Solid organ transplant (SOT) recipients are at greater risk than the general population for complications and mortality from influenza infection.
MethodsResearchers and clinicians with experience in SOT infections have developed this consensus document in collaboration with several Spanish scientific societies and study networks related to transplant management. We conducted a systematic review to assess the management and prevention of influenza infection in SOT recipients. Evidence levels based on the available literature are given for each recommendation. This article was written in accordance with international recommendations on consensus statements and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II).
ResultsRecommendations are provided on the procurement of organs from donors with suspected or confirmed influenza infection. We highlight the importance of the possibility of influenza infection in any SOT recipient presenting upper or lower respiratory symptoms, including pneumonia. The importance of early antiviral treatment of SOT recipients with suspected or confirmed influenza infection and the necessity of annual influenza vaccination are emphasized. The microbiological techniques for diagnosis of influenza infection are reviewed. Guidelines for the use of antiviral prophylaxis in inpatients and outpatients are provided. Recommendations for household contacts of SOT recipients with influenza infection and health care workers in close contact with transplant patients are also included. Finally antiviral dose adjustment guidelines are presented for cases of impaired renal function and for pediatric populations.
ConclusionsThe latest scientific information available regarding influenza infection in the context of SOT is incorporated into this document.
Los receptores de un trasplante de órgano sólido (TOS) presentan un riesgo mayor de complicaciones y una mortalidad más alta de la infección por el virus de la gripe que la población general.
MétodosDiversos clínicos e investigadores de las infecciones en portadores de TOS han desarrollado este documento de consenso en el que han participado varias sociedades científicas y grupos de trabajo relacionados con el trasplante de órganos. Hemos realizado una revisión sistemática para determinar el abordaje de la infección por virus de la gripe en receptores de TOS. En el documento se especifica el nivel de evidencia para cada recomendación basado en la literatura disponible. Este artículo se ha redactado de acuerdo con las recomendaciones internacionales sobre documentos de consenso y las recomendaciones del Intrumento para Evaluación de Guías de Práctica Clínica II (AGREE II).
ResultadosSe realizan recomendaciones sobre la obtención de órganos de donantes con sospecha o confirmación de infección por virus de la gripe. Se destaca la importancia de mantener un alto nivel de sospecha de infección gripal en cualquier portador de TOS que consulte por clínica de infección de vías respiratorias altas o bajas, incluida la neumonía. Se resalta la importancia de iniciar tratamiento antiviral precoz en todos los portadores de TOS con sospecha o confirmación de infección por virus de la gripe, así como la importancia de que anualmente reciban la vacuna frente a este virus. Se revisan las diferentes técnicas microbiológicas para la detección de la infección por virus de la gripe. Se aportan directrices para el empleo de profilaxis antiviral tanto en pacientes ambulatorios como en aquellos ingresados en el hospital. Se incluyen recomendaciones para los sujetos que conviven estrechamente con portadores de TOS infectados por virus de la gripe y para los trabajadores sanitarios que los atienden. Finalmente, se incluyen recomendaciones sobre el ajuste de dosis de antivirales en sujetos con deterioro de la función renal y para la población pediátrica.
ConclusionesSe incorpora a este documento la información científica más actualizada sobre la infección por virus de la gripe en el contexto del TOS.
Worldwide, 40,000 organ transplants are performed annually, with very high success rates. In Spain, 3706 organ transplantations were performed in 2011. Renal transplants were the most common, followed by liver, heart, lung, and others, including dual organ, pancreatic, and intestinal transplantation.
Solid organ transplant (SOT) recipients are at greater risk for complications and mortality from influenza infection than those in the general population due to their immunocompromised status.1,2 Compared with the immunocompetent population, patients who have received transplants might have a greater viral burden and shed virus for longer periods of time.3 After the 2009 influenza pandemic, important information regarding influenza infection have been published in the SOT setting. Based on this, experienced SOT researchers and clinicians, with expertise in adults and pediatrics infectious diseases, nephrology, cardiology and surgery, have developed and implemented this consensus document in collaboration with several Spanish scientific societies, the Spanish National Transplant Organization and the research study networks.
The target populations of this document are adults and children receiving SOT, organ donor and recipient candidates, health-care workers and SOT household contacts. The intended guideline audience is physicians involved in the care of SOT recipients (including primary care physicians), transplant coordinators and other health-care workers attending SOT recipients. Here we report a consensus from a public health policy perspective with the objective of assessing the available overall evidences and to propose recommendations on the following key questions:
- 1.
How should we proceed when the donor has suspected or confirmed influenza infection?
- 2.
When is influenza chemoprophylaxis indicated for SOT recipients?
- 3.
What should be recommended regarding influenza vaccination for SOT recipients?
- 4.
What recommendations can be given to household contacts of SOT recipients?
- 5.
What should be done to avoid nosocomial transmission of influenza among SOT recipients?
- 6.
When should influenza infection be suspected in SOT recipients?
- 7.
What are the prognostic factors for influenza in solid-organ transplant recipients?
- 8.
If influenza infection is suspected in a SOT recipient, which microbiological studies should be performed?
- 9.
When should a SOT recipient with suspicion of influenza be treated with antivirals?
- 10.
What other therapeutic measures should be adopted in solid-organ transplant recipients with influenza infection?
- 11.
When should antibiotic treatment be administered to SOT recipients with influenza infection?
- 12.
How should neuraminidase inhibitors be used in solid-organ transplant recipients with renal function impairment?
- 13.
What are the special recommendations regarding influenza in SOT pediatric recipients?
Several areas related to infections in SOT recipients that are unresolved and controversial are also discussed, including emerging issues such as donor-derived infection, impact of influenza infection in SOT recipients, time and extension of antiviral therapy, drug-resistant infections, and timing of influenza vaccination after transplantation, among others.
MethodsWe conducted a systematic review to assess the management of influenza infection in SOT recipients Data for this document were identified by search of PubMed and references from relevant articles using the search terms “transplant*” and “influenza”. The criteria for search included articles in English that involved human participants. We selected and revised a total of 949 articles from 1971 to October 2012.
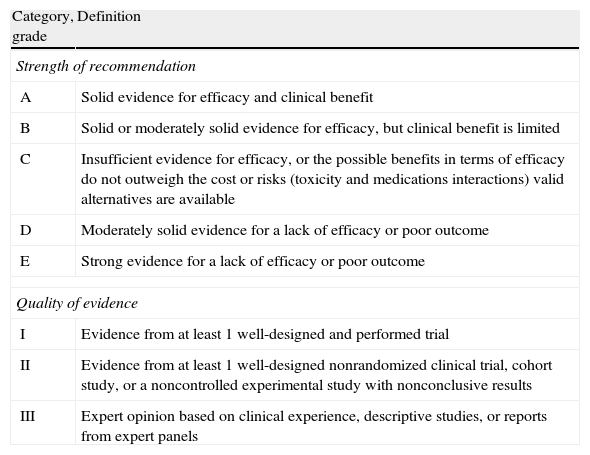
Evidence level based on the available literature is given for each recommendation to assess the strength of the evidence for risks and benefits of the procedure. This article was written in accordance with international recommendations on consensus statements (Table 1)4 and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II).5 The authors met twice for discussing the consensus and to achieve formal recommendations. The coordinators and authors agree on the content and conclusions. The consensus statement was sent to the 96 members of GESITRA for external revision of the manuscript. The board of directors of GESITRA will designate the coordinators for updating the statements within 5 years.
Classification of the recommendations of this consensus document, based on the strength and quality of the evidence analyzed.
| Category, grade | Definition |
| Strength of recommendation | |
| A | Solid evidence for efficacy and clinical benefit |
| B | Solid or moderately solid evidence for efficacy, but clinical benefit is limited |
| C | Insufficient evidence for efficacy, or the possible benefits in terms of efficacy do not outweigh the cost or risks (toxicity and medications interactions) valid alternatives are available |
| D | Moderately solid evidence for a lack of efficacy or poor outcome |
| E | Strong evidence for a lack of efficacy or poor outcome |
| Quality of evidence | |
| I | Evidence from at least 1 well-designed and performed trial |
| II | Evidence from at least 1 well-designed nonrandomized clinical trial, cohort study, or a noncontrolled experimental study with nonconclusive results |
| III | Expert opinion based on clinical experience, descriptive studies, or reports from expert panels |
- 1.
