Video capsule endoscopy (VCE) is a reliable noninvasive method for examination of small-bowel mucosa. However, it has some limitations. The aim of this article was to review the approach in patients with negative VCE. It is clear that a negative VCE should be interpreted based on the indication. In suspected small bowel bleeding (SSBB), patients with ongoing/recurrent overt bleeding, or occult bleeders who experience significant declines in hemoglobin after a negative VCE should proceed small bowel study; on the other hand, patients with occult SSBB and only mild-moderate anemia should be managed with supportive care. In inflammatory bowel disease, a normal VCE has a very high sensitivity and negative predictive value. In small bowel tumor suspicion there is a high risk of false negative results, so another imaging modality should be considered. In polyposis syndromes, if VCE is negative, patients should continue screening within 2–3 years.
La videocápsula endoscópica (VCE) es un método fiable no invasivo para la exploración de la mucosa del intestino delgado. Sin embargo, presenta algunas limitaciones. El objetivo de este artículo fue revisar el abordaje de algunos pacientes con VCE negativa. Está claro que una VCE negativa debe interpretarse en función de la indicación. En la sospecha de hemorragia del intestino delgado (SSBB, por sus siglas en inglés), los pacientes con hemorragia manifiesta persistente/recurrente o sangradores ocultos que sufren descensos considerables de la hemoglobina después de una VCE negativa deben continuar con un estudio del intestino delgado; además, los pacientes con SSBB oculta y solo anemia de leve a moderada deben ser tratados con tratamiento de apoyo. En la enfermedad inflamatoria intestinal, una VCE normal presenta una sensibilidad muy alta y un valor pronóstico negativo. Cuando se sospecha de tumor de intestino delgado, se corre un elevado riesgo de resultados falsos negativos, por lo que se debe considerar otra modalidad de prueba de diagnóstico por la imagen. En los síndromes de poliposis, si la VCE es negativa, se debe volver a realizar la prueba en los pacientes dentro de 2–3 años.
