Exocrine pancreatic insufficiency (EPI) is an important complication of chronic pancreatitis (CP). Guidelines recommend to rule out EPI in CP, to detect those patients who would benefit from pancreatic enzyme replacement therapy. The aim of this study was to evaluate the prevalence of EPI in patients with CP without follow-up in the last 2 years and to describe their nutritional status and quality of life (QoL).
MethodsThis was a cross-sectional, multicenter Spanish study. CP patients without follow-up by a gastroenterologist or surgeon in at least 2 years were included. EPI was defined as fecal elastase test <200mcg/g. For nutritional assessment, laboratory and anthropometric data were obtained. QoL was investigated using the EORTC QLQ-C30 questionnaire.
Results64 patients (mean age 58.8±10.3 years, 85.9% men) from 10 centers were included. Median time since diagnosis of CP was 58.7 months [37.7–95.4]. Forty-one patients (64.1%) had EPI. Regarding nutritional status, the following differences were observed (EPI vs. Non-EPI): BMI (23.9±3.5kg/m2 vs. 25.7±2.5, p=0.03); glucose (121 [96–189] mg/dL vs. 98 [90–116], p=0.006); HbA1c 6.6% [6.0–8.4] vs. 5.5 [5.3–6.0], p=0.0005); Vitamin A (0.44mg/L [0.35–0.57] vs. 0.53 [0.47–0.63], p=0.048) and Vitamin E (11.2±5.0μg/ml vs. 14.4±4.3, p=0.03). EPI group showed a worse EORTC QLQ-C30 score on physical (93.3 [66.7–100] vs. 100 [93.3–100], p=0.048) and cognitive function (100 [83.3–100] vs. 100 [100–100], p=0.04).
ConclusionsPrevalence of EPI is high in patients with CP without follow-up. EPI group had higher levels of glucose, lower levels of vitamins A and E and worse QoL.
la insuficiencia pancreática exocrina (IPE) es una importante complicación de la pancreatitis crónica (PC). Las guías recomiendan el seguimiento de la IPE en PC, para identificar a aquellos pacientes que puedan beneficiarse del tratamiento enzimático sustitutivo. El objetivo de este estudio fue evaluar la prevalencia de IPE en pacientes con PC sin seguimiento en los últimos 2 años y describir su estado nutricional y calidad de vida (QoL).
Métodosestudio trasversal, multicéntrico, español. Se incluyeron pacientes con PC sin seguimiento por un gastroenterólogo/cirujano en los últimos 2años. Se definió IPE como elastasa fecal<200mcg/g. Se recogieron parámetros de laboratorio y datos antropométricos para el análisis nutricional. Para la evaluación de QoL se utilizó el cuestionario EORTC QLQ-C30.
Resultadosse incluyeron prospectivamente 64 pacientes (58,8±10,3 años, media 85,9%) de 10 centros. Tiempo medio desde el diagnóstico de PC: 58,7meses [37,7-95,4]. 41 pacientes (64,1%) tenían IPE. Estado nutricional: se observaron las siguientes diferencias (IPE vs No-IPE): IMC (23,9±3,5kg/m2 vs. 25,7±2,5,p=0,03); glucosa 121 [96-189] mg/dL vs. 98 [90-116];p =0,006); HbA1c 6,6% [6,0-8,4] vs. 5,5 [5,3-6,0],p=0,0005); Vitamina-A (0,44mg/L [0,35-0,57] vs. 0,53 [0,47-0,63],p=0,048), Vitamina-E (11,2±5,0μg/ml vs. 14,4±4,3,p=0,03). El grupo de IPE mostró una peor puntuación en el EORTC QLQ-C30 en las funciones física (93,3 [66,7-100] vs. 100 [93,3-100], p=0,048) y cognitiva (100 [83,3-100] vs. 100 [100-100],p=0,04).
Conclusionesla prevalencia de IPE en pacientes con PC sin seguimiento es elevada. En el grupo de IPE se observaron niveles elevados de glucosa, bajos de vitaminas A y E y peor calidad de vida.
Chronic pancreatitis (CP) is a syndrome characterized by inflammation, fibrosis and loss of acinar and pancreatic islet cells.1 The amount of enzyme-rich fluid secreted by the pancreas is about 10 times the one needed to ensure a normal digestion. Thus, although subtle changes in exocrine function can be detected in patients with early pancreatic disease, overt steatorrhea as a manifestation of exocrine pancreatic insufficiency (EPI) does not occur until approximately 90 percent of glandular function has been lost.2 According to the recommendations of the Spanish Association of Pancreatology, the term EPI should be only used for the situation in which the alteration of pancreatic function is associated with the inability of the pancreas to perform a normal digestion process.3
Thirty percent of subjects with mild CP and 85% with severe CP will develop EPI 10–15 years after the beginning of the disease.4 Advanced EPI results in maldigestion of fat and protein leading to steatorrhea and weight loss. Vitamins and minerals deficiencies may also appear due to malabsorption. EPI is associated with a worse quality of life in patients with CP.5
There is evidence that incidence of CP and CP-related hospitalizations is increasing.6 Although the follow-up period for PC patients is not stabilised, the Spanish Association of Pancreatology recommend a clinical and analytical follow-up each six months for the stablished CP patients.3 Some patients with CP are not followed up adequately and, consequently, the diagnosis of possible complications like EPI could be delayed, limiting our therapeutic action.7 Early diagnosis of EPI is necessary to prevent symptoms, malnutrition and to minimize the development of possible complications. The aim of the present study is to analyze the exocrine pancreatic function by measuring the fecal elastase (FE-1) concentration in patients with CP that have not been followed up by a gastroenterologist or surgeon for at least two years, and to describe their nutritional status and quality of life (QoL).
MethodsThis cross-sectional, multicenter study included 64 patients with CP from 10 centers from Spain. We included patients with CP, that were visit by the gastroenterologist or surgeon some years ago and, who had not been followed up by a gastroenterologist or surgeon, non-necessarily expert in pancreas disease, for at least 2 years. The gastroenterologist participating in the study looked into their hospitals data bases and call these patients to attend to the hospital for review. Chronic pancreatitis was defined as patients that have one of the following criteria: pancreatic calcifications, duct dilation with parenchymal modifications (non-intraductal papillary neoplasia neither CP associated to NMPI), or 5 or more endoscopic criteria for CP.