If feasible, potential donors with upper or lower respiratory tract infection symptoms should be microbiologically tested to rule out influenza infection if deceased during the annual influenza epidemic, especially in cases of lung transplantation (B-III).
- 2.
Subjects who died in the context of confirmed or suspected influenza infection with or without having received antiviral treatment can be considered as donors for solid organ transplantation provided that the recipient is prophylactically treated with neuraminidase inhibitors. This recommendation is not applicable for lung or intestine transplantation (B-III).
- 3.
Subjects who died in the context of confirmed or suspected influenza infection should be ruled out as donors for lung or intestine transplantation independently of the treatment provided (A-III).
- 4.
Transplantation from a living donor with influenza infection should be postponed if feasible (A-III).
- 5.
All potential candidates for solid organ transplantation should receive inactivated influenza vaccine annually (A-I).
The benefits of transplantation have to be balanced with the risk of transmission of pathogens such as virus. The possibility of transmission of influenza infection from donor to recipient through the graft was a matter of concern during the 2009 pandemic.6–8 Several studies have demonstrated the spread of influenza virus to different organs such as the brain, kidney, pancreas, liver or heart.9–14 In contrast, several autopsy series during the 2009 pandemic did not demonstrate the presence of influenza A (H1N1) virus or pathological evidence of active disease in extrapulmonary organs.15–17 The possibility of transmission through the organ transplanted has not been well characterized. Lung and intestine transplantation deserves special consideration as they are considered target organs for influenza virus. Conversely, the risk of transmission to recipients of non-lung, non-intestine organs is considered to be very low due to the apparently infrequent viremia during influenza infection.
To the best of our knowledge, only one case of transmission had been communicated before the 2009 influenza pandemic and it was in a case of lung transplantation.18 During the pandemic there were some reports of transmission of influenza through lung transplantation, due to donors being diagnosed with microbiologically proven influenza after the implantation of the graft.19 Recipients were treated with oseltamivir and had a favorable outcome. Apart from lung transplantation, only one case of possible transmission of influenza has been reported in solid organ transplantation. It was the case of a kidney receptor from a donor who died with influenza infection that had not received antiviral treatment.19 The same type of influenza virus was detected by PCR in the donor and in the biopsy of the transplanted kidney. The recipient developed severe lung infection and was treated with oseltamivir. Influenza virus was not detected in any of the respiratory samples.
During 2009 pandemic several cases of successful transplantation were communicated in cases in which the donor was diagnosed with influenza infection. These cases involved liver, heart, lung and kidney transplantation.6,8,20,21 All donors received oseltamivir treatment for at least 48h before the procedure and none of the recipients developed influenza infection with most of them receiving a full oseltamivir treatment after transplantation.
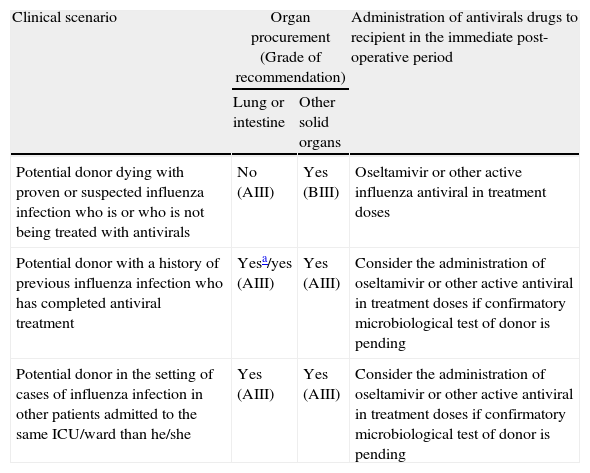
The criteria for the acceptance of organs from donors with suspected or confirmed influenza infection are expressed in Table 2, according to the experience and the recommendations after the 2009 pandemic.6–8,20,22,23 In cases of influenza infection of the donor, administration of antiviral treatment to the recipient is recommended. The transplantation team should inform the potential recipient of the risk and consequences of accepting or not accepting the transplant. A more conservative approach should be adopted in cases of influenza infection with more aggressive strains such as type A (H5N1) influenza virus (“avian flu”).
Guidelines for the management of potential donors for solid organ transplantation with suspected or confirmed influenza infection.
| Clinical scenario | Organ procurement (Grade of recommendation) | Administration of antivirals drugs to recipient in the immediate post-operative period | |
| Lung or intestine | Other solid organs | ||
| Potential donor dying with proven or suspected influenza infection who is or who is not being treated with antivirals | No (AIII) | Yes (BIII) | Oseltamivir or other active influenza antiviral in treatment doses |
| Potential donor with a history of previous influenza infection who has completed antiviral treatment | Yesa/yes (AIII) | Yes (AIII) | Consider the administration of oseltamivir or other active antiviral in treatment doses if confirmatory microbiological test of donor is pending |
| Potential donor in the setting of cases of influenza infection in other patients admitted to the same ICU/ward than he/she | Yes (AIII) | Yes (AIII) | Consider the administration of oseltamivir or other active antiviral in treatment doses if confirmatory microbiological test of donor is pending |
The most important measure for avoiding influenza transmission from donor to recipient in solid organ transplantation is the annual vaccination of the candidates.24,25 As SOT candidates have end-stage organ failure, they are candidates for annual vaccination regardless of the time when the transplantation is planned. Pneumococcal vaccine should also be updated in SOT candidates.
When is influenza chemoprophylaxis indicated for SOT recipients?Recommendations- 6.
Preexposure and post-exposure antiviral chemoprophylaxis should not routinely be used in solid organ transplant recipients, and might be only reserved for selected cases such as patients who are severely immunosuppressed and at high risk for influenza-related complications (BIII). For the rest of cases, solid organ transplant patients with known exposure to influenza should be advised to seek care if they experience symptoms of influenza infection in order to initiate early antiviral treatment (AII).
- 7.
As infection can occur during chemoprophylaxis, patients should seek medical evaluation if they develop fever or respiratory illness suggestive of influenza infection. In these cases the possibility of an infection with a resistant virus should be considered (BIII).
- 8.
Oseltamivir or zanamivir can be recommended for antiviral chemoprophylaxis of influenza A (H1N1), influenza A (H3N2), or influenza B viral infection (AI).
- 9.
Postexposure chemoprophylaxis should be administered for a total of 10 days after the most recent influenza contact.
- 10.
The duration of pre-exposure chemoprophylaxis should correspond to the entirety of the flu season (B-III). There are no data regarding the safety and tolerability of chemoprophylaxis for more than 6 weeks.