The small bowel has always been difficult to evaluate by endoscopic and radiologic techniques. Small bowel follow through was the only diagnostic tool for suspected small bowel disease until the end of the last century.1
Deep enteroscopy using balloon-assisted or spiral techniques, computerized tomography (CT) and magnetic resonance (MR) enteroclysis or enterography have emerged in recent years, facilitating diagnosis, monitoring, and management of patients with small bowel diseases. These technologies are complementary, each with their own advantages and limitations.2,3
Video capsule endoscopy (VCE) was introduced into clinical practice in 2001. Over the last years, an increasing number of publications have shown that VCE is a reliable and noninvasive method for endoscopic examination of the entire small-bowel mucosa.2,3
However, it has some limitations: not all VCE procedures result in complete examinations as the small-bowel VCE completion rate is about 80%4; the presence of food residue, air bubbles and turbid or green viscous intraluminal fluid limits small bowel visualization3; and there are lesions which might be easily overlooked like lesions in the duodenum and the proximal jejunum, lesions in the afferent limb of the reconstructed intestine after surgery or small bowel diverticula.5
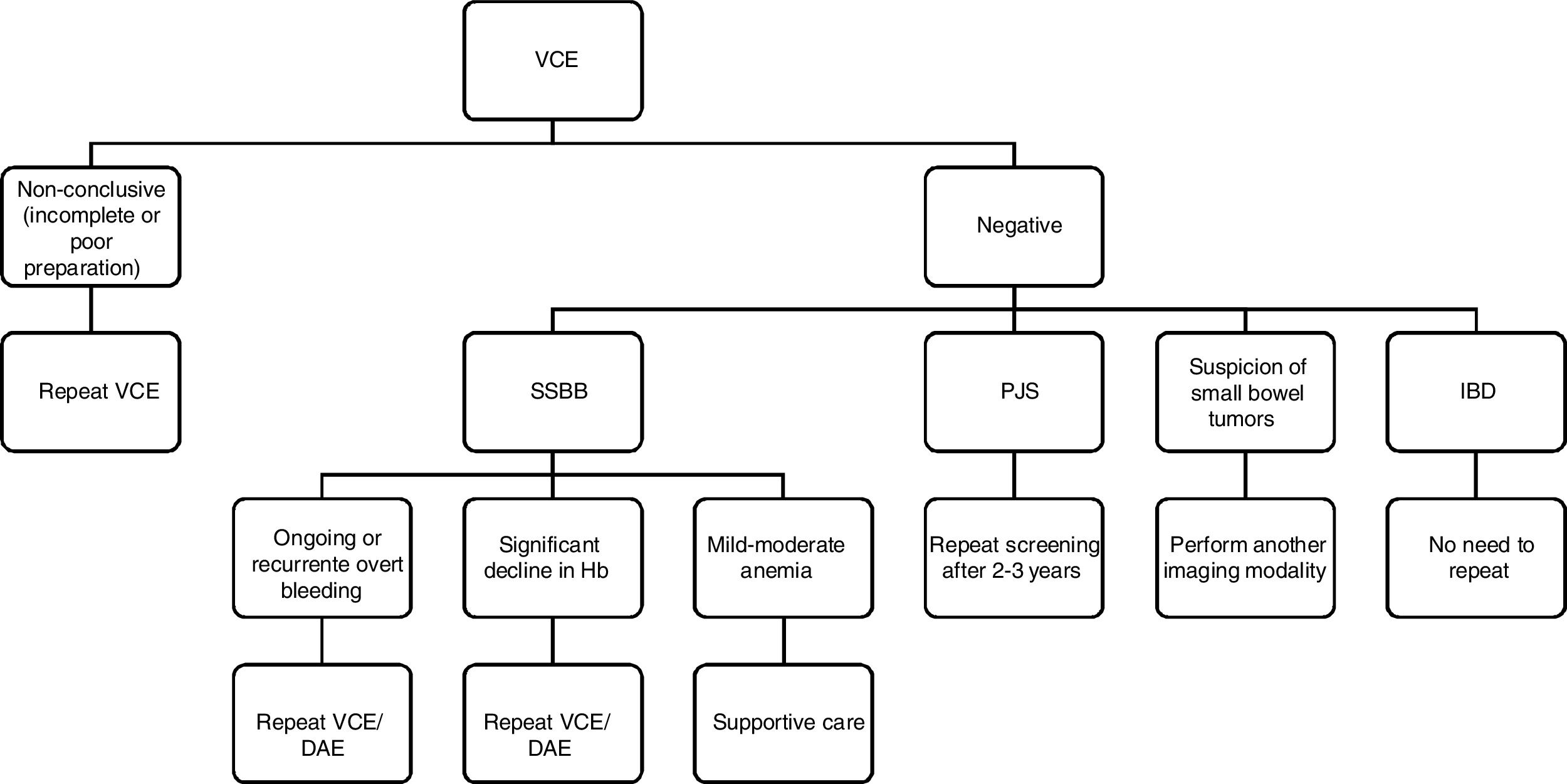
The aim of this article was to review the evidence in what should be the approach in a patient with a negative VCE. The difficulty begins in defining what is a negative VCE. For example, a negative VCE in the setting of a suspected Inflammatory bowel disease (IBD), is certainly more negative than in the setting of suspected small bowel bleeding (SSBB).
So the questions to be answered are: is a negative VCE non-conclusive, or simply negative? Should we always perform device assisted enterosopy (DAE) or another imaging modality when we have a negative VCE?
For the purpose of this review a non-conclusive VCE was defined as a VCE that did not reach the cecum or has not a clear visualization of the small bowel due to poor preparation or luminosity and a negative VCE was defined as a VCE that reach the cecum with good preparation but with negative findings.
