To analyse the relationship between doses of gemcitabine–carboplatin (GEM-CARBO) administered and incidence and level of haematological and renal toxicity, and the adherence to the treatment in patients with non-small cell lung cancer.
MethodsRetrospective study, which lasted for 37 months. We were able to obtain the minimum set of data needed to carry out the follow-up with the help of Farmis-Oncofarm® software and the medical and pharmacotherapeutic records.
The haematological toxicity was assessed in accordance with the Common Toxicity Criteria 3.0. Renal toxicity was evaluated using serum creatinine levels and creatinine clearance.
ResultsThirty-one patients were included in the study who were administered a total of 122 cycles. There was a 34.0% and 30.8% incidence of anaemia and grade 3 neutropenia, respectively. There was also a 3.8% and 7.7% incidence of grade 3 and grade 4 thrombocytopenia, respectively. No cases of renal toxicity were found. 65.0% of patients received more than 85.0% of the planned theoretical dosage of carboplatin and 58% of patients received more than 85.0% of the planned theoretical dosage of gemcitabine. Administration was delayed in 18.0% of the cycles prescribed.
ConclusionsThe indication and prescription of the GEM-CARBO regimen was adjusted in accordance with solid scientific evidence, but its haematological toxicity limited its use and made it difficult to maintain the dose intensity foreseen in the study. This compromised the effectiveness of the treatment.
Analizar la relación entre las dosis administradas de gemcitabina-carboplatino (GEM-CARBO) y la incidencia y grado de toxicidad, hematológica y renal, y la adherencia al tratamiento en pacientes con cáncer de pulmón no microcítico.
MétodosEstudio retrospectivo de 37 meses de duración. El conjunto mínimo de datos para realizar el seguimiento de los pacientes se obtuvo con ayuda del programa informático Farmis-Oncofarm® y de las historias clínicas y farmacoterapéuticas.
La toxicidad hematológica se evaluó de acuerdo con la Common Toxicity Criteria 3.0. La toxicidad renal se valoró a partir de los datos de concentración sérica de creatinina y el aclaramiento de creatinina.
ResultadosSe han incluido en el estudio 31 pacientes a los que se les administraron un total de 122 ciclos. La incidencia de anaemia y neutropenia grado III fue de un 34,0 y un 30,8%, respectivamente, de trombocitopenia de grado III del 3,8% y de grado IV del 7,7%. No se ha identificado ningún caso de toxicidad renal. El 65,0% de los pacientes recibieron más del 85,0% de la dosis de carboplatino teórica planeada y el 58,0% de los pacientes recibieron más del 85,0% de la dosis de gemcitabina teórica planeada. Se retrasó la administración en el 18,0% de los ciclos prescritos.
ConclusionesLa indicación y prescripción del esquema GEM-CARBO se ha ajustado con unas evidencias científicas sólidas, pero su toxicidad hematológica ha limitado su uso y ha dificultado la administración de la intensidad de dosis prevista comprometiendo la efectividad del tratamiento.
Lung cancer is the most common type of cancer around the world. It is estimated that its total incidence rate increases by 0.5% yearly, and it is the leading cancer-related cause of death in both men and women, with a male-to-female ratio of 11:1.1,2
Between 75% and 80% of the cases diagnosed with lung cancer are categorised as non-small cell (NSCLC), and at the time of diagnosis, more than half of all patients present inoperable or stage IV metastatic cancer.3 For patients who do not receive antineoplastic treatment, survival median time ranges between 4 and 5 months.4,5
Surgery is the best curative option when the disease is diagnosed in an early stage (1-II), or in certain select cases of locally advanced disease (stage IIIa).6 At the time of diagnosis, more than 40% of patients are not candidates for surgery, either because they present distant metastasis or because the cancer is advanced (stage IIIb-IV). For the latter, the best treatment option is antineoplastic chemotherapy, which has been shown to be better than the best supportive care options.7 The fact that there are so many available oncological regimens, added to their variability and complexity, requires the participation of an interdisciplinary team to guarantee better treatment quality for the patient.1,8
Doublets consisting of platin salts−cisplatin or carboplatin (CARBO)−in combination with gemcitabine (GEM), paclitaxel, vinorelbine, docetaxel or pemetrexed are the most frequently used pharmacotherapeutic regimes in advanced NSCLC; the cisplatin doublet is the first line of treatment.6–10 These doublets were compared to each other, and the results indicate that while their efficacies are similar, they produce different toxicity profiles.6–15 Analysis of the studies reveals that combinations with CARBO are less toxic and have a higher level of patient acceptance than combinations with cisplatin do.16
Studies performed in patients with advanced or metastatic NSCLC treated with the GEM-CARBO chemotherapy doublet show a similar degrees of efficacy measured in terms of global response, survival, mean progression time and 1-year survival when inter-cycle periodicity is maintained (21 days) with full doses of both CARBO (target area under curve [AUC] for plasma concentration-time 5mg/ml/min) and GEM (day 1 and 8 of the cycle with doses of 1200mg/m2). However, the profile and the degree of haematological toxicity vary depending on the literature one consults. Similarly, the incidence of grade III and IV anaemia varies between 2.1% and 18%; grade III and IV neutropenia, between 2.6% and 34%; and grade III and IV thrombocytopenia, between 13.8% and 32.6%.13–15
The purpose of this study is to analyse the relationship between the GEM-CARBO doses administered and the incidence and degree of treatment-derived haematological and renal toxicity in patients with advanced NSCLC (IIIb-IV), and adherence to that treatment.