Influenza vaccination is the primary tool used for preventing influenza infection, and antiviral chemoprophylaxis is not a substitute for influenza vaccination. Postexposure chemoprophylaxis with oseltamivir and zanamivir has been efficacious in placebo-controlled trials for the prevention of influenza illness among non-immunosuppressed subjects. Chemoprophylaxis was administered in subjects after contact with a household member or other close contact with laboratory-confirmed influenza. The efficacy ranged from 72% to 82% for zanamivir and from 68% to 89% for oseltamivir.26–31
In a retrospective case control study carried out in 25 hematopoietic stem cell transplant recipients, oseltamivir prophylaxis was evaluated for adverse events. Only one non-compliant patient developed an influenza B infection. The proportions of severe clinical and laboratory adverse events were not significantly different between patients who received oseltamivir prophylaxis and control subjects.32 The information related to postexposure chemoprophylaxis in SOT recipients is scarce and consists only of case reports.33,34
Preexposure chemoprophylaxis has been studied in the community in groups of healthy adults and patients living in institutional centers who received antiviral medications during influenza virus season. The efficacy of zanamivir and oseltamivir in preventing febrile, laboratory-confirmed influenza illness was 84% and 82%, respectively.27,35,36
Preexposure chemoprophylaxis must be administered only for the duration of the exposure. Prolongation of pre-exposure chemoprophylaxis (longer than 4 weeks), although it seemed to be highly efficacious for preventing symptomatic influenza infection among immunocompetent patients in a systematic review, was also associated with increased nausea and vomiting.37 However, all trials were industry-sponsored and none of the studies were powered to detect rare adverse events and they did not include immunocompromised patients.27,29,35,36,38,39
The efficacy of antiviral agents for preventing influenza infection among severely immunocompromised patients is not well established. Another concern is the selection of mutations that confer resistance to antivirals evidenced in some cases of postexposure chemoprophylaxis.40
A small non-randomized study with stem cell transplant recipients suggested that oseltamivir may prevent progression to pneumonia among influenza virus-infected patients.3 In a randomized placebo-controlled trial with 477 immunocompromised patients receiving kidney, liver or allogeneic hematopoietic stem cell transplantation, the incidence of laboratory confirmed influenza was 2.1% and 8.4% for patients receiving oseltamivir prophylaxis or placebo for 12 weeks, respectively. Four percent of patients were children (range, 1–12 years). No differences were found in the proportion of subjects with clinical symptoms of influenza infection or with positive viral culture and/or a rise in antibody titers from baseline ≥4-fold. No resistance to oseltamivir was reported and treatment was well tolerated.41
Primary antiviral chemoprophylaxis should be initiated at the onset of sustained influenza activity (B-II). When secondary chemoprophylaxis is indicated, a neuraminidase inhibitor medication should be started as early as contact with an influenza infected patient (preferably within the first 48h after the contact) to reduce the risk of developing symptomatic disease. If secondary chemoprophylaxis is not initiated, SOT recipients who are in close contact with a person with confirmed or suspected influenza infection should be counseled about the early signs and symptoms of influenza infection and advised to contact their health-care provider immediately for early treatment if clinical signs or symptoms develop.
Zanamivir and oseltamivir are the drugs of choice in cases of initiating influenza chemoprophylaxis. Given the high incidence of resistance to amantadine and rimantadine among circulating strains of influenza A and the intrinsic resistance of influenza B, they are not recommended for initiating chemoprophylaxis.42,43 They may be considered only in cases of suspicion of oseltamivir-resistant influenza A (H1N1) viral infection.
What should be recommended regarding influenza vaccination for SOT recipients?Recommendations- 11.
Seasonal inactivated influenza vaccination is strongly recommended every year in solid organ transplant recipients (AII).
- 12.
Influenza vaccine can be given after the first month of the transplant (BII).
- 13.
More evidence is needed for recommending intradermal vaccination, a second booster dose and high-dose influenza vaccine (CIII).
- 14.
Influenza vaccination is indicated in organ transplant children older than six months. Children younger than 9 years need two doses of the influenza vaccine in cases of not having received a previous vaccine (AII).
- 15.
The live attenuated influenza vaccine is not recommended in transplant patients (EIII).
- 16.
The pneumococcal vaccine is recommended in solid organ transplant recipients (AII).
Annual influenza vaccination is the most efficacious method for reducing the incidence and complications of influenza infection.44,45 Due to the continuous changes in the circulating influenza virus, the formulation of the influenza vaccine must be adapted yearly according to the recommendations of the World Health Organization (WHO).
Types of influenza vaccinesThe Influenza vaccine is usually composed of two influenza A subtypes (H1N1 and H3N2) and one influenza B virus. There are two vaccine manufactures: the trivalent inactivated vaccine, and the live-attenuated influenza vaccine.
The trivalent inactivated vaccine is the most commonly used and it is composed of antigens from the three virus strains combined and packaged in a single dose with 15μg of each strain.46 Although the majority of trivalent inactivated vaccines are administered by intramuscular injection, a new licensed intradermal preparation has been formulated.47 In addition to trivalent inactivated vaccine, a quadrivalent inactivated vaccine formulation containing two influenza A and two influenza B strains, is under development.
The live attenuated influenza vaccine is produced by reassortment of each of the three strains recommended each year with a cold adapted viral strain. Although the live attenuated influenza vaccine is approved by the FDA, it is not commercialized in Spain and it is not recommended for transplant patients.
Immunological response to influenza vaccination and related factorsThe response to influenza vaccination in the transplant population is discordant. Some studies have shown responses similar to that of the general population in renal48 and liver49 transplant recipients, but most of them suggest a clear reduction in efficacy in renal,50–53 liver,54–56 lung57 and cardiac transplant recipients,25,54 with a seroprotection rate that varies between 15% and 90%25,45,48–68 and a lower seroprotection (78%) after vaccination compared to healthy subjects.69
Some factors have been related with the diminished immune response to vaccination such as receiving lung transplantion57,69 and the type of immunosuppression. While mycophenolate mofetil48,69,70 and m-TOR inhibitors68 have been shown to diminish the antibody response to vaccination, there is no information on the use of other immunosuppressors such as thymoglobulin, rituximab or alentuzumab regarding the response to influenza vaccine.
The response to influenza vaccination is also related to the type of virus included in the formulation, with lower responses to influenza B70 and differences in the response to the different subtypes of influenza A virus.48,60,70,71 For instance, pediatric patients have a weaker response to influenza vaccination than SOT adults, and a second booster dose is usually needed.72,73
There are few studies regarding the impact of time from transplantation in the effectiveness of influenza vaccine. A concern has been raised about the safety of administering the vaccine within the first six months after transplantation and the hypothetical possibility of triggering acute rejection. Another aspect to take into consideration that may affect the response to the influenza vaccine is the strong immunosuppressive regimens given to patients, especially during the first months after transplantation.
Recommendations on administering influenza vaccination vary from 3 to 6 months after the transplant.23,74,75 However, there is little evidence regarding this aspect and most of the recommendations are based on expert opinions. There are only few studies with small series of patients vaccinated within the first six months after transplantation. Lawal et al. in a series of 51 liver transplant recipients observed that one (14%) of the 7 patients vaccinated within 4 months after the transplant responded to vaccination.76 Other larger cohort studies have shown no differences in the rate of seroprotection when patients vaccinated within the first six months after the transplant were compared with rest of transplant recipients.50,67,68 In all these studies, vaccination within the first 6 months after transplantation was safe and no cases of acute graft rejection were reported. Further studies are needed to completely clarify this issue, but based on previous data, influenza vaccination within the first six months after transplantation seems to be safe and efficacious. Given the high rate of complications of influenza infection, especially in the early post-transplant period, administration of the influenza vaccine may be considered after the first month of the transplant.
Clinical efficacyAlthough there are few studies addressing this matter, the clinical effectiveness of the administration of the adjuvanted influenza vaccine in SOT recipients ranged from 96.4% to 98.9%.65,67 However, during the 2010–2011 influenza season the antecedent of influenza vaccination among patients with confirmed flu disease was frequent. In a multicenter study carried out in SOT recipients with confirmed influenza infection, 53% had received one dose of the 2010–2011 trivalent seasonal influenza vaccine. Although influenza vaccination did not preclude symptomatic influenza, it reduced by 70% the risk of pneumonia.77
Strategies to improve immune response to influenza vaccine in SOT recipientsIt is essential to improve the effectiveness of the seasonal influenza vaccine in the population receiving solid organ transplantation. Different strategies have been proposed to improve the response. The intradermal administration of the vaccine have shown no benefit in a series of immunocompromised patients of renal and lung transplant recipients.57,78,79 Higher doses of vaccine have been related to better immunological response in the elderly. However, this approach has not been evaluated in SOT.80 The use of potent adjuvants increases the immunogenicity of influenza vaccine in immunocompetent persons81–83 but in SOT recipients no significant differences have been described.52,59,84–88
The presence of low baseline influenza antibody titers was associated with a better response in SOT patients receiving the 2009 seasonal and adjuvanted pandemic influenza A (H1N1) vaccines67,68. This suggests a potential booster effect of the vaccine in patients with baseline antibody titers which may facilitate a better immunological response. Based on these results, two strategies could be employed. Annual vaccination to maintain long-term antibody titers from prior year is hampered by two factors: first, the changes of the circulating strains between different epidemic seasons that are used for the formulation of the vaccine recommended by the WHO which may not match the strains of the previous year and second, the low rate of long term immunological response to influenza vaccination.67,89
Another strategy is to use a second vaccine dose (booster vaccination) in order to promote baseline antibody titers. Some studies have evaluated this strategy with different results25,48,54,56,57,59,86 which may be due to differences in the immunosuppression regimens employed, the circulating influenza strains and the rates of baseline seroprotection in the community. Further randomized clinical trials are warranted to generate strong evidences.
