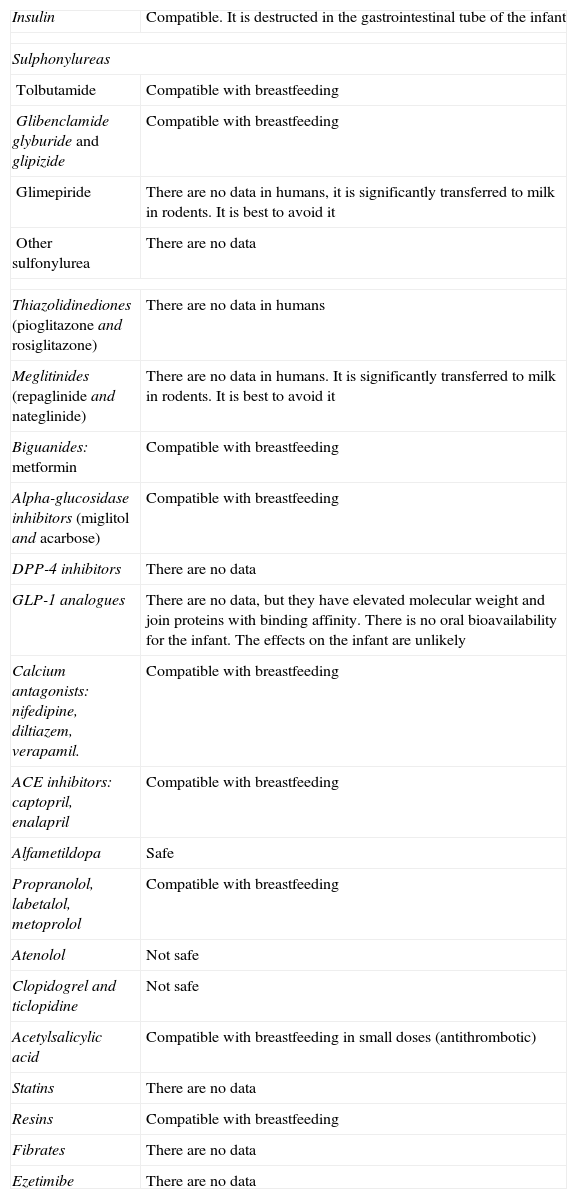
The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years.
© Clarivate Analytics, Journal Citation Reports 2025
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact.
See moreSNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field.
See more