MethodRetrospective study was carried out in Valencia's Hospital Universitario Dr. Peset with patients diagnosed with advanced NSCLC who began treatment with the GEM-CARBO regimen between January 2006 and January 2009 (37 months). In Hospital Universitario Dr. Peset in Valencia, this regimen is the first line of treatment for NSCLC patients who are elderly, have a confirmed intolerance of cisplatin, and/or have kidney failure.16–19
The GEM-CARBO regimen consists of GEM 1250mg/m2, administered by intravenous perfusion during 30min on days 1 and 8 of the cycle, and CARBO dosed to reach a target AUC of 5mg/ml/min, administered in a continuous 60min perfusion on day 1 of the cycle. Patients older than 65 years and a performance status less than or equal to 2 (Eastern Cooperative Oncology Group [ECOG])20 received doses to achieve a target AUC of 4mg/ml/min in order to decrease the probability of morbidity associated with carboplatin.19,21,22
The CARBO dose was calculated using the Calvert23 formula: D=AUCtarget×(GFR+25), in which the Cockcroft–Gault (CG)24 formula is used as the standard for calculating glomerular filtration rate (GFR).
The mean number of predefined cycles for this regimen, with a pre-defined inter-cycle period of 21 days, is 4 cycles if the disease is stable or 6 cycles if the patient responds to treatment.6,9,19 This regimen was suspended in patients who experienced disease progression or high levels of toxicity during treatment.
Haematological toxicity was managed by delaying the chemotherapy period in order for haematological values to recover, adjusting the dose and secondary prophylaxis with erythropoietin and colony-stimulating factors (CSFs).13–19,22
Data needed for clinical and drug treatment follow-up on patients were obtained from the Farmis-Oncofarm® programme, and from patients’ medical and drug histories.
We recorded the anthropometric variables of age, weight, height, and performance status.
The theoretical doses (TD) of antineoplastic drugs were calculated on a case-by-case basis for the first cycle of the GEM-CARBO regimen in order to evaluate variability with respect to the prescribed dose (PD). We accepted dosage differences (DD) of ±10% for CARBO based on the fact that we