Safety of influenza vaccination in SOT recipientsAdverse event data after influenza vaccination of SOT recipients are scarce. A recent meta-analysis published by Beck et al. did not identify consistent evidences of disease progression or worsening clinical symptoms related to underlying immunosuppressive conditions following vaccination.90
A controversy concerning the efficacy and safety and the theoretical risk of allograft rejection triggered by the immune response to influenza vaccination has been raised. Multiple studies involving SOT patients receiving seasonal influenza vaccine (without adjuvant), found no link between vaccination and rejection episodes.91 Studies of kidney48,53,91,92 and heart transplantation58,63,93 have not linked allograft rejection to influenza vaccination. The addition of an adjuvant to the formulation of the influenza vaccine may maximize the immunogenicity of a single-dose vaccine, at the expense of increasing rejection.94 However, some studies have shown no increase93 or effect on rejection.68
It has been speculated that influenza vaccination of renal allograft recipients may be associated with de novo production and/or increased anti-HLA antibodies titers95; however, minimal data have been accumulated concerning the influence of vaccination on anti-HLA antibody production.51,96 Based on these results further larger studies are necessary in order to definitively prove the influence of vaccination on anti-HLA antibody production.
Episodes of acute allograft rejection and permanent graft dysfunction have been related with seasonal and pandemic influenza virus infection. In these circumstances, the benefits of the vaccine outweigh the potential risk of infection in SOT recipients and support the administration of the vaccine in this population.
Seasonal influenza vaccination is recommended in pregnant women that have received solid organ transplantation during the whole pregnancy period.
Bacterial coinfection is associated with increased severity and mortality in SOT recipients.2 Additive benefits of pneumococcal and influenza vaccines have been shown in different groups of patients at risk of influenza complications.97,98 Pneumococcal vaccination prevents invasive pneumococcal disease, pneumonia hospitalization and reduces mortality in nonimmunosuppressed and some immunosuppressed patients.98–102 Although, there is no evidence regarding SOT recipients, given the safety of the vaccine and the high morbidity and mortality of pneumococcal-influenza coinfection, it seems reasonable to recommend pneumococcal vaccination.
What recommendations can be given to household contacts of SOT recipients?Recommendations- 17.
All persons, including children older than 6 months, who live with or care for solid organ transplant recipients should receive the influenza vaccine every year (AI).
- 18.
These persons should receive the trivalent inactivated vaccine unless contraindicated due to severe adverse reactions (AI).
- 19.
In cases of antecedent of severe adverse reaction to trivalent inactivated vaccine, the live attenuated influenza vaccine can be administered. As a precautionary measure, contacts of solid organ transplant recipients receiving the live attenuated vaccine should avoid providing care for severely immunosuppressed patients for 7 days after vaccination. In case of householders, they should minimize the exposure of respiratory secretions (AI).
- 20.
To avoid influenza transmission, in cases of recipient's household contacts with upper respiratory disease during influenza season, good hand hygiene with water and soap or an alcohol-based hand rub should be encouraged. Linens, eating utensils, and dishes belonging to those who are sick should not be shared without washing thoroughly first. Exposure to respiratory secretions should be minimized by the use of correct cough etiquette and tissues (AI).
- 21.
Household contacts of a solid organ transplant recipient should avoid contact with people who are known to have influenza (BII).
- 22.
In cases of household contact with influenza like illness, solid organ transplant recipients should be advised to seek care and start antiviral therapy if developing respiratory symptoms or fever (AIII).
- 23.
Exceptionally, in selected cases of severely immunosuppressed organ transplant recipients at high risk of influenza infection when a household contact is suspected of having influenza disease, all family members can be administered prophylaxis with oseltamivir or zanamivir (AII).
Given the high rate of morbidity and mortality of influenza infection among transplant patients and the reduced effectiveness of influenza vaccine in this population, it is essential to decrease transmission of influenza from household. Mild illness and subclinical infection is frequent in hospitals and nursing homes, with a rate of seroconversion of 23% during influenza season.103 In a study carried out during 2010–2011, influenza A(H1N1)pdm virus was detected in 58% of healthy workers and caregivers in SOT units and was a predictive factor for developing an influenza like illness in the following 15 days.104 Healthy contacts with influenza infection, even if subclinical, may transmit the infection to organ recipients.
With the objective of protecting the SOT recipients from influenza infection, two types of measures may be considered: avoiding influenza infection among household contacts and, in case of becoming infected, avoiding the transmission to transplant recipients.
Within household contacts, there is evidence that suggest that influenza vaccination of preschool and school-aged children reduces the risk of transmission of influenza in households, and the protection achieved in children is extended to adults at risk of influenza complications. These randomized trials have used either the trivalent inactivated vaccine105 or live attenuated influenza vaccine.106 Live attenuated influenza vaccination was shown to reduce the incidence of clinically suspected influenza among contacts of vaccine recipients in nonrandomized community-based studies, although in these studies no microbiological confirmation of the suspected influenza diseases was carried out.107,108
None of these studies were performed in the transplant setting. In other debilitated populations, such as the elderly, it has been demonstrated that influenza vaccination reduces mortality among household contacts and it is likely that this statement may be also applicable to the SOT recipient. All household contacts, including children older than six months, should receive influenza vaccination every year. It is important to include school-aged children in these recommendations since they have the highest rate of influenza disease109 and they are the main source for transmitting influenza into the household.110
The type of influenza vaccine recommended for contacts is the trivalent inactivated influenza vaccine. In cases of severe reactions to the trivalent inactivated influenza vaccine, the live attenuated vaccine can be used. Although it has never been demonstrated, there is a concern about the possibility of transmission of influenza infection from subjects receiving the live attenuated vaccine to severely immunosuppressed patients. As a precautionary measure, contacts of SOT recipients receiving the live attenuated vaccine should avoid providing care to severely immunosuppressed patients for 7 days after vaccination. In the case of household contacts, they should minimize the exposure of respiratory secretions.
Influenza is transmitted through large respiratory droplets. These droplets are usually produced when coughing or sneezing and reach a distance of 1–2m. They can directly reach the mucosa of the SOT patients or contaminate the hands or surfaces where virus can live from 3 to 48h, depending on the type of material. The virus can subsequently be spread when touched by a susceptible person. If a transplant recipient household contact is suspected of being infected with influenza, good hand hygiene with water and soap or an alcohol-based hand rub should be encouraged. Linens, eating utensils, and dishes belonging to those who are sick should not be shared without washing thoroughly first. Exposure to respiratory secretions should be minimized by the use of correct cough etiquette that consist on covering the nose and mouth with a tissue when coughing or sneezing and throwing the tissue in the trash or sneezing or coughing in the sleeves to avoid contaminating the hands.111
Antiviral chemoprophylaxis is not usually recommended, although it might be used for the prevention of influenza in families. In a double-blind, placebo-controlled study the use of inhaled zanamivir was investigated for the treatment and prevention of influenza infection in families. The index case was also randomized to receive zanamivir or placebo. The proportion of families with at least one initially healthy household contact with influenza infection was significantly smaller in the zanamivir group than in the placebo group (4% vs. 19%, P<0.001) which represented a 79% reduction in the proportion of families with at least one affected contact. Zanamivir provided protection against both influenza A and influenza B infection and there was no evidence of the emergence of resistant variants.28
None of these studies included organ transplant household contacts but it is theoretically plausible that chemoprophylaxis might reduce the risk of the transplant recipients becoming infected with influenza. However, antiviral chemoprophylaxis, as previously stated, should only be reserved for few selected cases with severe immunosuppression and with high risk of influenza complications.
What should be done to avoid nosocomial transmission of influenza among SOT recipients?Recommendations- 24.
All health-care workers attending solid organ transplant recipients should receive influenza vaccine annually (AI).