A great amount of data about a positive VCE and its therapeutic and prognostic implications are available. However, there is insufficient evidence on the outcomes of patients with a negative VCE.6
Non-conclusive capsule endoscopy – incomplete examination or poor preparationThe completion rate of small-bowel VCE is about 80%.7,8 Retrospective studies have identified predictive factors of incomplete small-bowel VCE examinations such as inpatient status, previous abdominal surgery and poor bowel cleansing.8 The effects of diabetes mellitus and of a greater age are controversial. In a patient with incomplete VCE the procedure should be repeated. Furthermore, patients at increased risk for incomplete examinations may benefit from the use of the real-time viewer during the procedure or/and intervention with prokinetics or endoscopic placement of the capsule in the duodenum.7,8
Cleansing is of great importance for VCE because there is no possibility of flushing or suctioning and mucosal visualization becomes impaired by the presence of air bubbles, bile and intestinal debris.9,10
Purgative bowel cleansing before VCE improves the quality and increases the diagnostic yield of the examination in comparison to a clear liquids diet.4
The current standard preparation remains to be established, although the majority of the evidence recommends PEG-based regimens as the first line but with no defined recommendation regarding the timing and dosing.11
Taking into account that after a colonoscopy with poor preparation it is recommended to repeat it within a year, it would be reasonable to apply the same rationale and repeat VCE with poor preparation, but data and universal recommendations are lacking.
Impact of positive or negative VCE findings in different pathologiesSuspected small bowel bleedingBleeding from the small bowel occurs in approximately 5% of patients with gastrointestinal hemorrhage and this represents the most frequent indication for small-bowel VCE.2,3 Obscure gastrointestinal bleeding (OGIB) is defined as occult or overt bleeding of unknown origin that persists or reoccurs after a negative endoscopic evaluation (endoscopy and colonoscopy). Recently, with the advances in small bowel imaging, this term has been proposed for patients whose source of bleeding cannot be identified anywhere in the gastrointestinal tract.2
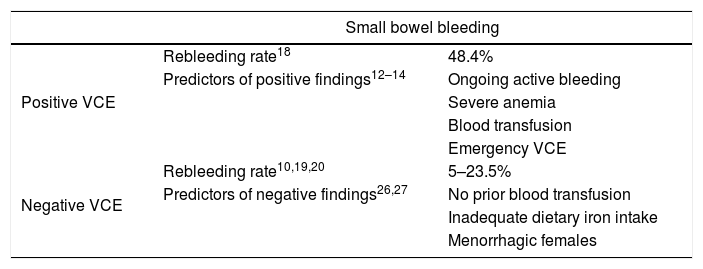
The diagnostic yield of VCE is significantly higher in ongoing, active bleeding compared to occult bleeding and in patients with more severe anemia and increased blood transfusion requirements (Table 1).12–14
Rebleeding and predictors of positive and negative VCE in small bowel bleeding.
| Small bowel bleeding | ||
|---|---|---|
| Positive VCE | Rebleeding rate18 | 48.4% |
| Predictors of positive findings12–14 | Ongoing active bleeding | |
| Severe anemia | ||
| Blood transfusion | ||
| Emergency VCE | ||
| Negative VCE | Rebleeding rate10,19,20 | 5–23.5% |
| Predictors of negative findings26,27 | No prior blood transfusion | |
| Inadequate dietary iron intake | ||
| Menorrhagic females | ||
VCE: video capsule endoscopy.