used several formulas to calculate it and approximated GFR by means of CrCl. We accepted a DD of ±5% for GEM.16,25–27 DD was calculated using the following formula: DD=[(PD−TD)/TD]×100.
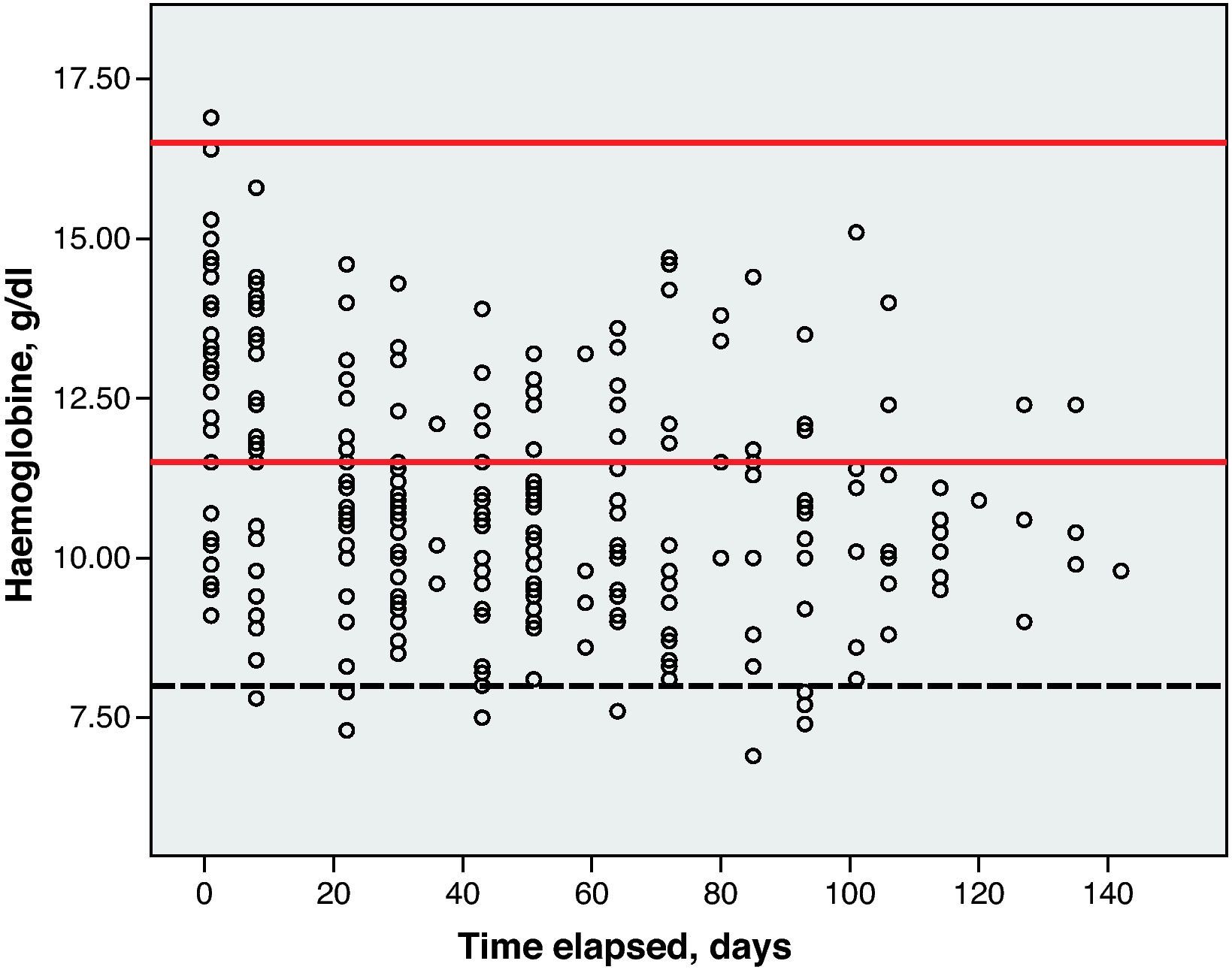
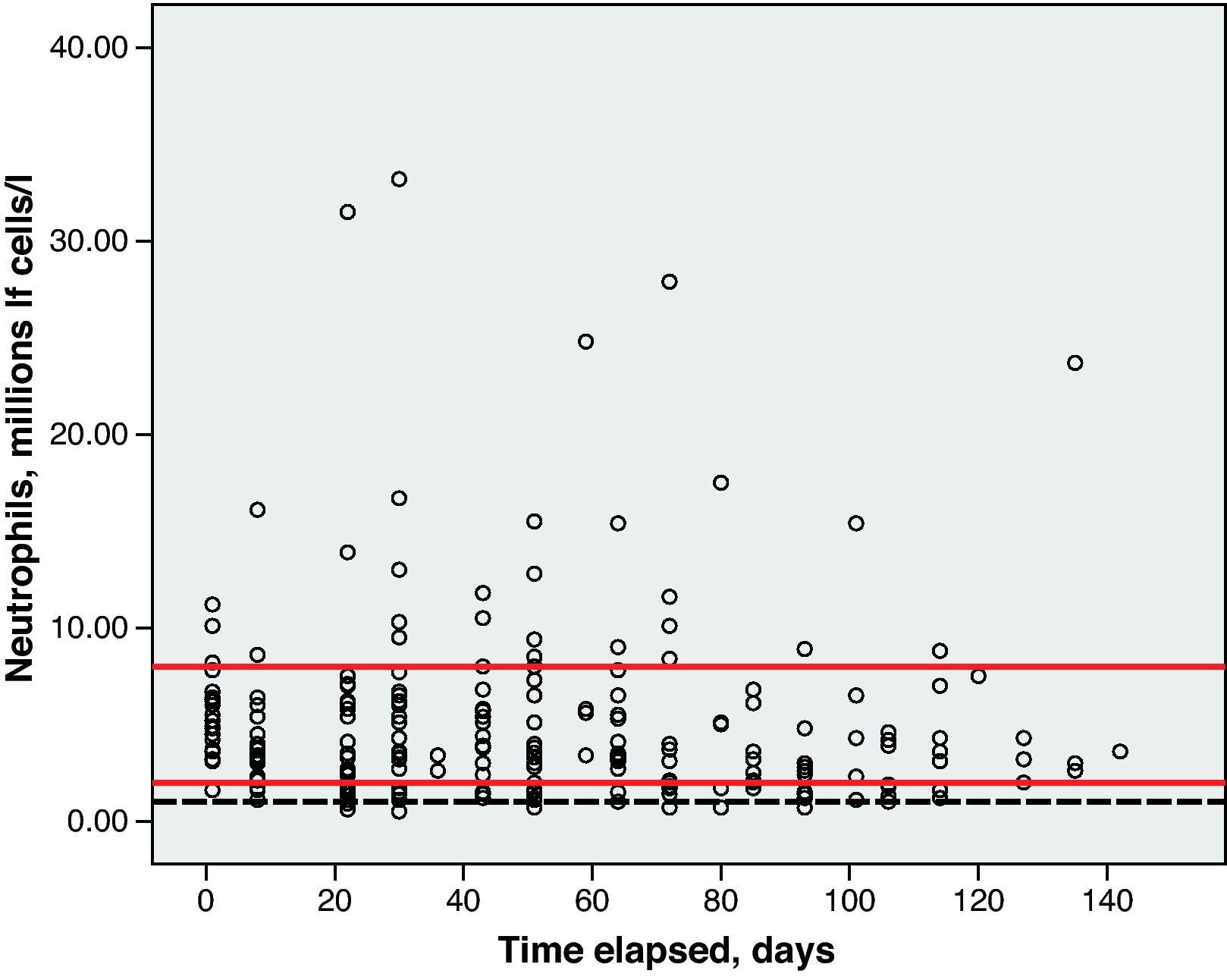
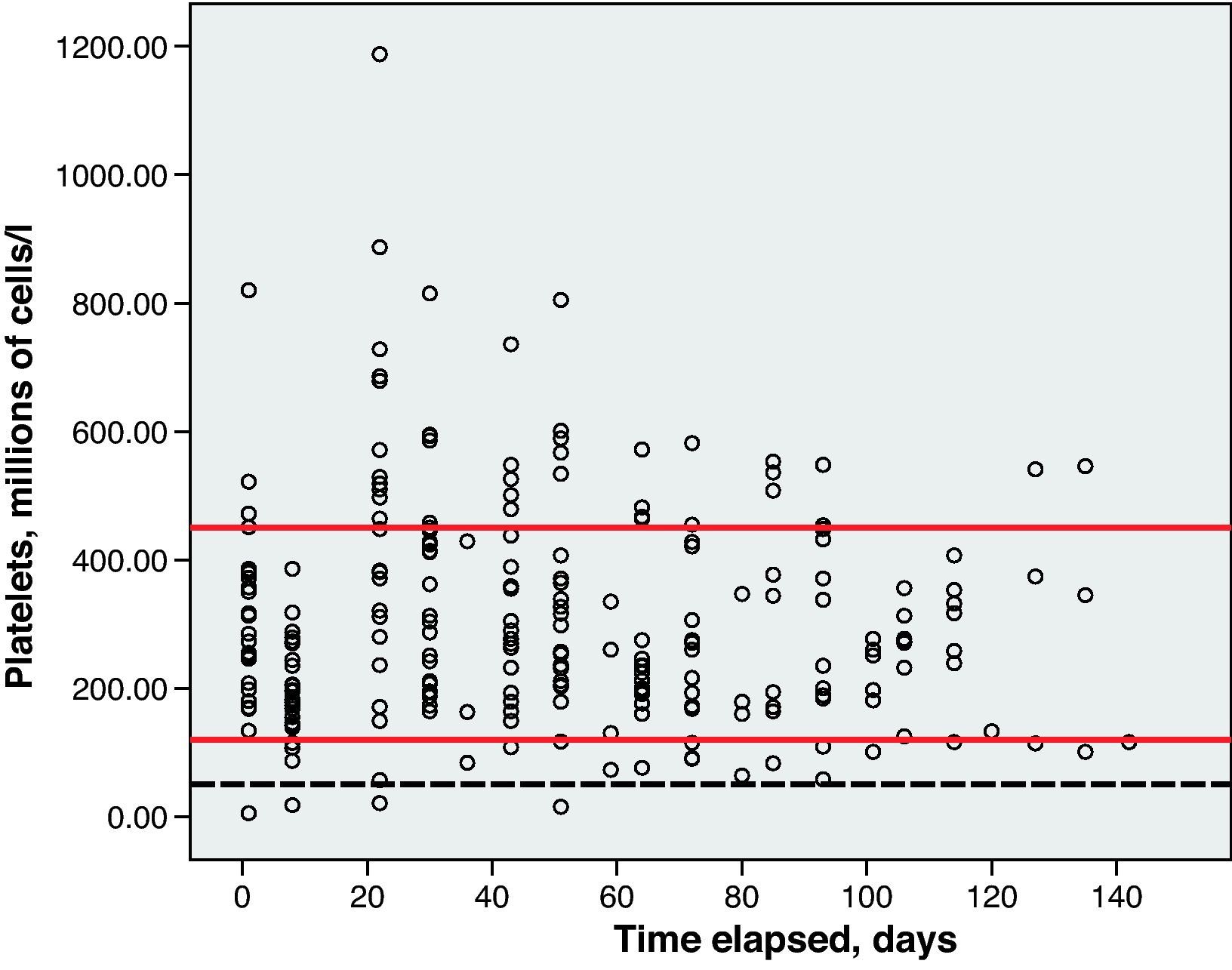
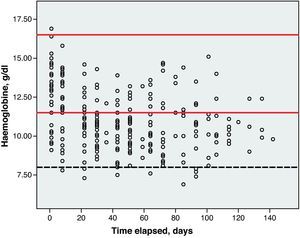
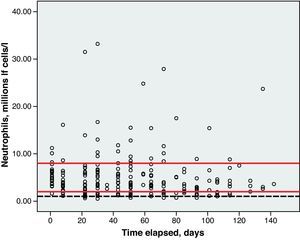
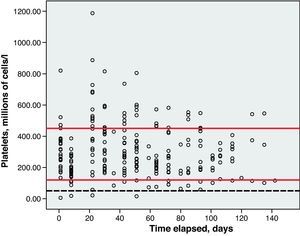
Haematological toxicity was evaluated according to the Common Toxicity Criteria (CTC) 3.0 based on haemoglobin (Hb), absolute neutrophil count (ANC) and platelet counts measured during treatment. The normal range for Hb defined in this hospital is between 11.5 and 16.5g/dl; for ANC, 2–8×109cells/l; and for platelets, values between 120 and 450×109cells/l.
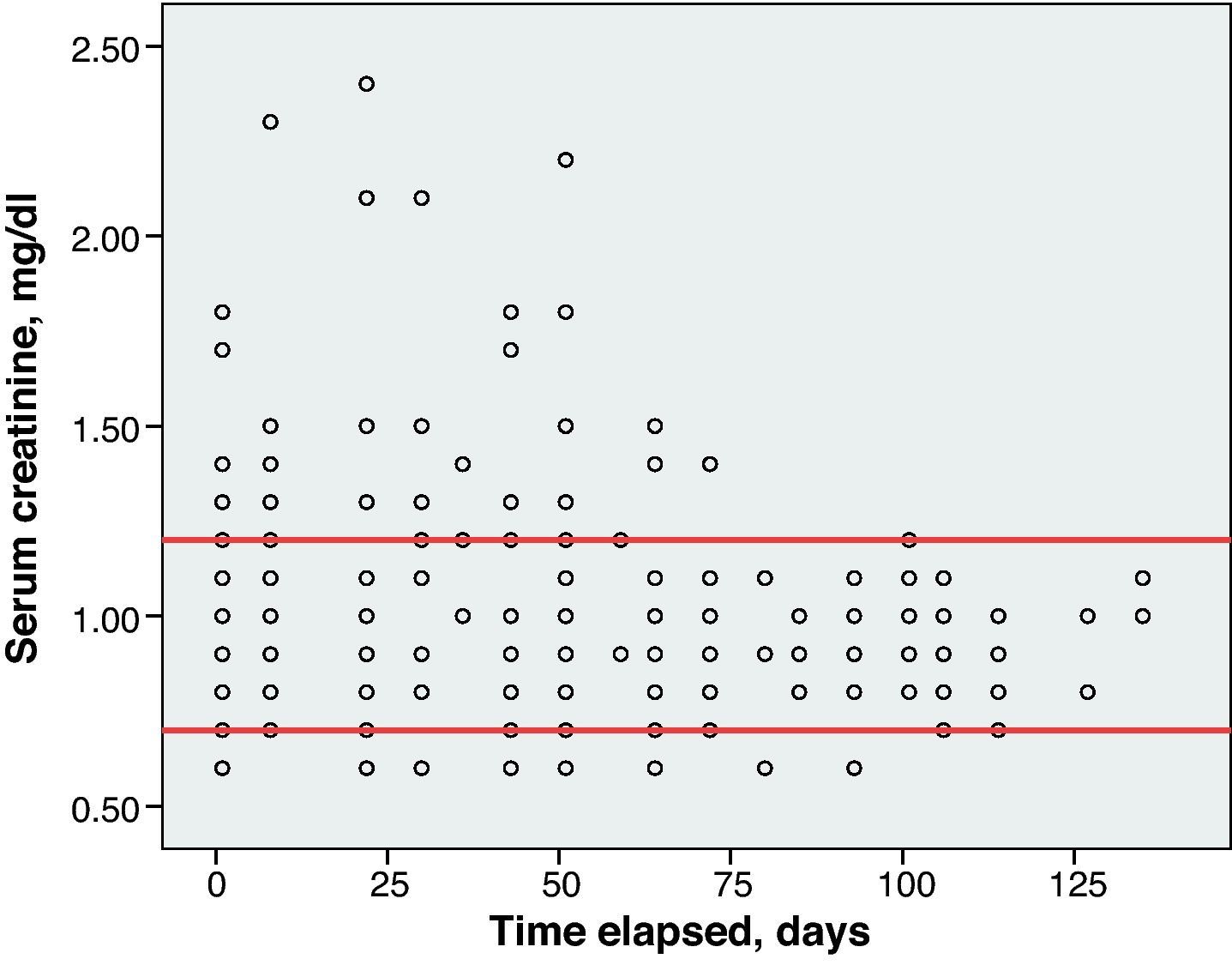
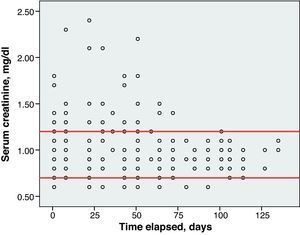
Renal toxicity was evaluated based on the data for serum creatinine (Cr) levels and creatinine clearance (CrCl) calculated using the Cockcroft–Gault formula. An increase of 0.5mg/dl or more above baseline CR was considered positive for renal toxicity.