- 25.
All health-care workers and visitors to the hospital should strictly comply with hand hygiene recommendations and cough etiquette (AII).
- 26.
All visitors and health-care workers presenting symptoms of upper or lower respiratory tract infection should avoid entering wards where solid organ transplant recipients are admitted (AIII).
- 27.
Influenza should be considered in the differential diagnosis for fever and/or respiratory symptoms in any hospitalized solid-organ transplant recipient during seasonal epidemic (AII).
- 28.
Solid organ transplant recipients admitted to a hospital with suspected or confirmed influenza virus infection should be placed in a private room where standard and droplet precautions should be adopted (AIII).
- 29.
Wearing a surgical mask and other measures (for example correct hand hygiene) to avoid droplet transmission are recommended when entering the room or cubicle of a patient with confirmed or suspected influenza infection (AI).
- 30.
Wearing a particulate respirator, a non-sterile long sleeved gown and gloves is recommended when performing aerosol-generating procedures (AII).
- 31.
Antiviral prophylaxis with oseltamivir or other neuraminidase inhibitors may be considered for solid organ transplant recipients sharing the hospital room with patients with confirmed influenza infection, regardless of the influenza vaccination status (especially in the case of a lung transplant recipients or severely immunosuppressed solid organ transplant recipients). Early recognition of illness and prompt initiation of treatment is an alternative to antiviral prophylaxis after suspected exposure of solid organ transplant recipients (except for lung transplant recipients or severely immunosuppressed solid organ transplant recipients) (BII).
- 32.
If two or more cases of influenza infection are confirmed in subjects admitted to different rooms of the same ward (outbreak), prophylaxis could be considered for all solid organ transplant recipients admitted to that ward. In this context, the measures to avoid transmission are especially emphasized (AII).
In 1972, a nosocomial influenza outbreak was described in a medical ward attending kidney transplant recipients.112 Two of five patients with influenza infection developed pneumonia. Other outbreaks have been described in wards admitting SOT recipients113 involving both patients and health-care workers. Nosocomial outbreaks have also affected other type of immunosuppressed patients.114–120
Influenza vaccination for health-care workersVaccine immunoprophylaxis is the main option for preventing influenza infection in the hospital environment. All healthcare workers attending SOT recipients should receive influenza vaccine annually.103,121–126 Vaccination of health-care workers has been associated with a substantial reduction in mortality in elderly patients admitted to long-term care hospitals.127 Compliance with this recommendation among health-care workers has been a constant problem.126,128,129 Common explanations for not receiving vaccination include concerns about the safety of the vaccine, belief of being at low risk for influenza infection or complications from infection, doubts about protection of patients and about the efficacy of the vaccine in preventing illness.122,128 Different measures have been proposed to improve vaccination compliance in this context, such us providing vaccination directly in the wards or in the offices for multiple days or developing marketing campaigns among the health-care professionals.122,130
All visitors and healthcare workers presenting symptoms of upper or lower respiratory tract infection should avoid entering wards where SOT recipients are admitted. Placing signs with this advice at the access points to the hospital and wards where SOT recipients are admitted is recommended.131
SOT recipients admitted to the hospital with influenza infection. Recommendations for patients, health-care workers and visitorsSOT recipients should wear surgical masks when showing signs or influenza-like illness while staying in waiting areas or being transported within the hospital.131 They should strictly adhere to recommendations for hand hygiene and cough etiquette. Influenza infection should be considered in the differential diagnosis for fever and/or respiratory symptoms in any hospitalized SOT recipient during seasonal epidemic.
The WHO developed recommendations for prevention and control of influenza infection during the 2009 pandemic.132 SOT recipients admitted to the hospital with suspected or confirmed influenza virus infection should be placed in a private room where standard and droplet precautions to avoid transmission can be adopted.131 Exceptionally, different patients with confirmed infection by the same strain could be placed in the same room.
Health-care workers and visitors should follow recommendations to avoid nosocomial transmission. Both should strictly comply with hand hygiene recommendations and cough etiquette. Hand hygiene is the main component of standard precautions and one of the best methods to prevent transmission of influenza in the health-care setting.132,133 Hand hygiene has been considered even more important than wearing a mask in order to prevent influenza transmission.134 Cough etiquette consists of covering the nose and mouth with a disposable tissue when coughing or sneezing and throwing the tissue in the trash after using it. If no tissue is available, the person should cough or sneeze into their sleeve to avoid contaminating their hands.
Wearing a surgical mask is recommended to avoid droplet transmission when entering the room or cubicle of a patient with confirmed or suspected influenza infection.134–138 The surgical mask should be tightly sealed to the mouth.139 Wearing a particulate respirator (e.g. FPP2 or FPP3 masks in the European Union and N95, N99 and N100 in the United States), a non-sterile long sleeved gown and gloves are recommended when performing aerosol-generating procedures. These procedures include aspiration or open suctioning of the respiratory tract including collection of lower respiratory tract specimens, nebulization, intubation, resuscitation, bronchoscopy, autopsy, etc. The same recommendations are considered for care of patients under mechanical ventilation. For the attendance of patients with suspected or confirmed infection by type A (H5N1) influenza virus (“avian flu”) special recommendations can be consulted in the WHO web page (www.who.int).
Viral replication is known to be able to persist for extended periods of time despite antiviral treatment in immunosuppressed subjects. All patients should remain in droplet precautions for a minimum of 7 days following the symptoms onset. If feasible, a microbiological study to confirm the absence of the virus is recommended before precautions are stopped. It is frequent that cough persists beyond the period of infectivity.
Prophylaxis recommended for SOT recipients when exposed to influenza while admitted to the hospitalAntiviral prophylaxis with oseltamivir or other neuraminidase inhibitor could be considered for SOT recipients sharing the hospital room with patients with confirmed influenza infection, regardless of the influenza vaccination status.140,141 This recommendation is especially applicable for lung transplant recipients or for severely immunosuppressed SOT recipients. Prophylaxis may also be considered for SOT recipients who had close contact with the index case during hospitalization, even if they were not sharing the same room.140 Early recognition of illness and prompt initiation of treatment is an alternative to antiviral prophylaxis after suspected exposure of SOT recipients (except for a lung transplant recipient or severely immunosuppressed SOT recipient).
If two or more influenza infection confirmed cases in subjects admitted to different rooms of the same ward (outbreak), prophylaxis should be considered for all SOT recipients admitted to that ward.131 In this context, the measures to avoid transmission are especially emphasized.
The standard oseltamivir dose for prophylaxis is 75mg per day for 10 days. Dosage should be adapted in cases of renal failure according to the recommendations included in this document. Vaccination should be “re-offered” to unvaccinated SOT recipients and healthcare workers in the context of an outbreak.
When should influenza infection be suspected in SOT recipients?Recommendations- 33.
Influenza infection should be clinically suspected in all solid organ transplant recipients presenting with flu-like symptoms such as fever, rhinorrhea, headache, cough, sore throat, myalgias and dyspnea in an epidemiological setting, (AII) especially in the presence of household contacts with influenza symptoms (AII).
- 34.
Moreover, influenza should be considered in the differential diagnosis of every case of pneumonia occurring in solid organ transplant recipients during the influenza season, (AII) particularly in patients with flu-like symptoms and bilateral pulmonary infiltrates (AII).