Patients were included between June 2014 and April 2015. We excluded patients receiving pancreatic enzyme replacement therapy. The main objective of the study was to evaluate the prevalence of EPI among these patients. Secondary objectives included to describe other CP-related complications, assessment of the nutritional status and quality of life (QoL) of these patients.
All patients gave their written informed consent to take part in the study. The study was conducted according to the requirements stated in the Declaration of Helsinki and the current Spanish legislation. The protocol was submitted to the Spanish Agency of Medicines and Medical devices (AEMPS) and approved by the Ethic Committee of all the centers involved. Data were acquired prospectively during the first and subsequent outpatient clinic visits. Confidentiality of personal data was preserved following the guidelines of Spanish Organic law and all personal data were properly anonymized.
CP patients diagnosed before 2012 and without follow-up for at least two years were invited to participate. Data were retrieved from the medical records of each participating center. Those who agreed to participate, signed the informed consent were included and were followed by a gastroenterologist.
Exocrine pancreatic function was analyzed by measuring the FE-1 in a stool sample, considering concentrations below 200μg/g as indicative of EPI and <100μg/g consistent with severe EPI.
For the evaluation of the nutritional status, anthropometric (weight, body mass index (BMI)) and laboratory parameters (vitamins A, D, E, K and B12, lipoproteins, triglycerides, magnesium, hemoglobin, glycated hemoglobin, retinol binding protein (RBP), albumin and prealbumin), were compared between groups with and without EPI. Collection of laboratory data was done according to usual practice of each participant center and some of these data could not be available.
For the assessment of QoL, the EORTC-QLC-C30 scale was used. Although originally designed for cancer patients, the QLQ-C30 was previously shown to be suitable as an assessment tool for the CP population.8–11 The questionnaire consists of 30 questions, of which 24 form nine multiquestion scales: one global scale, five functional scales (physical, role, emotional, cognitive, and social) and three symptoms scales (pain, nausea, and fatigue). The six remaining questions aim at reporting six symptoms: dyspnea, insomnia, appetite, constipation, diarrhea, and financial difficulties. The QLQ-C30 produces a complex assessment of the health-related quality of life (HR-QOL). Results are linearly recalculated to a uniform scale of 0–100 points, in which a higher score indicates a more favorable outcome.
Proportion and types of CP-related complications apart from EPI, are described.
Statistical methodsContinuous variables have been analyzed using the following descriptive statistics: sample size, mean, standard deviation (SD), median [Q1–Q3], minimum and maximum. The Shapiro–Wilk test has been used to check if a variable has a normal distribution.
Mean (SD) was used for normal distribution and Median (Q1–Q3) for non-normal distribution.
Categorical variables are presented in contingency tables, calculating absolute and relative frequencies. A category of missing data has been included if needed.
For the comparison between groups of continuous variables, parametric (T-test) or non-parametric tests (Wilcoxon) have been used, depending on the distribution of the analyzed variables in each case.
For the comparison between groups of categorical variables, the Chi-square test or the Fisher test have been used, choosing the most appropriate in each case.
Relative frequencies and descriptive parameters are shown with 2 decimal digits. Descriptive p-values ≥0.001 are shown with a four decimal digit precision and p-values <0.001 are shown as <0.001).
All analyses have been performed using the SAS® v9.3 software.
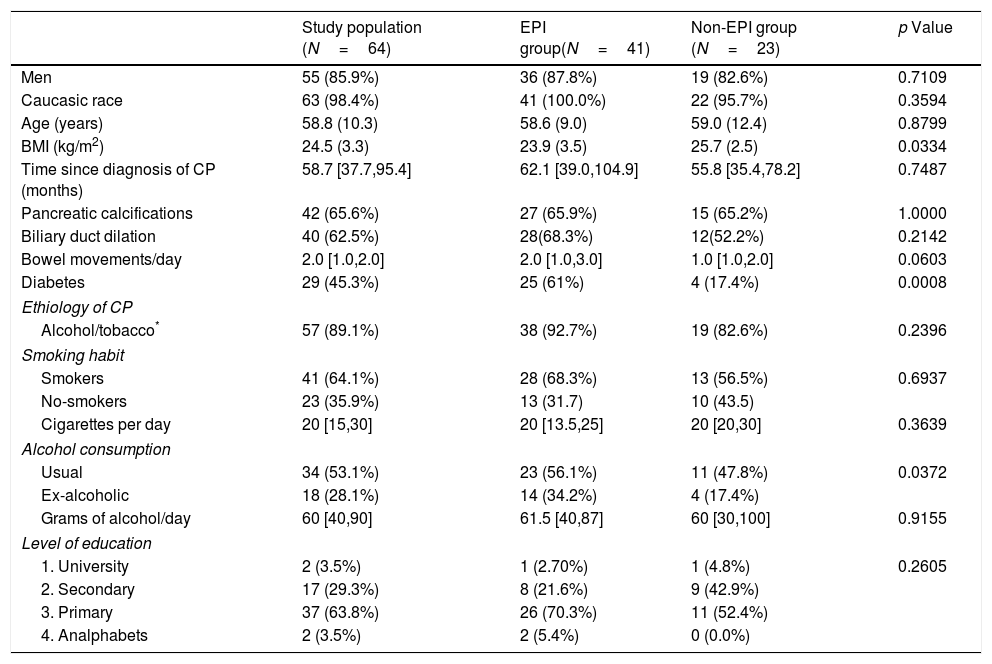
Results64 patients with CP were finally included in the study. The original diagnosis of CP was made by clinical suspicion and it was confirmed by computerized tomography in most patients (64.8%). Forty-two patients (65.6%) had pancreatic calcifications and 40 (62.5%) biliary duct dilation. The median (Q1–Q3) time since diagnosis of CP was 58.7 [37.7–95.4]). Table 1 shows the baseline characteristics of the study population (64 patients) and the comparison between the EPI and non-EPI group. Forty-one (64.1%) had FE-1 levels <200μg/g and were diagnosed with EPI, according to the criteria stated in the study protocol. Twenty-nine patients (45.3%) had levels of FE-1<100μg/g and were classified as severe EPI. Five patients had already been diagnosed with EPI when they entered the study. All of them fulfilled the criteria for severe EPI. Median time from the diagnosis of CP was longer in those with severe EPI (68 [36.3–103.3] months vs. 57.3 [45.6–88.4] months) than in the group with mild EPI, though this difference was not statistically significant (p=0.8286). When we analyzed in patients with EPI the average time from diagnosis of CP according to its etiology (alcohol, tobacco, obstructive, other) we did not find significant differences either. Number of bowel movements was higher in the group with EPI (median 2 [1–3] vs. 1 [1–2]) though this difference did not reach statistical significance (p=0.0603).