The inherent toxicity of the GEM-CARBO pharmacotherapeutic regimen was determined by evaluating haematological and renal toxicity, after the first cycle was completed, in those patients who had been given antineoplastic drugs according to the regimen's predefined standard dose and who did not receive erythropoietic factor or CSF as an adjuvant treatment.
Treatment adherence was evaluated based on the parameters described in the 4 following points25–27:
- (1)
Number of patients receiving all cycles of the regimen.
- (2)
Dose intensity: the dose of one antineoplastic drug administered to patients within a specific time frame (until disease progression and/or completion of predefined cycles according to treatment response) as a percentage of the total theoretical dose.26
- (3)
Number of patients with a dose intensity greater than 85%, since lower dose intensities compromise treatment effectiveness. This 85% threshold is used in clinical trials to analyse proper treatment adherence or compliance.25–27
- (4)
Inter-cycle period changes of more than ±3 days. The care team established that delaying or moving up the scheduled date by 3 days or less did not change compliance with the regimen period.
Of the 31 patients included in the study, 30 were male, with 55% of the population (17/31) aged 65 years or older and 5/31 having a low performance status (ECOG scale of 2 or above). They received a total of 122 cycles (Day 1: 122; Day 8: 115).
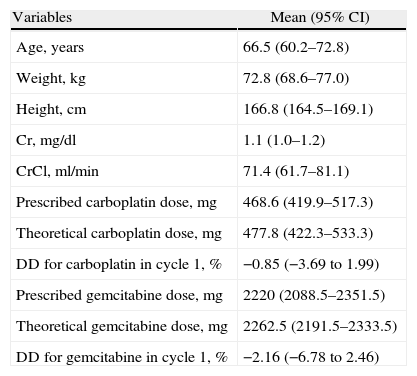
Table 1 shows the central tendency and dispersion measurements for the anthropometric, biochemical and dosage variables that were measured during cycle 1 of the treatment.
Anthropometric, Biochemical and Dosage Variables for Cycle 1.
| Variables | Mean (95% CI) |
| Age, years | 66.5 (60.2–72.8) |
| Weight, kg | 72.8 (68.6–77.0) |
| Height, cm | 166.8 (164.5–169.1) |
| Cr, mg/dl | 1.1 (1.0–1.2) |
| CrCl, ml/min | 71.4 (61.7–81.1) |
| Prescribed carboplatin dose, mg | 468.6 (419.9–517.3) |
| Theoretical carboplatin dose, mg | 477.8 (422.3–533.3) |
| DD for carboplatin in cycle 1, % | −0.85 (−3.69 to 1.99) |
| Prescribed gemcitabine dose, mg | 2220 (2088.5–2351.5) |
| Theoretical gemcitabine dose, mg | 2262.5 (2191.5–2333.5) |
| DD for gemcitabine in cycle 1, % | −2.16 (−6.78 to 2.46) |
DD, dosage difference; 95% CI, 95% confidence interval.
Upon beginning treatment with the GEM-CARBO regime, all patients had Hb, platelet and ACN values within the normal range. Another myelosuppressive therapy had been used as previous treatment in 19% of the population; this group also received treatment with erythropoietin and CSF as prophylaxis against anaemia and neutropenia secondary to chemotherapy when the GEM-CARBO regimen was initiated.
CARBO doses were personalised so as to achieve a target AUC of 5mg/ml/min in 84% of the population (26/31 patients). In the remaining 16% (5/31), we selected a target AUC of 4mg/ml/min. For these 5 patients, the median dose of CARBO prescribed in cycle 1 was 380mg, with a theoretical median calculated at 372mg.
In the first cycle of treatment, variation between the prescribed GEM dose and the calculated theoretical dose was less than ±5%. One patient did not meet this condition due to having a low performance status. After administering cycle 1 day 1 of the GEM-CARBO regimen, a new pharmacotherapeutic plan was drawn up for this patient which excluded GEM administration on day 8 of each cycle.
Five patients died prior to receiving the second cycle of treatment, and were therefore excluded from the subsequent toxicity and adherence analysis; a sample of 26 patients remained for these studies.
Toxicity AnalysisFig. 1 shows the dispersion graph with patients’ Hb values from the time they began chemotherapy with the GEM-CARBO regimen (day 1) until the day they finished treatment. Figs. 2–4 present the dispersion graphs for the ANC, platelet and Cr measurements, respectively.