Influenza is an acute, usually self-limited, febrile illness caused by infection with influenza A or B virus that occurs in seasonal outbreaks. Its clinical spectrum ranges from asymptomatic infection to life-threatening illness.142–144
The clinical presentation of influenza infection in SOT recipients does not differ substantially from that described in the general population.145 Although clinical findings in the general population identify patients with influenza-like illness, they are not particularly useful for confirming or excluding diagnosis. No single symptom or symptom complex correlates sufficiently to make a conclusive clinical diagnosis of influenza infection, and timely epidemiological data are also needed in order to ascertain whether influenza is circulating in the community.74,146,147
Most clinical signs and symptoms of influenza infection are the result of cytokine release correlating with the local replication of influenza in the respiratory mucosa.148 SOT recipients are on medication that modulates the inflammatory response, and symptoms associated with influenza may be less common than in normal healthy hosts,149 particularly in patients with severe lymphopenia.150
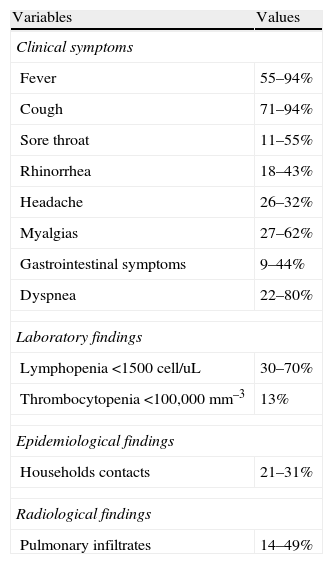
In SOT recipients with influenza the most common presenting symptoms are fever, cough, myalgias and dyspnea. (Table 3).1,2,148,151–157 The presence of fever strongly suggests influenza infection in SOT recipients with respiratory viral infection.158 In a comparison in adult versus pediatric SOT recipients with influenza A (H1N1)pdm virus infection presenting symptoms (1), children were more likely to present fever, rhinorrhea, sore throat, and headache than adults. Length of symptoms until influenza diagnosis in SOT recipients varies from 2 to 6 days (range 1–15 days).1,2,148,151–157 The most frequent laboratory abnormalities reported include lymphopenia and low platelet count,1,2,151 but SOT recipients also tend to have low lymphocyte counts due to immunosuppressive therapy. The presence of pulmonary infiltrates in SOT recipients with influenza infection ranges from 14% to 49%, and bilateral involvement is frequently observed.1,2,151,152 Other less frequent clinical manifestations of influenza in SOT recipients include encephalitis,1,155 myocarditis, myositis149 and bronchiolitis obliterans syndrome.153,159
Reported clinical symptoms, laboratory data and radiological findings in SOT recipients with influenza infection.
| Variables | Values |
| Clinical symptoms | |
| Fever | 55–94% |
| Cough | 71–94% |
| Sore throat | 11–55% |
| Rhinorrhea | 18–43% |
| Headache | 26–32% |
| Myalgias | 27–62% |
| Gastrointestinal symptoms | 9–44% |
| Dyspnea | 22–80% |
| Laboratory findings | |
| Lymphopenia <1500cell/uL | 30–70% |
| Thrombocytopenia <100,000mm–3 | 13% |
| Epidemiological findings | |
| Households contacts | 21–31% |
| Radiological findings | |
| Pulmonary infiltrates | 14–49% |
References 1,2,149,151–156.
In early reports of influenza infection in renal transplant recipients, the most common symptoms were cough and fever.155,156 Vilchez et al.149 reported 30 cases of influenza A and B among 30 adult SOT recipients (lung=19, liver=5, kidney=6) during a 10-year period at a single center. Symptoms reported included malaise, myalgia, fever, cough and dyspnea. About half of patients had pulmonary infiltrates. In this cohort of SOT recipients, influenza infection was more frequent in lung recipients than in the others.
In an Australian study of 22 lung transplant recipients with confirmed influenza A (H1N1)pdm infection,153 26% of patients presented isolated upper respiratory tract infection symptoms. Other common manifestations were dyspnea 80%, productive cough 71%, myalgias 58% and fever 55%. Five patients (23%) reported household contacts with flu-like symptoms prior to their infection.
In a study conducted in Singapore including 22 SOT recipients with influenza A (H1N1)pdm infection152 (renal=18, lung=2, heart=1, liver=1) the most frequent presenting symptoms were: fever 86%, cough 77%, sore throat 55%, productive cough 32%, myalgia 27%, rhinorrhea 18%, and dyspnea 14%. Another study carried out in Argentina151 included 77 transplant recipients with symptoms compatible with pandemic influenza infection (renal=49, kidney-pancreas=8, lung=5, kidney-heart=1, liver=3), although only 23 cases were microbiologically confirmed. Reported clinical symptoms at the time of the first visit were as follows: fever 89%, cough 84%, rhinorrhea 33%, headache 26%, sore throat 24%, dyspnea 22%, and diarrhea 9%. Laboratory findings include leukopenia in 16% of cases, and lymphopenia in 30%. About half of patients had pulmonary infiltrates, which were bilateral in 84% of cases.
In a study by Kumar et al.,1 including 242 adult and child SOT recipients with H1N1 infection from Canada, United States and Netherlands (kidney=87, liver=47, lung=33, heart=45, intestinal=5, other combination=20), the most common presenting symptoms were cough 91%, fever 85%, myalgias 51%, rhinorrhea 43%, sore throat 43% and headache 32%. Interestingly, gastrointestinal symptoms were reported in 44% of cases. Lymphopenia was present at diagnosis in 61% of recipients. In 32% of patients an alveolar consolidation in chest radiograph or CT was documented. Significantly, 31% of patients had ill household contacts.
In a multicenter study in Spain (2), including 51 hospitalized adult SOT recipients with influenza A (H1N1)pdm infection (kidney=24, liver=11, lung=8, heart=5, pancreas-kidney=2, liver-kidney=1), 78% of cases occurred beyond the first year post-transplantation. The clinical manifestations reported were fever 94%, cough 80%, sore throat 11%, arthralgias 62%, headache 31%, dyspnea 29%, rhinorrhea 21%, diarrhea 9% and vomiting 17%. Twenty-nine per cent of patients had pulmonary infiltrates, which were bilateral in 73% of cases. The only laboratory findings that differed from previous basal values at diagnosis were lower lymphocyte and platelet counts.
It is of paramount importance to consider influenza in the differential diagnosis of pneumonia in SOT recipients during the influenza season, particularly in patients with flu-like symptoms. Moreover, it should be noted that influenza can be transmitted to recipients by health care providers or visiting family members. Therefore, influenza should also be ruled out in hospitalized recipients with flu-like symptoms and/or pneumonia, including those cases occurring in the early post-transplant period.
What are the prognostic factors for influenza in solid-organ transplant recipients?Recommendations- 35.
Influenza appears to be most severe in the early post-transplant period (<3 months) in solid organ transplant recipients (AII).
- 36.
The most significant factors associated with a worse outcome are diabetes mellitus, septic shock at presentation, pneumonia and secondary/concomitant pulmonary infection (AII).
- 37.
Early antiviral therapy is associated with better outcomes, and is the only prognostic factor that may be modified by medical intervention (AII).
- 38.
The use of antilymphocyte globulin has also been associated with poor outcomes in solid organ transplant recipients with influenza infection (AII).
- 39.
Influenza infection may also cause indirect effects including allograft rejection (AII).
Influenza infection in SOT recipients has been associated with high rates of complications and mortality.160 SOT recipients with influenza are hospitalized in about 57% to 70% of cases, and in 13% to 20% ICU admission is required.1,2,149,151–155 Associated mortality of influenza in this population ranges from 4% to 8%,1,2,151,152 rising as high as 21% in a small series of lung recipients during the influenza A H1N1 pandemic.153 In addition, influenza has been associated with both acute and chronic allograft dysfunction. The incidence of acute allograft rejection during influenza infection varies from 9% to 61%, with higher rates in lung transplant recipients.2,149,152 Chronic allograft rejection or bronchiolitis obliterans syndrome is mainly reported in lung allograft recipients.159
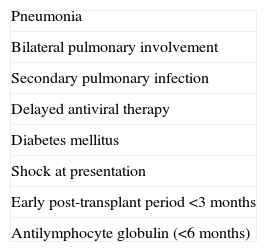
Prognostic factors of influenza in SOT recipients are similar to those in the general population.144,161–163 The prognostic factors identified for influenza infection in SOT recipients are summarized in Table 4. The most important prognostic factors are the presence of pneumonia, secondary pulmonary infection and delayed antiviral therapy.