Baseline characteristics of the study population (N=64).
| Study population (N=64) | EPI group(N=41) | Non-EPI group (N=23) | p Value | |
|---|---|---|---|---|
| Men | 55 (85.9%) | 36 (87.8%) | 19 (82.6%) | 0.7109 |
| Caucasic race | 63 (98.4%) | 41 (100.0%) | 22 (95.7%) | 0.3594 |
| Age (years) | 58.8 (10.3) | 58.6 (9.0) | 59.0 (12.4) | 0.8799 |
| BMI (kg/m2) | 24.5 (3.3) | 23.9 (3.5) | 25.7 (2.5) | 0.0334 |
| Time since diagnosis of CP (months) | 58.7 [37.7,95.4] | 62.1 [39.0,104.9] | 55.8 [35.4,78.2] | 0.7487 |
| Pancreatic calcifications | 42 (65.6%) | 27 (65.9%) | 15 (65.2%) | 1.0000 |
| Biliary duct dilation | 40 (62.5%) | 28(68.3%) | 12(52.2%) | 0.2142 |
| Bowel movements/day | 2.0 [1.0,2.0] | 2.0 [1.0,3.0] | 1.0 [1.0,2.0] | 0.0603 |
| Diabetes | 29 (45.3%) | 25 (61%) | 4 (17.4%) | 0.0008 |
| Ethiology of CP | ||||
| Alcohol/tobacco* | 57 (89.1%) | 38 (92.7%) | 19 (82.6%) | 0.2396 |
| Smoking habit | ||||
| Smokers | 41 (64.1%) | 28 (68.3%) | 13 (56.5%) | 0.6937 |
| No-smokers | 23 (35.9%) | 13 (31.7) | 10 (43.5) | |
| Cigarettes per day | 20 [15,30] | 20 [13.5,25] | 20 [20,30] | 0.3639 |
| Alcohol consumption | ||||
| Usual | 34 (53.1%) | 23 (56.1%) | 11 (47.8%) | 0.0372 |
| Ex-alcoholic | 18 (28.1%) | 14 (34.2%) | 4 (17.4%) | |
| Grams of alcohol/day | 60 [40,90] | 61.5 [40,87] | 60 [30,100] | 0.9155 |
| Level of education | ||||
| 1. University | 2 (3.5%) | 1 (2.70%) | 1 (4.8%) | 0.2605 |
| 2. Secondary | 17 (29.3%) | 8 (21.6%) | 9 (42.9%) | |
| 3. Primary | 37 (63.8%) | 26 (70.3%) | 11 (52.4%) | |
| 4. Analphabets | 2 (3.5%) | 2 (5.4%) | 0 (0.0%) | |
Data are presented as n (%), mean (SD: standard deviation) or median [Q1, Q3]. BMI: body mass index; CP: chronic pancreatitis.
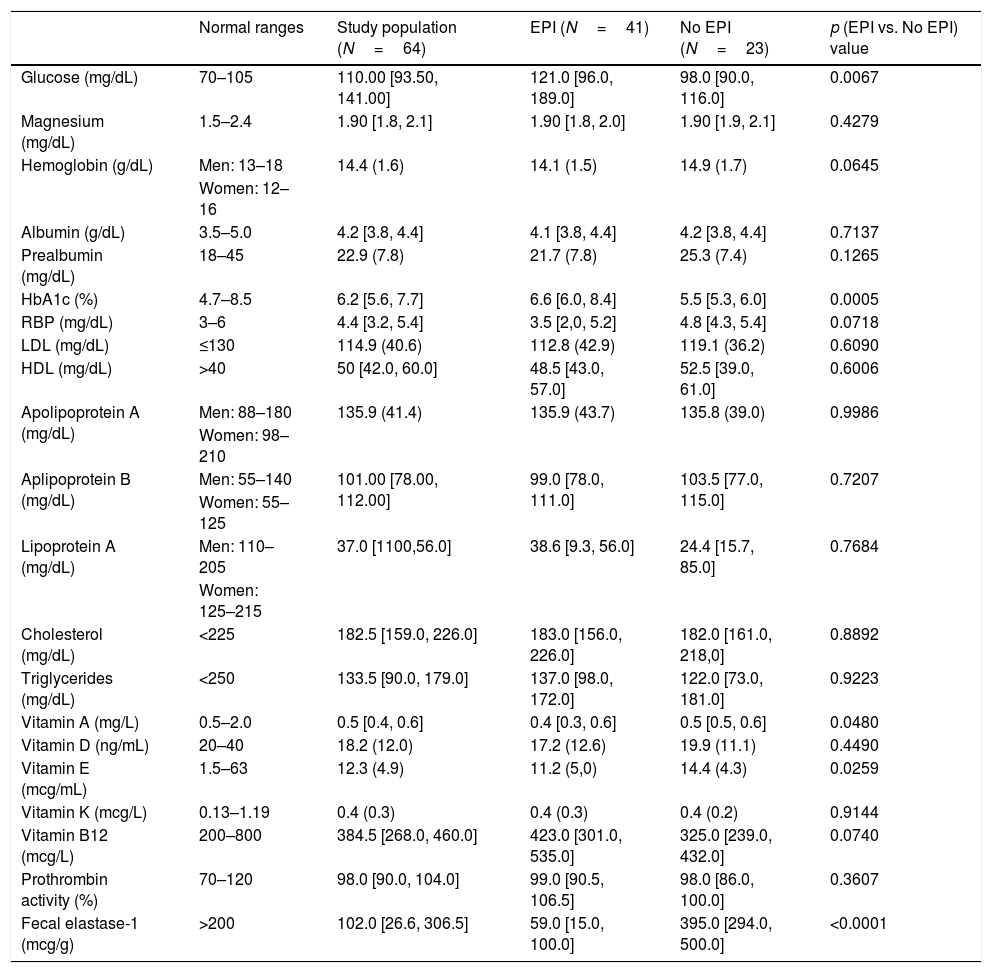
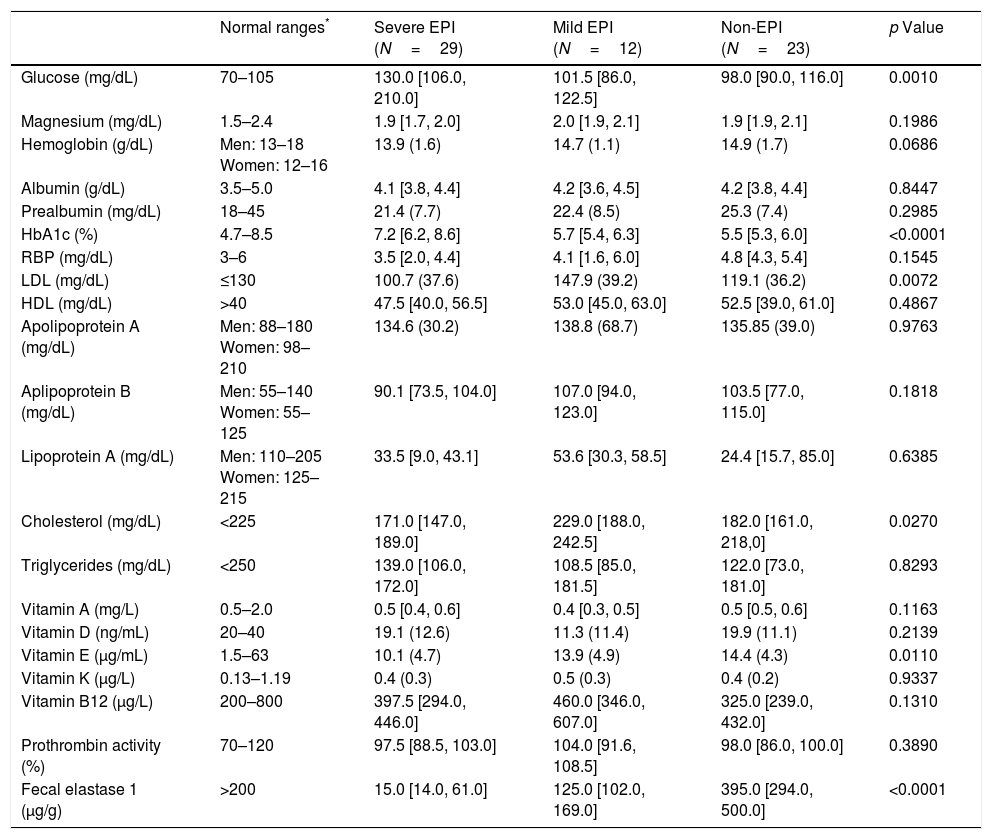
Regarding nutritional status, we found differences (EPI group vs. non EPI group) in BMI (23.9+3.5kg/m2 vs. 25.7+2.5, p=0.0334) and in the following laboratory parameters (Table 2): fasting glucose levels (121 vs. 98mg/dL, p=0.0067), glycated hemoglobin (6.6 vs. 5.5%, p=0.0005), vitamin A (0.44 vs. 0.53mg/L, p=0.0480) and vitamin E (11.2 vs. 14.4μg/ml, p=0.0259). All these parameters were lowers in the PEI group than in the Non EPI group, although media and median were always into the normal ranges in both groups. When we compared patients with severe EPI with those with mild EPI (Table 3) we observed the following differences: fasting glucose levels (130mg/dL vs. 101.5mg/dL, p=0.0010), HbA1c (7.2% vs. 5.7%, p<0.0001), LDL cholesterol (100.7mg/dL vs. 147.9mg/dL, p=0.0072), total cholesterol (171 vs. 229mg/dL, p=0.0270) and vitamin E (10.1 vs. 13.9μg/ml, p=0.0110).