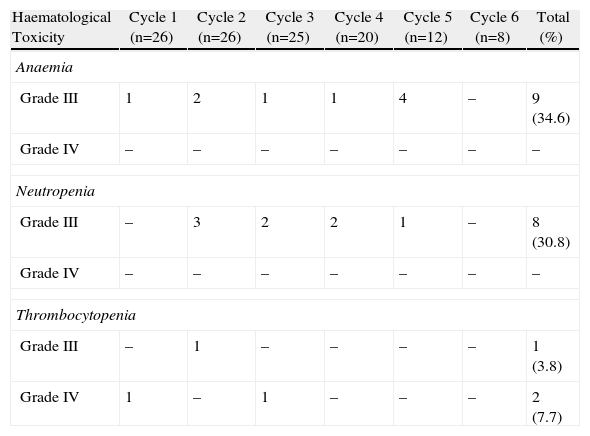
The incidence rate of grade III and IV haematological toxicity for the GEM-CARBO regimen was 53.8% among the patients included in the study (14/26). Table 2 shows the different episodes of haematological toxicity recorded in the study population per cycle.
Incidence of Grade III and IV Haematological Toxicity Caused by Treatment With the GEM-CARBO Regimen per Cycle in the Study Population.
| Haematological Toxicity | Cycle 1 (n=26) | Cycle 2 (n=26) | Cycle 3 (n=25) | Cycle 4 (n=20) | Cycle 5 (n=12) | Cycle 6 (n=8) | Total (%) |
| Anaemia | |||||||
| Grade III | 1 | 2 | 1 | 1 | 4 | – | 9 (34.6) |
| Grade IV | – | – | – | – | – | – | – |
| Neutropenia | |||||||
| Grade III | – | 3 | 2 | 2 | 1 | – | 8 (30.8) |
| Grade IV | – | – | – | – | – | – | – |
| Thrombocytopenia | |||||||
| Grade III | – | 1 | – | – | – | – | 1 (3.8) |
| Grade IV | 1 | – | 1 | – | – | – | 2 (7.7) |
At the beginning of the second treatment cycle, we evaluated haematological variables only in those patients who had received a CARBO dose during the first cycle that differed by no more than ±10% from the calculated theoretical dose, and who had not begun treatment with erythropoietin or CSF. This was the case for 19 patients, which allowed us to evaluate the haematological toxicity incidence rate following administration of the first cycle of the standard GEM-CARBO regimen in patients with advanced-stage NSCLC. We identified grade III neutropenia in 1 patient (5.3%) and grade IV thrombocytopenia in another (5.3%).
One patient (1/26, 4% of the population) suffered a Cr spike greater than 0.5mg/dl between 2 consecutive cycles. However, this increase was not clinically significant, since the patient had chronic renal failure, CrCl and the CARBO dose did not undergo significant changes.
Adherence AssessmentDuring the first treatment cycle, the DD between prescribed CARBO and the calculated theoretical dose was within ±10% in 90% of the patients (28/31). In 2 patients (7%), the delivered CARBO doses were 17% and 28% less, respectively, than the calculated theoretical doses. The decision to decrease these doses was a preventive measure, since patients who had previously been treated with other myelosuppressive drug regimens had already developed haematological toxicity.18,19,22 One patient (3%) received a 15% overdose compared to the calculated theoretical dose because a Cr value that had not been updated was used at the time of prescription.
The number of patients receiving the maximum number of cycles indicated in the GEM-CARBO regimen was 16/26 (61.5%). A maximum of 4 cycles were administered to 8 of the 26 patients whose disease remained stable; 8 of 26 patients received 6 cycles due to their disease responding to treatment. The median number of administered cycles was 4 (interquartile range 3.0–5.5).
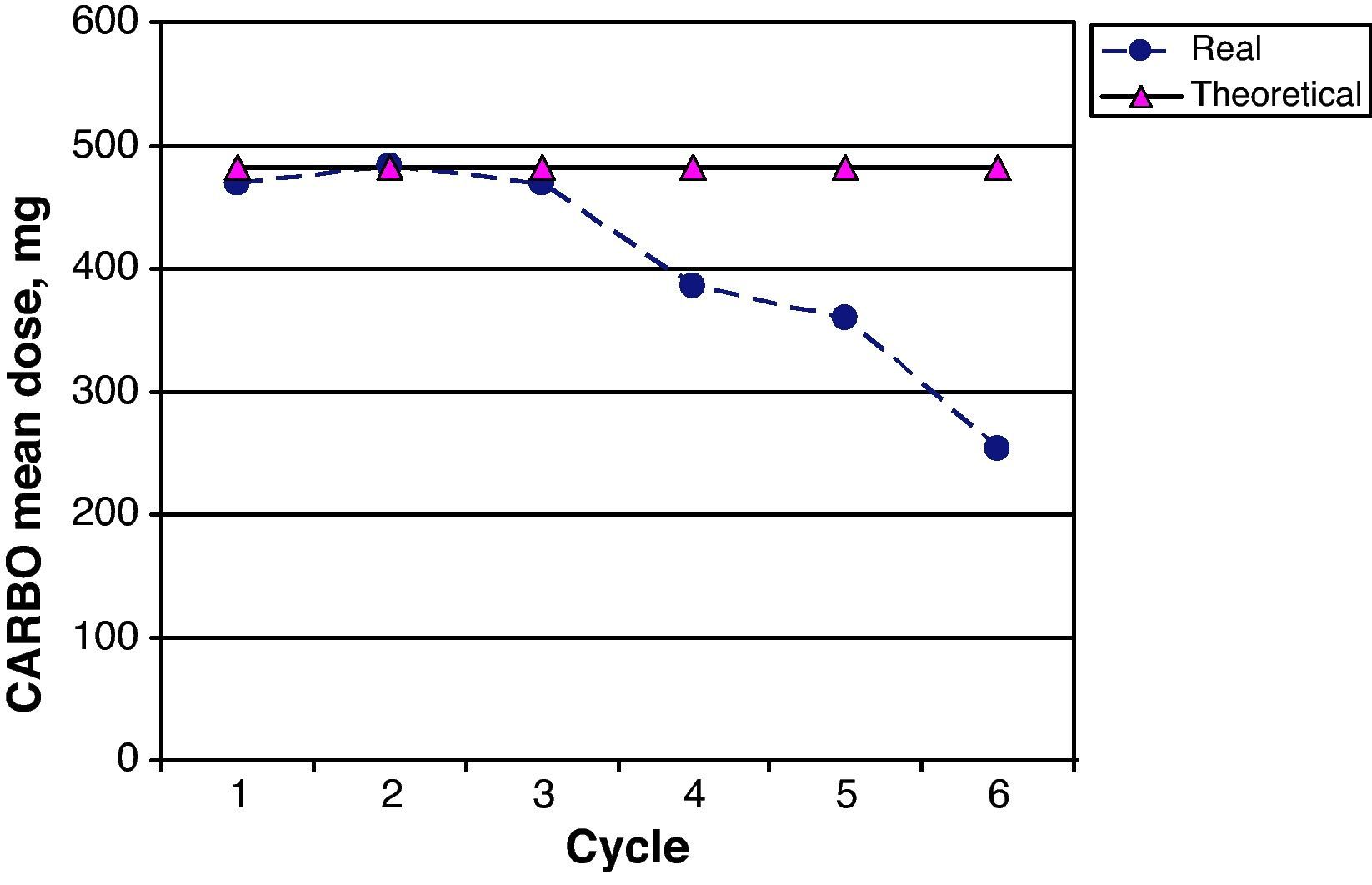
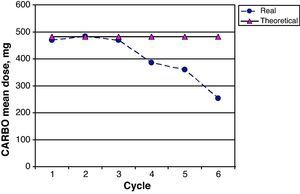
