28 |
| Vcycle | ||||
| Healthy | 47 (77%) | 13 (21%) | 1 (16%) | 61 |
| ICD | 9 (52%) | 10 (47%) | 0 (0%) | 19 |
| NPH | 0 (0%) | 4 (14%) | 24 (85%) | 28 |
| Fmean | ||||
| Healthy | 45 (74%) | 15 (24%) | 1 (1%) | 61 |
| ICD | 9 (47%) | 10 (52%) | 0 (0%) | 19 |
| NPH | 0 (0%) | 6 (21%) | 22 (78%) | 28 |
| Combination | ||||
| Healthy | 33 (54%) | 27 (44%) | 1 (2%) | 61 |
| ICD | 3 (16%) | 16 (84%) | 0 (0%) | 19 |
| NPH | 0 (0%) | 5 (18%) | 23 (82%) | 28 |
Units: Vmaxd Vmaxs Vdif in mm/s; Fmean in mL/min; Vcycle in μl.
ICD: ischaemic cerebrovascular disease; Fmean: mean flow; NPH: normal pressure hydrocephalus; Vcycle: volume per cycle; Vdif: velocity amplitude; Vmaxd: maximum diastolic velocity; Vmaxs: maximum systolic velocity.
Normal pressure hydrocephalus is difficult to diagnose clinically. On many occasions, it shows considerable clinical and RM imaging similarities to other entities, including ICD.30
This clear association between white matter changes and hydrocephalus is what led us to attempt to distinguish between patients with NPH and those with ICD; both processes show similar clinical and RM imaging features.
Different diagnostic guidelines have been proposed to aid in diagnosing this disease20; the most recent one was published by Mori et al.31–33 However, none of these guidelines includes an in-depth analysis of the utility of phase-contrast MRI.
Based on our understanding of the difficulties involved in diagnosing NPH and grading its symptoms, we have developed a method that uses a postprocessing technique to minimise the partial volume and aliasing effects without the repeat examinations that were needed in earlier studies.31–33
Our study confirms hyperdynamic flow in the aqueduct, which has been observed in previous studies.3,4 Volume per cycle is the isolated parameter that best distinguishes between patients with ICD and NPH. Our volume per cycle values were higher than those reported by Bradley. Mean volume per cycle was 138μl, whereas Bradley found a mean of 42μl for patients with NPH.35 Bradley's group did not correct for aliasing or use automatic segmenting. Our normal values resemble those presented by El Sankari et al.36 who established a normal cut-off level at 71μ1 (mean of normal values plus 2 standard deviations). The mean in Bradley's NPH patients was 175±71, which was higher than that in our group.
The second best parameter for differentiating between patients with NPH and ICD was mean flow. Several studies have used this parameter for diagnosing NPH. They include the study by Luetmer et al.,34 which concluded that patients with NPH had a mean flow of more than 18mL/min. These results are similar to our own, except that in Luetmer's case, aliasing was corrected by repeating acquisitions.
Neither volume per cycle nor mean flow presented false positives. As such, these criteria will not result in any patient undergoing unnecessary shunting, which is important considering the risks of surgical procedures in these older patients. In unclear cases, we propose performing an imaging test 6 months later to assess any changes in flow dynamics. Maximum diastolic velocity, on the contrary, did produce 4 false positives. Using this parameter alone would therefore result in shunting being performed on patients who would not benefit from the procedure. A discriminant analysis using all of the parameters did not improve classification results, probably because there were certain statistical correlations between the study variables.
Although some studies have evaluated the completed dynamics of CSF flow, both in the aqueduct of Sylvius and in the cervical space, these studies generally find no differences in CSF flow in the cervical space and focus exclusively on flow in the aqueduct.37
The main limitation of our study is patient selection. Firstly, the number of patients with NPH is quite low (n=28), despite the length of our study period (5 years). This occurred because NPH is an uncommon entity. In addition, the selection process used a combination of clinical, radiological, and treatment response criteria since no tests of reference have been developed so far.20
Our acquisition parameters were similar to those used by Luetmer et al.34 and Lee et al.38 The encoding velocity should be higher than expected to avoid aliasing, but still as low as possible since it is inversely proportional to the signal/noise index (SNI). Because of this, we changed it during the course of the study.39 We minimised measurement errors by selecting the encoding velocity and taking thinner slices perpendicular to the aqueduct. The most frequent of these errors is partial volume produced by tissues adjacent to the aqueduct. Background error, caused by cerebral movement, was minimised using the segmentation and post-processing technique developed by our group. According to this method, the measurement obtained is highly reproducible and objective.
In conclusion, PC-MRI is a very useful method of identifying patients with NPH and distinguishing them from patients with vascular changes. Quantitative parameters obtained using PC-MRI, mainly volume per cycle and mean flow, can be used to separate patients with initial or established NPH from patients with ICD.
FundingPart of this study was financed by a grant from the Spanish Society of Radiology (SERAM_Industria_2008).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Forner Giner J, Sanz-Requena R, Flórez N, Alberich-Bayarri A, García-Martí G, Ponz A, et al. Estudio cuantitativo del flujo de líquido cefalorraquídeo mediante resonancia magnética en contraste de fase: método para identificar a los pacientes con hidrocefalia a presión normal. Neurología. 2014;29:68–75.