Laboratory parameters in study population, EPI and non-EPI groups.
| Normal ranges | Study population (N=64) | EPI (N=41) | No EPI (N=23) | p (EPI vs. No EPI) value | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 70–105 | 110.00 [93.50, 141.00] | 121.0 [96.0, 189.0] | 98.0 [90.0, 116.0] | 0.0067 |
| Magnesium (mg/dL) | 1.5–2.4 | 1.90 [1.8, 2.1] | 1.90 [1.8, 2.0] | 1.90 [1.9, 2.1] | 0.4279 |
| Hemoglobin (g/dL) | Men: 13–18 | 14.4 (1.6) | 14.1 (1.5) | 14.9 (1.7) | 0.0645 |
| Women: 12–16 | |||||
| Albumin (g/dL) | 3.5–5.0 | 4.2 [3.8, 4.4] | 4.1 [3.8, 4.4] | 4.2 [3.8, 4.4] | 0.7137 |
| Prealbumin (mg/dL) | 18–45 | 22.9 (7.8) | 21.7 (7.8) | 25.3 (7.4) | 0.1265 |
| HbA1c (%) | 4.7–8.5 | 6.2 [5.6, 7.7] | 6.6 [6.0, 8.4] | 5.5 [5.3, 6.0] | 0.0005 |
| RBP (mg/dL) | 3–6 | 4.4 [3.2, 5.4] | 3.5 [2,0, 5.2] | 4.8 [4.3, 5.4] | 0.0718 |
| LDL (mg/dL) | ≤130 | 114.9 (40.6) | 112.8 (42.9) | 119.1 (36.2) | 0.6090 |
| HDL (mg/dL) | >40 | 50 [42.0, 60.0] | 48.5 [43.0, 57.0] | 52.5 [39.0, 61.0] | 0.6006 |
| Apolipoprotein A (mg/dL) | Men: 88–180 | 135.9 (41.4) | 135.9 (43.7) | 135.8 (39.0) | 0.9986 |
| Women: 98–210 | |||||
| Aplipoprotein B (mg/dL) | Men: 55–140 | 101.00 [78.00, 112.00] | 99.0 [78.0, 111.0] | 103.5 [77.0, 115.0] | 0.7207 |
| Women: 55–125 | |||||
| Lipoprotein A (mg/dL) | Men: 110–205 | 37.0 [1100,56.0] | 38.6 [9.3, 56.0] | 24.4 [15.7, 85.0] | 0.7684 |
| Women: 125–215 | |||||
| Cholesterol (mg/dL) | <225 | 182.5 [159.0, 226.0] | 183.0 [156.0, 226.0] | 182.0 [161.0, 218,0] | 0.8892 |
| Triglycerides (mg/dL) | <250 | 133.5 [90.0, 179.0] | 137.0 [98.0, 172.0] | 122.0 [73.0, 181.0] | 0.9223 |
| Vitamin A (mg/L) | 0.5–2.0 | 0.5 [0.4, 0.6] | 0.4 [0.3, 0.6] | 0.5 [0.5, 0.6] | 0.0480 |
| Vitamin D (ng/mL) | 20–40 | 18.2 (12.0) | 17.2 (12.6) | 19.9 (11.1) | 0.4490 |
| Vitamin E (mcg/mL) | 1.5–63 | 12.3 (4.9) | 11.2 (5,0) | 14.4 (4.3) | 0.0259 |
| Vitamin K (mcg/L) | 0.13–1.19 | 0.4 (0.3) | 0.4 (0.3) | 0.4 (0.2) | 0.9144 |
| Vitamin B12 (mcg/L) | 200–800 | 384.5 [268.0, 460.0] | 423.0 [301.0, 535.0] | 325.0 [239.0, 432.0] | 0.0740 |
| Prothrombin activity (%) | 70–120 | 98.0 [90.0, 104.0] | 99.0 [90.5, 106.5] | 98.0 [86.0, 100.0] | 0.3607 |
| Fecal elastase-1 (mcg/g) | >200 | 102.0 [26.6, 306.5] | 59.0 [15.0, 100.0] | 395.0 [294.0, 500.0] | <0.0001 |
Figures are expressed as mean (standard deviation) or median [Q1,Q3]. RBP: retinol binding protein. HbA1c: glicated hemoglobin; LDL: low density lipoproteins; HDL: high density lipoproteins.