The CARBO dose intensity for the 26 patients in the study was 82.2% (95% CI 74.3–91.3); 17/26 patients (65.0%) received more than 85% of the planned theoretical CARBO dose. Fig. 5 shows a comparison of the mean real prescribed CARBO doses and the theoretical calculated doses for each cycle in the GEM-CARBO regimen. We observe a significant difference beginning with the fourth cycle, which is the point at which doses are decreased and adapted on a case-by-case basis according to haematological toxicity; prior to this, toxicity had been managed by delaying the inter-cycle period.
The first day of the drug administration cycle was delayed in 16 of the 91 potential prescribed cycles in the regimen (18%); 13 of these delays occurred before cycle 4 of treatment.
The CARBO dose was modified with respect to the dose prescribed in the first cycle due to haematological toxicity in 10 of the 91 prescribed cycles (11.0%). The reduced dose in all cases was calculated for a target AUC of 4mg/ml/min.
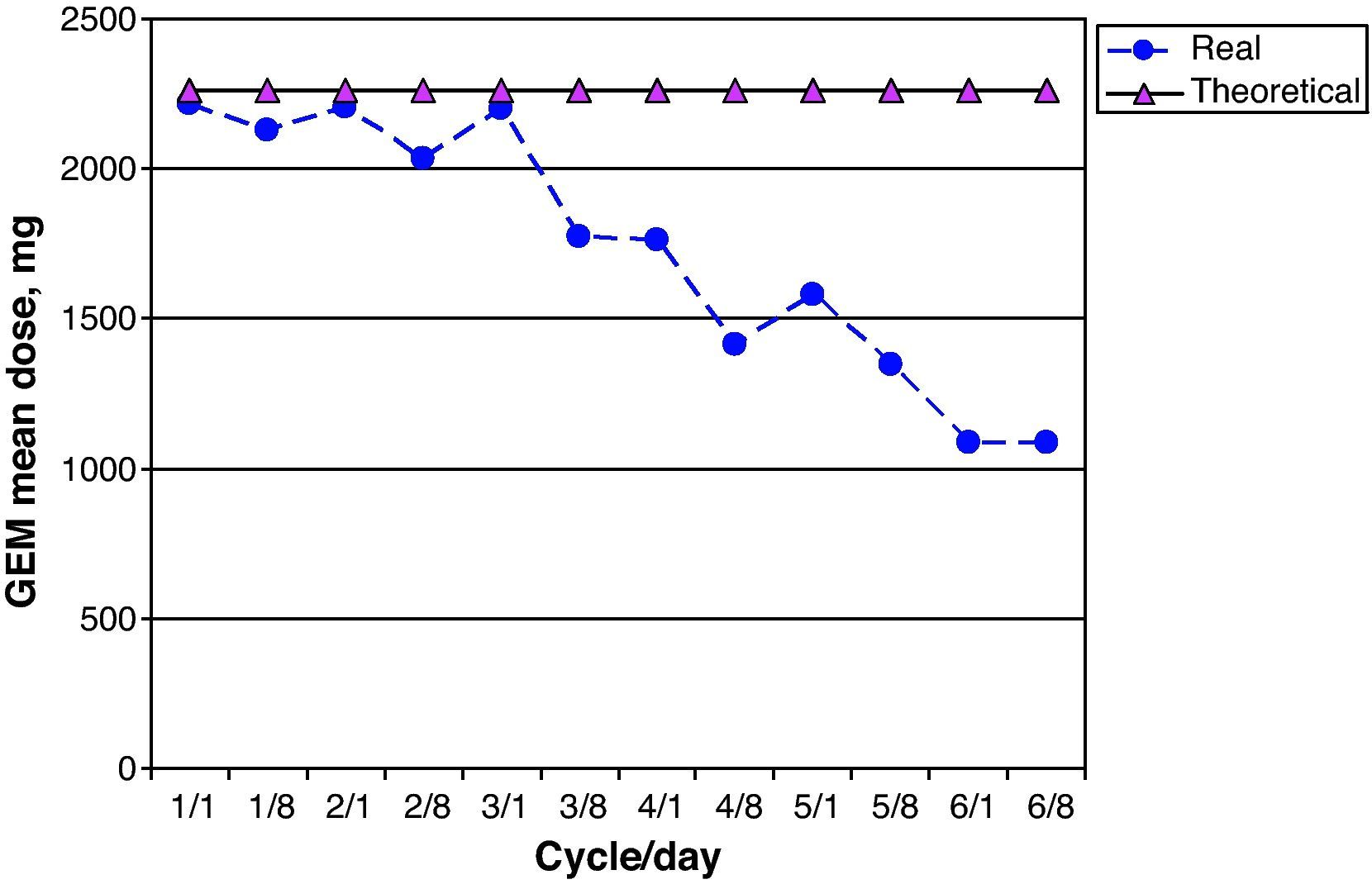
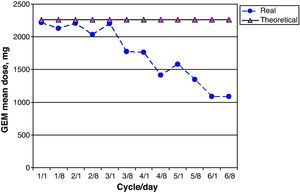
GEM dose intensity was 78.0% (95% CI 67.1–88.9); 15 of 26 patients (58.0%) received more than 85% of the theoretical cumulative GEM dose. Fig. 6 shows a comparison of the real prescribed mean GEM doses compared to the calculated theoretical doses for each cycle. Decreases in GEM dosage were more common beginning with cycle 4 of treatment, and therefore coincided with the reductions in CARBO doses. GEM doses were modified due to haematological toxicity by eliminating GEM on day 8 of the corresponding cycle in 19 of 213 possible doses (9.0%).
No grade III or IV haematological toxicity occurred in 3 of 26 patients (11%) who received a CARBO dose calculated for a target AUC of 4mg/ml/min beginning with the first cycle, as they were older than 65 and had a low performance status. These patients received the maximum number of cycles predefined for the regimen without any period delays or modifications to the dosage of either drug.
DiscussionThe fact that our study population is predominantly male coincides with the higher incidence rate of NSCLC in the male sex.2
The antineoplastic drugs that make up the standard GEM-CARBO regimen in cycle 1 were prescribed according to the characteristics described in the bibliography.6,7,17,18 We determined that patients who had not previously undergone myelosuppressive treatment and were not receiving erythropoietin and CSF were unlikely to suffer haematological toxicity after completion of the first cycle, but that any toxicity tended to be severe: grade III neutropenia and grade IV thrombocytopenia were the first consequences to appear. In the study population, the incidence rate of grade III and IV haematological toxicity increased as the accumulated dose of antineoplastic drugs in patients increased. Grade III anaemia and neutropenia occurred in 34.0% and 30.8% of patients, respectively; the literature reports rates of 18.0% for anaemia and 34.0% for neutropenia, grade III and IV. Our population had a lower incidence rate, but severer cases of thrombocytopenia: 3.8% for grade III and 7.7% for grade IV, compared with a 32.6% incidence of grade III and IV thrombocytopenia reported in some of the studies we consulted.13–15