Prognostic factors of influenza infection in SOT recipients associated with poorest outcome.
| Pneumonia |
| Bilateral pulmonary involvement |
| Secondary pulmonary infection |
| Delayed antiviral therapy |
| Diabetes mellitus |
| Shock at presentation |
| Early post-transplant period <3 months |
| Antilymphocyte globulin (<6 months) |
Viral pneumonia is the most common cause of pulmonary infiltrates in patients with influenza infection.164–166 In the general population, patients with pneumonia more frequently presented shock, required ICU admission, underwent mechanical ventilation, and had longer length of hospital stay and higher mortality than other patients.164 Pneumonia in SOT recipients is also associated with ICU admission and fatal outcome, especially when bilateral involvement is present.1,2,151
It has been estimated that up to 17% of SOT recipients with seasonal influenza infection develop secondary bacterial pneumonia.149 Conversely, in studies performed during influenza A (H1N1) pandemic infection, secondary bacterial infection in SOT recipients ranged from 4% to 13%.2,149 Association of influenza infection with fungal coinfection has also been described in immunosuppressed patients.2,149,167 SOT recipients with influenza who developed secondary pulmonary infection had a worse outcome, with longer hospital stays and higher rates of severe disease and mortality.1,2
Delayed antiviral therapy increases illness severity and mortality in patients with influenza infection in the general population as well as in immunosuppressed patients.3,23,74,144,161–163,168,169 In a study of SOT recipients with influenza A (H1N1)pdm infection, time from onset of symptoms to treatment was longer in SOT recipients requiring hospitalization and ICU admission.151 Moreover, in a large cohort of SOT patients diagnosed with influenza A (H1N1)pdm, delayed antiviral treatment was a factor independently associated with ICU admission.1 Mortality was also higher in the group receiving delayed antiviral therapy (6% vs. 1%).1 Time to antiviral therapy was also independently associated with severe influenza infection in another cohort of SOT recipients in Spain.2 In fact, during an episode of influenza infection, the earlier antiviral drugs are started, the better the clinical outcomes (i.e. lower rates of hospitalization, death and complications).169
Diabetes mellitus has been recognized as an independent factor of poor outcome in SOT recipients with influenza infection and is associated with severe influenza infection2 and higher rates of ICU admission.1 Diabetes mellitus has also been identified as a risk factor for secondary pulmonary infection.2 In this population, the development of post-transplant diabetes mellitus related to the use of anticalcineurin inhibitors is a matter of concern.
SOT recipients with influenza presenting with shock had a greater risk of secondary bacterial infection and severe illness,1,2 as also reported in the general population.160 Early post-transplantation influenza infection has been recognized as a risk factor for more severe illness.164 Considering that influenza may be transmitted to SOT patients by the health care team or by visiting family members, the vaccination of all close contacts of SOT recipients is critical to avoid influenza transmission. In addition, any patient with influenza-like symptoms should avoid contact with immunocompromised patients.74
Antilymphocyte globulin administered in the previous six months is the only immunosuppressive drug that has been associated with poor outcomes in SOT recipients with influenza infection.1
Influenza infection has been associated with the development of acute allograft rejection in SOT recipients.2 In lung transplant recipients, influenza has also been linked to the occurrence of bronchiolitis obliterans syndromes.159 Therefore, influenza infection can cause indirect effects with possibly devastating consequences for graft function.2
If influenza infection is suspected in a SOT recipient, which microbiological studies should be performed?Recommendations- 40.
It is recommended to confirm a diagnosis by specific testing when influenza infection is suspected in a transplant patient (AII).
- 41.
Specimens should be collected as soon as possible after illness onset, preferentially within the first 48h after the onset of symptoms (AII).
- 42.
Appropriate samples include nasal and oropharyngeal swabs, both placed in a single viral transport medium. If there is clinical or radiological evidence of lower respiratory tract involvement, bronchoalveolar lavage should be considered (AII).
- 43.
Respiratory specimens should be refrigerated at 4°C pending testing and should be tested for influenza as soon as possible after collection (AII).
- 44.
Reverse transcriptase polymerase chain reaction (RT-PCR) is the most sensitive and specific testing modality for influenza and it is useful for differentiating quickly between influenza types and subtypes; if RT-PCR is not available, rapid antigen detection assays may be used, although due to the lower sensitivity of this method compared to RT-PCR, a negative test result does not rule out influenza virus infection that should be confirmed (AII).
- 45.
During influenza season, in patients with lower respiratory tract disease, both influenza and bacterial etiologies should be considered, regardless of a positive microbiological result in any of them (AII).
- 46.
In any patient undergoing treatment who fails to have an appropriate clinical response within 3–5 days of initiating antiviral therapy or who has a relapsing course despite ongoing therapy, the possibility of antiviral resistance should be considered (AII).
Influenza A and B are common causes of viral infections each year in transplant recipients with predictable complications including viral pneumonia, secondary bacterial pneumonia and possible acute allograft rejection in the weaning of setting immunosuppression.170,171
Since influenza virus infection is not associated with a unique clinical syndrome and transplant recipients often present atypical symptoms, only clinical features cannot be used to diagnose influenza infection. Therefore, it is convenient to confirm influenza diagnosis by specific testing when this is suspected in a transplant patient.74,172
The benefit of a more accurate viral diagnosis is crucial in terms of receiving appropriate antiviral therapy.147 Early treatment may reduce the severity and duration of symptoms, hospitalization and complications, the extension and quantity of viral shedding, and possibly mortality. In addition, a precise diagnosis, will establish isolation measures of in-patients to prevent transmission to other patients, as well as to health care workers. Moreover, several studies indicate that testing for influenza may decrease the use of antibiotics and possibly reduce the necessity of performing some other diagnostic tests.74,160
A key to successfully manage patients with influenza infection is the collection of high quality respiratory tract samples for laboratory testing. Specimens should be collected as soon as possible, preferentially within the first 48h after illness onset, although immunocompromised patients may shed influenza viruses for weeks to months. Appropriate samples for testing respiratory viruses include nasal (one collected deeply from each nostril) and oropharyngeal swabs, both placed in a single tube containing viral transport medium. Nasopharyngeal washes are the sample of choice from children younger than 3 years. False negative results can occur with upper respiratory tract samples in patients with pneumonia, so, if there is clinical or radiological evidence of lower tract involvement, bronchoalveolar lavage should be considered.144 In patients with severe respiratory illness, the disappearance of detectable virus in the upper airway seems to correlate with a worsening of respiratory status.173 The detection of influenza virus in serum is not frequent and more studies are needed to determine its meaning.
All specimens should be kept at 4°C for no longer than 72h before testing and ideally should be analyzed within 24h after collection. If storage longer than 72h is necessary, clinical specimens should be kept at −80°C and specimens for virus isolation preferably in liquid nitrogen.
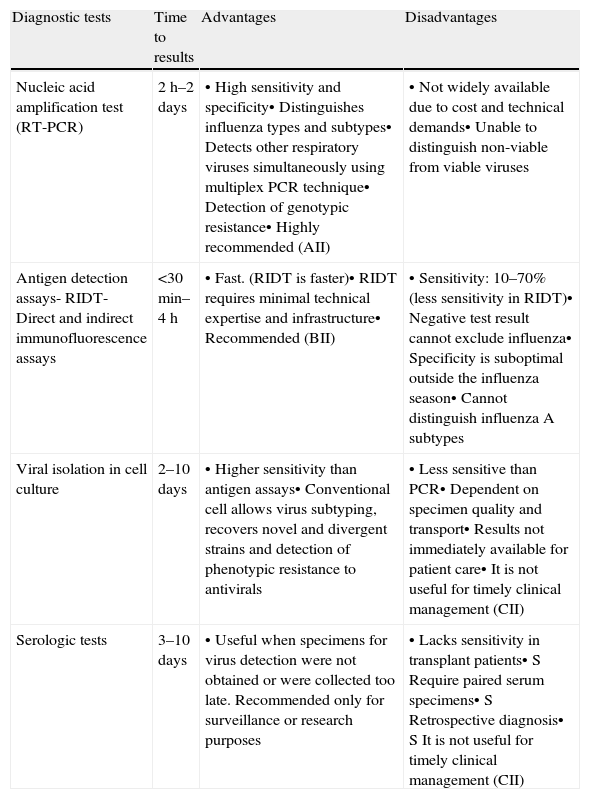
Microbiological diagnosis of influenza infection can be achieved by serology, virus culture, antigen detection and nucleic acid testing (Table 5).