*Normal ranges according: http://www.msdmanuals.com/es-es/professional/apéndices/valores-normales-de-laboratorio/pruebas-de-sangre-valores-normales#v8508814_es and https://medlineplus.gov/.
Laboratory parameters in patients with severe EPI vs. patients with mild EPI.
| Normal ranges* | Severe EPI (N=29) | Mild EPI (N=12) | Non-EPI (N=23) | p Value | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 70–105 | 130.0 [106.0, 210.0] | 101.5 [86.0, 122.5] | 98.0 [90.0, 116.0] | 0.0010 |
| Magnesium (mg/dL) | 1.5–2.4 | 1.9 [1.7, 2.0] | 2.0 [1.9, 2.1] | 1.9 [1.9, 2.1] | 0.1986 |
| Hemoglobin (g/dL) | Men: 13–18 Women: 12–16 | 13.9 (1.6) | 14.7 (1.1) | 14.9 (1.7) | 0.0686 |
| Albumin (g/dL) | 3.5–5.0 | 4.1 [3.8, 4.4] | 4.2 [3.6, 4.5] | 4.2 [3.8, 4.4] | 0.8447 |
| Prealbumin (mg/dL) | 18–45 | 21.4 (7.7) | 22.4 (8.5) | 25.3 (7.4) | 0.2985 |
| HbA1c (%) | 4.7–8.5 | 7.2 [6.2, 8.6] | 5.7 [5.4, 6.3] | 5.5 [5.3, 6.0] | <0.0001 |
| RBP (mg/dL) | 3–6 | 3.5 [2.0, 4.4] | 4.1 [1.6, 6.0] | 4.8 [4.3, 5.4] | 0.1545 |
| LDL (mg/dL) | ≤130 | 100.7 (37.6) | 147.9 (39.2) | 119.1 (36.2) | 0.0072 |
| HDL (mg/dL) | >40 | 47.5 [40.0, 56.5] | 53.0 [45.0, 63.0] | 52.5 [39.0, 61.0] | 0.4867 |
| Apolipoprotein A (mg/dL) | Men: 88–180 Women: 98–210 | 134.6 (30.2) | 138.8 (68.7) | 135.85 (39.0) | 0.9763 |
| Aplipoprotein B (mg/dL) | Men: 55–140 Women: 55–125 | 90.1 [73.5, 104.0] | 107.0 [94.0, 123.0] | 103.5 [77.0, 115.0] | 0.1818 |
| Lipoprotein A (mg/dL) | Men: 110–205 Women: 125–215 | 33.5 [9.0, 43.1] | 53.6 [30.3, 58.5] | 24.4 [15.7, 85.0] | 0.6385 |
| Cholesterol (mg/dL) | <225 | 171.0 [147.0, 189.0] | 229.0 [188.0, 242.5] | 182.0 [161.0, 218,0] | 0.0270 |
| Triglycerides (mg/dL) | <250 | 139.0 [106.0, 172.0] | 108.5 [85.0, 181.5] | 122.0 [73.0, 181.0] | 0.8293 |
| Vitamin A (mg/L) | 0.5–2.0 | 0.5 [0.4, 0.6] | 0.4 [0.3, 0.5] | 0.5 [0.5, 0.6] | 0.1163 |
| Vitamin D (ng/mL) | 20–40 | 19.1 (12.6) | 11.3 (11.4) | 19.9 (11.1) | 0.2139 |
| Vitamin E (μg/mL) | 1.5–63 | 10.1 (4.7) | 13.9 (4.9) | 14.4 (4.3) | 0.0110 |
| Vitamin K (μg/L) | 0.13–1.19 | 0.4 (0.3) | 0.5 (0.3) | 0.4 (0.2) | 0.9337 |
| Vitamin B12 (μg/L) | 200–800 | 397.5 [294.0, 446.0] | 460.0 [346.0, 607.0] | 325.0 [239.0, 432.0] | 0.1310 |
| Prothrombin activity (%) | 70–120 | 97.5 [88.5, 103.0] | 104.0 [91.6, 108.5] | 98.0 [86.0, 100.0] | 0.3890 |
| Fecal elastase 1 (μg/g) | >200 | 15.0 [14.0, 61.0] | 125.0 [102.0, 169.0] | 395.0 [294.0, 500.0] | <0.0001 |
Figures are expressed as mean (standard deviation) or median [Q1,Q3]. RBP: retinol binding protein. HbA1c: glicated hemoglobin. LDL: low density lipoproteins; HDL: high density lipoproteins.