In patients who undergo emergency VCE (within 48h) the diagnostic return and therapeutic yield are higher and the rebleeding rate is lower.15,16
In a large retrospective study, the diagnostic yield of VCE decreased each day after admission from 55% at day 1, 48% at day 2, 29% at day 3, 27% at day 4 and 18% at day 5.17
Nevertheless, the true long-term outcomes of a positive VCE in terms of patient's overall outcome (intervention, rebleeding and mortality) remains poorly defined.18 Although some studies have reported a higher rebleeding rate in patients with positive VCE than in those with negative VCE, other studies reported no differences in outcomes (although specific treatments may be responsible for lowering the rebleeding rates after positive VCE).18
After a negative VCE, the rate of rebleeding in patients with SSBB and negative VCE is around 5–23.5%.19,20
This rate is significantly higher in patients who need more transfusions of packed red cells before VCE and in overt bleeding.20 Rebleeding occurs mostly within the following 2 years so medical surveillance during the above-mentioned period seems advisable.21
In a recent meta-analysis from Yung et al, the re-bleeding after a negative VCE for OGIB was 0.19 compared to 0.29 in patients with a positive VCE (p<0.001). However, this study did not demonstrate any statistically significant effect of receiving treatment for OGIB.22
So, how to manage patients with ongoing bleeding or persistent anemia despite iron therapy after a negative VCE? In 2 randomized control trials comparing different capsules in patients who underwent 2 consecutive VCE examinations, discordant results between the 2 VCE procedures were reported in approximately 16% of the cases.23,24
The occurrence of overt bleeding and a hemoglobin decrease of 4g/dL or more have been found to be significant predictive factors for a positive second VCE.25
Contrarily, patients with less severe anemia (no prior blood transfusions), those with inadequate dietary iron intake and menorrhagic females are less likely to present with clinically relevant findings in VCE (Table 1).26,27
In Western counties, two thirds of patients bleed from vascular lesions, half of patients treated with argon plasma coagulation rebleed within 3–5 years and the spontaneous cessation of bleeding is ∼45%/year.28,29 Further endoscopic treatment sessions for angioectasias may be beneficial due to the relative effective reduction of rebleeding in a sub-group of patients.30
So, after a negative VCE, only a small percentage of patients that did a second VCE will benefit from DAE for treatment, and, of these, only half will have a long-term response. The counter argument is that there is evidence that DAE improves hemoglobin and decreases blood transfusion requirements. And, a 50% rebleed rate at 3–5 years means that the other 50% of patients are no longer bleeding.29,31
In conclusion, and according to ESGE guidelines, after a negative VCE, patients with ongoing or recurrent overt bleeding, or occult bleeders who experience significant declines in hemoglobin, should proceed with either repeat VCE or with DAE, after an initially negative VCE. On the other hand, patients with occult OGIB and only mild-moderate anemia persisting after iron therapy, should be managed with supportive care only.3 If the initial VCE is non-conclusive, then the patient should undergo further diagnostic work-up, such as repeat VCE, CT/MR enterography or DAE to exclude pathology from the unexamined small bowel.
Inflammatory bowel diseaseVCE has a role in the diagnostic work-up and management of selected patients with suspected or established Crohn's Disease (CD) and in Inflammatory Bowel Disease Unclassified (IBDU). The exact place of VCE within the diagnostic algorithm is yet to be clearly defined.
According to ECCO guidelines from 2017, VCE should be reserved for patients in whom the clinical suspicion for CD remains high despite negative evaluations with ileocolonoscopy and radiological examinations.32
In 2 studies in patients who have negative or inconclusive initial evaluations, VCE led to an incremental diagnostic yield of 24% and showed good sensitivity (93%).33,34
VCE also as an important role in inflammatory bowel disease type unclassified (IBDU) since it enables visualization throughout the small bowel and it contributes for the reclassification of IBDU – a Lewis Score >95% has a sensitivity of 90% and specificity of 100% for the diagnosis of CD.35
VCE is only limited by a lack of specificity. Indeed, over 10% of healthy subjects demonstrate mucosal breaks and erosions in their small bowel.32
In conclusion, a normal VCE examination in IBD has a very high sensitivity and negative predictive value, essentially ruling out small bowel CD. Taking that into account, there is no need to repeat VCE, except in cases of incomplete capsule examinations or poor bowel preparation.