In daily care, haematological toxicity was managed according to the recommendations described below13–19,22: administering erythropoietin and colony-stimulating factors, providing blood and platelet transfusions, delaying inter-cycle periods to promote recovery of the patient's haematological parameters, and reducing doses of antineoplastic drugs. Even so, compliance with the treatment programme was poor; the standard GEM-CARBO regimen was not well tolerated by patients, mainly due to the haematological toxicity, which they developed. These results contradict the good tolerability of both antineoplastic agents described in phase II and phase III trials of the GEM-CARBO regimen, which we consulted. The differences may be due to younger patients with fewer co-morbidities having been included in the clinical trials.12–14 In this study, a little more than half of the patients (61.5%) received the total number of cycles of the regimen that were planned at the start of treatment. The mean dose intensity for both antineoplastic agents was less than 85%, which is the threshold used in clinical trials to indicate proper adherence or compliance; cumulative doses below 85% of the theoretical dose compromise treatment efficiency.25–27
No significant cases of renal toxicity were identified. This justifies our choice of a regimen evaluated for patients with NSCLC and associated renal failure for whom cisplatin use could cause renal toxicity and aggravate morbidity.
Increasing safety by administering CARBO for a target AUC of 4 coincides with different studies performed on the GEM-CARBO combination. In these studies, a significant decrease in grade III and IV haematological toxicity was identified21 when CARBO was dosed for a target AUC of 4mg/ml/min. No grade III or IV haematological toxicity was identified in the patients in our study who received doses for a target AUC of 4. We did not have to recur to delaying any of the cycles, and all the patients completed their scheduled cycles according to their individual clinical responses to the chemotherapy. Likewise, patients whose original CARBO doses corresponded to a target AUC of 5mg/ml/min and whose doses had to be decreased later due to the appearance of haematological toxicity had their CARBO doses adjusted on a case-by-case basis to achieve a target AUC of 4mg/ml/min.
In conclusion, the GEM-CARBO regimen indication and prescription was adjusted according to solid scientific evidence16–19,21; however, its safety profile, mainly referring to the appearance of grade III and IV haematological toxicity, limited its use and made it difficult to administer the planned dose intensity, which compromised treatment effectiveness. We therefore have need of a dose-response study for the GEM-CARBO regimen that modifies the standard CARBO prescription to reach a target AUC of 4mg/ml/min in patients with advanced NSCLC, since adherence to the treatment would be better owing to its lower haematological toxicity profile.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Gómez Herrero D, et al. Perfil de toxicidad y adherencia del esquema farmacoterapéutico gemcitabina-carboplatino en cáncer de pulmón no microcítico. Farm Hosp. 2011;35:298–304.