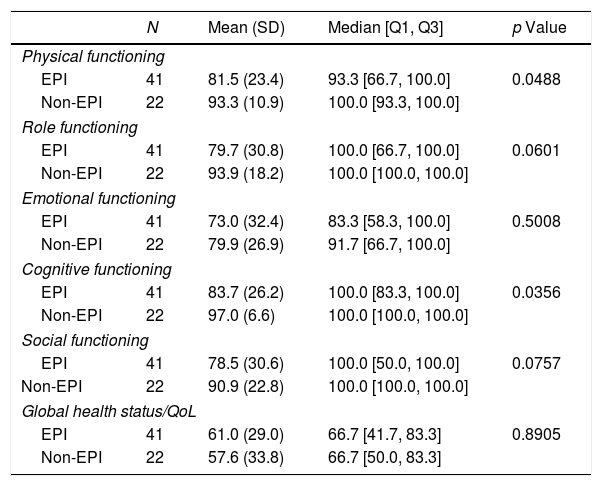
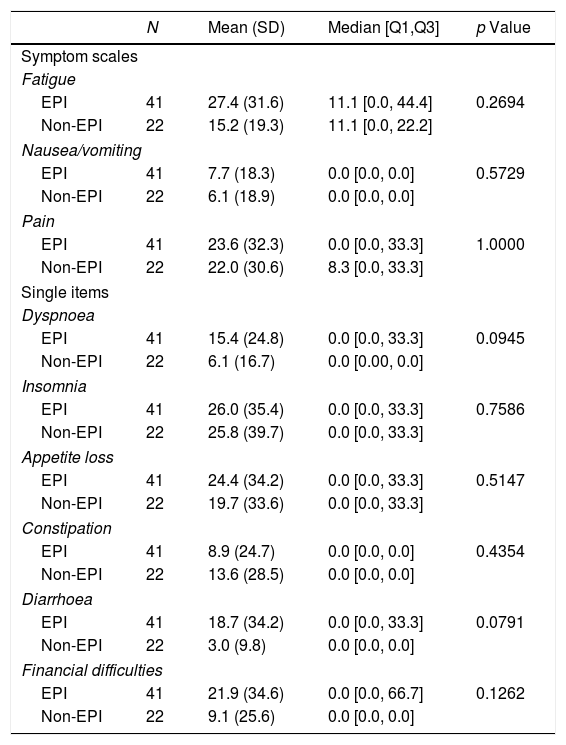
In relation to quality of life, significant differences were detected in functional scores (physical and cognitive functioning) between patients with and without EPI but not in global health status/quality of life, symptoms scales or in single items either (Tables 4 and 5).
Results of the functional scales and global health status/Quality of life of the QLQ-C30 quality of life questionnaire: EPI vs. non-EPI groups.
| N | Mean (SD) | Median [Q1, Q3] | p Value | |
|---|---|---|---|---|
| Physical functioning | ||||
| EPI | 41 | 81.5 (23.4) | 93.3 [66.7, 100.0] | 0.0488 |
| Non-EPI | 22 | 93.3 (10.9) | 100.0 [93.3, 100.0] | |
| Role functioning | ||||
| EPI | 41 | 79.7 (30.8) | 100.0 [66.7, 100.0] | 0.0601 |
| Non-EPI | 22 | 93.9 (18.2) | 100.0 [100.0, 100.0] | |
| Emotional functioning | ||||
| EPI | 41 | 73.0 (32.4) | 83.3 [58.3, 100.0] | 0.5008 |
| Non-EPI | 22 | 79.9 (26.9) | 91.7 [66.7, 100.0] | |
| Cognitive functioning | ||||
| EPI | 41 | 83.7 (26.2) | 100.0 [83.3, 100.0] | 0.0356 |
| Non-EPI | 22 | 97.0 (6.6) | 100.0 [100.0, 100.0] | |
| Social functioning | ||||
| EPI | 41 | 78.5 (30.6) | 100.0 [50.0, 100.0] | 0.0757 |
| Non-EPI | 22 | 90.9 (22.8) | 100.0 [100.0, 100.0] | |
| Global health status/QoL | ||||
| EPI | 41 | 61.0 (29.0) | 66.7 [41.7, 83.3] | 0.8905 |
| Non-EPI | 22 | 57.6 (33.8) | 66.7 [50.0, 83.3] | |
QoL: quality of life; SD: standard deviation.
Results of the symptom scales and single ítems of the QLQ-C30 quality of life questionnaire: EPI vs. non-EPI groups.
| N | Mean (SD) | Median [Q1,Q3] | p Value | |
|---|---|---|---|---|
| Symptom scales | ||||
| Fatigue | ||||
| EPI | 41 | 27.4 (31.6) | 11.1 [0.0, 44.4] | 0.2694 |
| Non-EPI | 22 | 15.2 (19.3) | 11.1 [0.0, 22.2] | |
| Nausea/vomiting | ||||
| EPI | 41 | 7.7 (18.3) | 0.0 [0.0, 0.0] | 0.5729 |
| Non-EPI | 22 | 6.1 (18.9) | 0.0 [0.0, 0.0] | |
| Pain | ||||
| EPI | 41 | 23.6 (32.3) | 0.0 [0.0, 33.3] | 1.0000 |
| Non-EPI | 22 | 22.0 (30.6) | 8.3 [0.0, 33.3] | |
| Single items | ||||
| Dyspnoea | ||||
| EPI | 41 | 15.4 (24.8) | 0.0 [0.0, 33.3] | 0.0945 |
| Non-EPI | 22 | 6.1 (16.7) | 0.0 [0.00, 0.0] | |
| Insomnia | ||||
| EPI | 41 | 26.0 (35.4) | 0.0 [0.0, 33.3] | 0.7586 |
| Non-EPI | 22 | 25.8 (39.7) | 0.0 [0.0, 33.3] | |
| Appetite loss | ||||
| EPI | 41 | 24.4 (34.2) | 0.0 [0.0, 33.3] | 0.5147 |
| Non-EPI | 22 | 19.7 (33.6) | 0.0 [0.0, 33.3] | |
| Constipation | ||||
| EPI | 41 | 8.9 (24.7) | 0.0 [0.0, 0.0] | 0.4354 |
| Non-EPI | 22 | 13.6 (28.5) | 0.0 [0.0, 0.0] | |
| Diarrhoea | ||||
| EPI | 41 | 18.7 (34.2) | 0.0 [0.0, 33.3] | 0.0791 |
| Non-EPI | 22 | 3.0 (9.8) | 0.0 [0.0, 0.0] | |
| Financial difficulties | ||||
| EPI | 41 | 21.9 (34.6) | 0.0 [0.0, 66.7] | 0.1262 |
| Non-EPI | 22 | 9.1 (25.6) | 0.0 [0.0, 0.0] | |
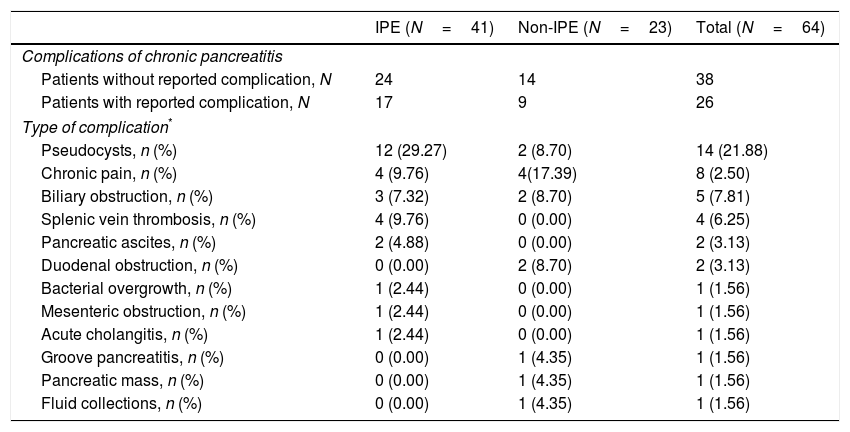
Table 6 shows other complications of CP apart from EPI. Most frequent were pseudocysts (21.9%), chronic abdominal pain (12.5%), biliary obstruction (7.8%) and splenic vein thrombosis (6.3%).