Small bowel tumorsSmall bowel tumors are rare and account for <5% of all gastrointestinal neoplasms.36
There is no definite clinical manifestation suggestive of a small bowel tumor so they are detected with a frequency of around 4% in VCE with various indications such as SSBB, iron deficiency anemia, unexplained abdominal pain and others.37
The findings of small bowel tumors in VCE are not always easily interpreted and there is a high detection miss rate for isolated mass lesions up to 20%.38–40
According to Japanese guidelines, it is recommended that large tumors in the small bowel be examined first with CT, MRI or enteroclysis rather than CE, because the latter can result in a false negative finding. On the other hand small neoplasms or flat lesions are better detected with CE.5
In patients with suspected small bowel tumors, CT or MR enterography allow examination of the lumen, bowel wall and external structures.
For patients with suspicion of a small bowel tumor, MRE showed an overall sensitivity, specificity, and accuracy in identifying patients with small-bowel lesions of 86%, 98%, and 97%, respectively.40
CT enteroclysis can detect tumors as small as 5mm with a reported sensitivity and specificity of 95% and 100%.41
In DAE, when we have a suspicion by other imaging modalities, the detection rate is high (47–52%).29,42
The majority of small-bowel malignancies found by VCE are gastrointestinal stromal and neuroendocrine tumors that originated from the submucosal layer. VCE presents limitations since the lesions cannot be characterized by washing, insufflating air or taking a biopsy specimen.43,44
In conclusion, if a small bowel tumor is highly suspected after a negative VCE another imaging modality should be performed regarding the risk of false negatives, especially in tumors located in the duodenum or proximal jejunum due to rapid transit or in patients with subepithelial tumors with an intact overlying mucosa.
Polyposis syndromesSmall-bowel polyps occur in more than 75% of familial adenomatous polyposis (FAP) and Peutz-Jeghers syndrome (PJS) patients.44,45
VCE has been shown to detect more and smaller jejunal–ileal polyps than other imaging modalities including contrast radiography and MR enteroclysis and enterography and has similar detection rates to DAE.46,47
MR imaging provides a better estimation of the exact site and size of the polyps but VCE is superior at detecting smaller small bowel polyps; polyps of 15mm and above are equally detected by both modalities.48,49
Because of high risk of gastrointestinal polyp-related complications and the demonstrated diagnostic yield of VCE it is recommended as part of ongoing surveillance for patients with polyposis syndromes, especially those with PJS.
If VCE is negative or small polyps are found, screening should be repeated within 2–3 years. If polyps have a size above 10–15mm a DAE should be performed.50
Patients with Peutz–Jeghers syndrome (PJS) and small-bowel polyps are currently managed almost exclusively endoscopically precluding complications like intussusception, bleeding, and eventually malignancy, avoiding multiple surgeries.51,52
In conclusion, if VCE is non-conclusive, screening should be repeated, either with another VCE examination or MRE. If VCE is negative screening should be repeated within 2–3 years
ConclusionAfter reviewing the evidence available for VCE, it is clear that a negative VCE should be interpreted based on the indication of the procedure (Fig. 1).
On the other hand, in all indications, if VCE is incomplete or non-conclusive due to bowel preparation or light artifacts, it should be repeated to allow visualization of the entire small bowel mucosa.
In SSBB, after a negative VCE, patients with ongoing or recurrent overt bleeding, or occult bleeders who experience significant declines in hemoglobin, should proceed with either repeat VCE or with DAE, after an initially negative VCE; conversely, patients with occult SSBB and only mild-moderate anemia persisting after iron therapy, should be managed with supportive care.
In IBD, a normal VCE examination has a very high sensitivity and negative predictive value so there is no need to repeat the procedure.
When there is suspicion of small bowel tumors, even after a negative VCE, another imaging modality or even DAE should be considered, since VCE has a high miss rate for isolated mass lesions.
In polyposis syndromes, if VCE is negative or only small polyps are found, VCE should be repeated for screening within 2–3 years.
Supportive foundationsThe authors declare no source of funding for this article.
Conflicts of interestThe authors declare no conflicts of interest.