Complications of chronic pancreatitis: EPI vs. non-EPI groups.
| IPE (N=41) | Non-IPE (N=23) | Total (N=64) | |
|---|---|---|---|
| Complications of chronic pancreatitis | |||
| Patients without reported complication, N | 24 | 14 | 38 |
| Patients with reported complication, N | 17 | 9 | 26 |
| Type of complication* | |||
| Pseudocysts, n (%) | 12 (29.27) | 2 (8.70) | 14 (21.88) |
| Chronic pain, n (%) | 4 (9.76) | 4(17.39) | 8 (2.50) |
| Biliary obstruction, n (%) | 3 (7.32) | 2 (8.70) | 5 (7.81) |
| Splenic vein thrombosis, n (%) | 4 (9.76) | 0 (0.00) | 4 (6.25) |
| Pancreatic ascites, n (%) | 2 (4.88) | 0 (0.00) | 2 (3.13) |
| Duodenal obstruction, n (%) | 0 (0.00) | 2 (8.70) | 2 (3.13) |
| Bacterial overgrowth, n (%) | 1 (2.44) | 0 (0.00) | 1 (1.56) |
| Mesenteric obstruction, n (%) | 1 (2.44) | 0 (0.00) | 1 (1.56) |
| Acute cholangitis, n (%) | 1 (2.44) | 0 (0.00) | 1 (1.56) |
| Groove pancreatitis, n (%) | 0 (0.00) | 1 (4.35) | 1 (1.56) |
| Pancreatic mass, n (%) | 0 (0.00) | 1 (4.35) | 1 (1.56) |
| Fluid collections, n (%) | 0 (0.00) | 1 (4.35) | 1 (1.56) |
CP is the most common cause of EPI in adults. Pancreatic function tests have been used over decades for the diagnosis of the disease. Direct tests such as the secretin-pancreozymin test and the endoscopic test are sensitive enough but they are also invasive and cumbersome for routine use in clinical practice. Other tests like the quantification of the coefficient of fat absorption and the 13C-mixed triglyceride breath test12,13 are complex to perform and not widely available. Fecal elastase-1 test is non-invasive and easily applicable in clinical practice. It is useful for detecting moderate to severe EPI but its sensitivity for the diagnosis of patients with non-advanced CP is limited.14
In the present study, we included only patients with CP that had not been followed-up in the last 2 years by a gastroenterologist or a surgeon. These patients are usually managed by general practitioners and may not be included in hospital or gastroenterologist-based surveys. Prevalence of EPI in these patients, with an average time of 5–6 years since diagnosis of CP, was high (64.1%). 70% of patients diagnosed with EPI had levels of FE-1 <100μg/g (widely considered as severe EPI) what means they were in an advanced stage of the disease. An early diagnosis of EPI allows starting treatment with supplemental enzymes in order to avoid morbidity and mortality linked to patient malnutrition. Randomized studies have demonstrated an improvement in coefficients of fat and nitrogen absorption, fecal appearance and quality of life with this treatment.15–18
It has been suggested that, in patients with CP, morphological findings (e.g. pancreatic calcifications, main duct dilation) can be used as an indirect way to diagnose pancreatic exocrine insufficiency.19 In our study 27 (65.9%) and 28 (68.3%) patients from the EPI group had pancreatic calcifications or pancreatic duct dilation, respectively. Corresponding figures in the non-EPI group were 15 (65.2%) and 12 (52.2%). Thus, 3 out of 10 patients with EPI did not show these changes but they were present in more than 50% of patients without EPI. In addition, about 10% of patients with advanced CP had a preserved exocrine function and may not need enzyme supplementation.4 This reinforces the necessity of using a pancreatic function test to confirm the presence of EPI.
In a cross-sectional study of patients with alcoholic and idiopathic CP, exocrine dysfunction was present in 63% of patients after 5 years of CP and in 94% of those with disease lasting 10 years or more.20 Our results are in accordance with the ones of that study, as prevalence of EPI was 64% after an average time of 5–6 years after diagnosis of CP. However, other studies have reported a longer time from the diagnosis of CP to the development of EPI.21 The main etiology of CP in our study was alcohol and tobacco consumption. Smoking is currently proposed to be the driving force behind the progression of pancreatitis.22 In our study 68.3% of patients with EPI were current smokers consuming an average of 20 cigarettes/day and this could have played a role in the development of the exocrine dysfunction. However, in our study we have not observed differences in the smokers’ consumers.
The nutritional status of a patient with CP depends, at least in part, on the duration of the disease, its severity and the etiology of CP. The reasons for a compromised nutritional status are mainly low food intake (due to abdominal pain or nausea after eating), maldigestion and malabsorption. The most frequently studied markers of the nutritional status of patients with CP are: liposoluble vitamins (A, D, E and K which correlate well with severity of steatorrhea), serum cholesterol,23 albumin24 and serum magnesium levels.25 Osteopathy (osteoporosis, osteomalacia, osteopenia) may occur in at least 25% of CP patients and it may be related to vitamin D deficiency.26 Due to inadequate protease secretion by the pancreas, vitamin B12 deficiencies can occur.27 Zinc deficiency may be seen especially in association with diabetes.28 Besides, deficiencies in calcium, thiamine and folic acid have been reported. None of these markers can be taken as an isolated parameter for the diagnosis of EPI, but a combination of them could help in its diagnosis.25
For the assessment of the patients’ nutritional status in our study, anthropometric and laboratory parameters were analyzed. BMI was significantly lower in patients with EPI. This may reflect a worse nutritional status of EPI patients, but we have to bear in mind that both, weight and BMI, do not take body composition into account and may be misinterpreted as a result of fluid changes including ascites and edema.
Regarding laboratory parameters, we only found differences between the EPI and Non-EPI groups in levels of glucose, vitamin A, E and glycated hemoglobin. Significant differences were also present when comparing the severe and mild EPI groups, except for vitamin A. Levels of total cholesterol and LDL cholesterol were also significantly lower, but only in patients with severe EPI compared with those with mild EPI or without EPI. Thus, a decrease in total cholesterol levels could be a marker of advanced stages of EPI. The most frequent vitamin deficiency in patients with CP is vitamin E. It may cause neuromuscular diseases like cerebellar ataxia and amyotrophic myopathy.29
There were no significant differences between groups in the levels of magnesium, albumin, hemoglobin, prealbumin or RBP. Mean levels of magnesium in patients without EPI were 1.93±0.21mg/dL. A study25 suggested that chronic pancreatitis patients with a magnesium value above 2.05mg/dL and normal hemoglobin, albumin, prealbumin, retinol binding protein and HbA1C are very unlikely to suffer from PEI and do not require further evaluation of pancreatic exocrine function. Due to the relatively low levels of magnesium in our study population, this rule could not have been applied for the majority of our patients.
Diabetes due to the destruction of islets in patients with CP is termed type 3c diabetes. It is more common than previously believed and might represent at least 8% of all patients with diabetes.30 It has been suggested that β-cell regeneration is disturbed in pancreatic diseases, which could explain reduced β-cell mass and diabetes in CP. In our study, the prevalence of diabetes was significantly higher in the EPI group (61% vs. 17.4%, p=0.0008). Glucose levels and glycated hemoglobin were significantly higher in patients with EPI compared with those without EPI. We also found a relationship between severity of EPI and levels of glucose and HbA1c, being higher in those with severe EPI. Exocrine pancreatic and diabetes are usually developed in parallel. In contrast, clinical observations lead to the notion that exocrine pancreatic disease is a critical factor for development rather than a sequel to diabetes.31,32
CP is a long-term, sometimes progressive and debilitating condition that may have an important impact on QoL. Symptoms like severe abdominal pain and consequences of diabetes mellitus, malabsorption and weight loss could severely affect health-related QoL.8 In our study, the presence of EPI contributed to aggravate even more the QoL of patients with CP. Cognitive and physical functioning were significantly worse in patients with EPI compared with those without EPI. It has been shown that QoL in patients with EPI improves with pancreatic enzyme supplements.33,34 Thus, an early diagnosis of EPI in these patients could lead to a better QoL by starting an appropriate treatment.
Regarding other complications of CP, presence of pseucocysts was the most frequent (19.4% of study population). This prevalence is higher than previously described35 (about 10%) and could be related to a more advanced stage of the disease in our patients. Chronic pain (12.5%), biliary obstruction (7.8%) and splenic vein thrombosis (6.3%) were other frequent reported complications.
Our study have some possible limitations, we used an indirect test (FE-1) to diagnose EPI. It is well known that indirect tests have a lower sensitivity for the diagnosis of early stages of EPI and, because of that, prevalence of EPI in our study population could be underestimated. Though we found some differences in laboratory parameters between both groups, it doesn¿t necessarily mean that EPI was the cause of those differences, because vitamin deficiencies and cholesterol levels could have been explained by other diseases or treatments.
In conclusion, according to the results of our study, prevalence of EPI is high in a population that is not usually included in epidemiological studies of CP (patients without follow-up by gastroenterologists). Presence of EPI was associated with a poorer quality of life and some nutritional deficiencies. Thus, an adequate follow-up of these patients is warranted.
Funding statementThis Study was funded by Mylan Spain.
Together with investigators from the Pancreatic Unit, H. General Universitario de Alicante and Hospital Universitario Araba, Vitoria-Gasteiz, the sponsor was involved in the design of the study and review of planned study procedures.
Pivotal S.L., Madrid, Spain, which was contracted by the study sponsor, carried out trial monitoring. The sponsor provided logistical support during the trial. Investigators and study staff at each site collected data. The sponsor worked with Pivotal S.L., Madrid, Spain, to prepare the statistical analysis plan and the analysis was performed by Pivotal. Together with the study investigators, the sponsor participated in the interpretation of data, writing of the manuscript, and the decision to submit the manuscript for publication. Medical writing assistance for this manuscript was provided by Javier Ortega, PhD, Pivotal S.L., Madrid, Spain and was funded by the study sponsor.
Conflict of interest statementE. Labrador Barba and M.L. Orera Peña are employees of Mylan.
Enrique de-Madaria and Carlos Marra-Lopez have received a grant from Mylan and have consulted Mylan in the development and performance of clinical research in pancreatic exocrine insufficiency.
Emma Martinez-Moneo, Esperanza Perez, Andres del Pozo and María Francisco have given lectures sponsored by Mylan partially related with the submitted work.
The authors would like to express gratitude to the following co-investigators for their contributions to this study: Fidencio Bao-Hospital San Eloy, Aitor Orive-Calzada – Hospital Universitario Araba Vitoria-Gasteiz, Maria Luisa Vignote Alguacil – Hospital Universitario Reina Sofia and Eduardo Bajador Andreu – Hospital Universitario Miguel Servet; and Jorge Puig de la Bellacasa - Mylan.
Previous presentations: Preliminary analysis has been successfully presented at the 47th Annual Meeting of the European Pancreatic Club, 24–26th of June 2015 in Toledo, Spain; at the XIV Meeting of the Spanish Pancreatic Club, 26–27th of June in Toledo, Spain, at the United European Gastroenterology Week, 24–28th of October 2015 in Barcelona, Spain. Final analysis has been successfully presented at the Sociedade Portuguesa de Gastroenterología, 1st to 4th of June 2016, Algarve Portugal; at the LXXV Congress of the National Digestive Society 17–19th of June, 2016, in Santiago, Spain; 48th Annual Meeting of the European Pancreatic Club, 6–8th of June 2015 in Liverpool, United Kingdom and at the United European Gastroenterology Week, 15–19th of October, 2016. Vienna, Austria.