Comparison of diagnostic techniques for influenza virus infection.
| Diagnostic tests | Time to results | Advantages | Disadvantages |
| Nucleic acid amplification test (RT-PCR) | 2h–2 days | • High sensitivity and specificity• Distinguishes influenza types and subtypes• Detects other respiratory viruses simultaneously using multiplex PCR technique• Detection of genotypic resistance• Highly recommended (AII) | • Not widely available due to cost and technical demands• Unable to distinguish non-viable from viable viruses |
| Antigen detection assays- RIDT- Direct and indirect immunofluorescence assays | <30min–4h | • Fast. (RIDT is faster)• RIDT requires minimal technical expertise and infrastructure• Recommended (BII) | • Sensitivity: 10–70% (less sensitivity in RIDT)• Negative test result cannot exclude influenza• Specificity is suboptimal outside the influenza season• Cannot distinguish influenza A subtypes |
| Viral isolation in cell culture | 2–10 days | • Higher sensitivity than antigen assays• Conventional cell allows virus subtyping, recovers novel and divergent strains and detection of phenotypic resistance to antivirals | • Less sensitive than PCR• Dependent on specimen quality and transport• Results not immediately available for patient care• It is not useful for timely clinical management (CII) |
| Serologic tests | 3–10 days | • Useful when specimens for virus detection were not obtained or were collected too late. Recommended only for surveillance or research purposes | • Lacks sensitivity in transplant patients• S Require paired serum specimens• S Retrospective diagnosis• S It is not useful for timely clinical management (CII) |
RIDT – rapid influenza diagnostic tests.
Serologic testing is usually not recommended for initial diagnosis of respiratory viral infections since it has a reduced sensitivity among transplant recipients. Influenza serologic test data for a single serum specimen cannot be reliably interpreted. Paired acute and convalescent phase serum specimens are needed for determination of antibody titers; the most reliable method for serological diagnosis is the demonstration of a greater than 4-fold increase in virus-specific IgG levels.174 Results are useful only for retrospective diagnosis and for epidemiological and research purposes but it will not influence clinical management.
Antigen detection assaysDirect and indirect immunofluorescence assays use commercial type-specific monoclonal antibodies to detect viral antigen directly on clinical specimens or from cell culture to confirm viral cytophatic effect. Immunofluorescence assays have a specificity greater than 90% and a sensitivity of 47–93% compared with cell culture and PCR methods.175
In the last years, rapid antigen detection assays, sometimes called point-of-care tests, have been developed. These tests give visual results on immunochromatographic strips using influenza A or B nucleoprotein specific monoclonal antibodies within 10–30min. These assays exhibit a high specificity but limited sensitivity (10–70%) compared with PCR and with viral culture.176
Sensitivity of antigen detection assays is significantly higher in children and when specimens are collected within the first few days of illness. The clinical usefulness of these tests is associated with their positive and negative predictive values and is greater during the peak influenza season, when false positive results are less likely and positive predictive value is higher.176
Given the limited sensitivity of these assays, a negative result does not rule out influenza virus infection and follow-up testing with PCR and/or viral culture should be considered to confirm negative results. However, a positive result is useful because the specificity of these tests is high. Although these tests do not provide information on the influenza A subtype, if most circulating influenza A viruses have similar antiviral susceptibilities, influenza A subtype data may not be needed to guide clinical care.
Virus isolationInfluenza virus replication within cell culture has been the reference technique.177,178 However, this technique has considerable limitations. Usually, it requires specific technical expertise, it is labor-intensive and expensive and it takes several days to the virus to grow and be identified.
Nevertheless, virus isolation is usually more sensitive than antigen detection assays and it is capable of recovering novel or highly divergent strains missed by other tests, providing an isolate for subsequent characterization. In addition and in contrast to the nucleic acid amplification tests, it only detects viable viruses which can be very useful for patient management.
Nucleic acid amplification testsViral RNA detection using nucleic acid amplification is considered the most sensitive, specific and versatile test for the diagnosis of influenza and it has replaced viral isolation as reference standard. Compared with isolation in cell culture, sensitive PCR assays can more readily identify influenza viruses in immunosuppressed transplant recipients for whom frequent lower respiratory tract infections are often associated with low viral levels.179 Although the turnaround time for nucleic acid testing is intermediate between cell culture and direct antigen detection, newer techniques can reduce this to 4–5h or less.
Specimen quality, timing and transportation conditions although are important may be less critical for nucleic acid testing than culture and antigen detection, since it is not necessary to preserve viable virus and intact infected cells. In addition, influenza virus RNA is detectable for several days longer into the clinical course than cultivable virus.
In the literature there is a lot of information about commercial tests and in-house assays that use a variety of amplification methods. RT-PCR is the most commonly method used allowing the identification of type and subtype of influenza virus.180
Conventional end-point RT-PCR including nested PCR assays have been developed and provides high sensitivity181; however, most clinical laboratories do not use these techniques because they increase the work load and the risk for contamination is high. Real time RT-PCR assays for influenza combine the reverse transcription, amplification and detection steps, shortening the time of the assay and reducing cross contamination.175 Moreover, real time RT-PCR can quantify the viral load in clinical specimens to assess prognosis and measure antiviral efficacy.182,183
Determination of the specific genetic sequence of amplified DNA is the highest level of genetic characterization. Genetic-sequence data allow the detailed analysis necessary for evolutionary characterization as well as for the identification of specific mutations with biological significance, such as those leading to changes in antiviral resistance, antigenic variability, and pathogenicity in different hosts. Transplant recipients usually experience prolonged viral shedding, even in the setting of active antiviral therapy. This fact may contribute to increasing the risk of emergence of resistant variants. Any patient undergoing treatment who fails to have an appropriate clinical response within 3–5 days of antiviral therapy or who has a relapsing course despite ongoing therapy, should be suspected of being infected with a resistant virus. Sanger sequencing and pyrosequencing have been used to study the resistance to the neuraminidase inhibitors and to M2 inhibitors.7,182
However, it should be noted that molecular methods also have limitations, such as the lack of immediate access in some hospitals, and the fact that they do not distinguish viable viruses from non-viable viruses. Laboratories must be alert to the fact that even small changes in nucleic acid sequences of circulating viruses may affect the sensitivity of molecular methods and new viruses may appear. Home-brew tests can be more rapidly adapted than commercial kits to accommodate these changes. The WHO web site is a valuable tool for clinicians and laboratories, providing guidance on test indications and performance and informing on test limitations.184
It is important to take into account that influenza like illness can be attributed to a wide range of respiratory viruses,185 therefore, it is advisable for patient management to differentiate infections caused by influenza viruses from those caused by other respiratory viruses. Currently, newer multiplex RT-PCR techniques can simultaneously detect influenza and other respiratory viruses.180
On the other hand, bacterial pneumonia is a known complication of influenza infection in immunosuppressed patients. It should also be considered that a positive influenza result does not exclude bacterial coinfection and the evaluation for the potential necessity of antibiotics.2
When should a SOT recipient with suspicion of influenza be treated with antivirals?Recommendations- 47.
Adequate antiviral therapy should be initiated, as soon as possible, in all solid organ transplant recipients with suspected influenza infection, (AII) independently of the severity of illness and duration of symptoms (AII) and without waiting for diagnostic results (AII).
- 48.
Early antiviral treatment should be especially recommended in solid organ transplant recipients with influenza pneumonia, (AII) as the presence of this complication is an important factor for poor outcome (AII).
Antiviral treatment should be initiated as soon as possible if suspected influenza infection in SOT recipients regardless of the severity of illness and without waiting for diagnostic results.1,2,74,147,186 Early antiviral treatment in SOT recipients with influenza infection should be especially encouraged in cases of pneumonia, as the presence of this disease is known to be an important factor for poor outcome.1,74,187 Moreover, antivirals should be administered even if patients have presented symptoms for longer than two days, given the beneficial effect on outcomes in patients with severe illness and those at high risk of complications such us SOT recipients.1,2,74,188,189
The optimal antiviral drug in influenza infection depends on the prevalence of antiviral resistance in the circulating influenza viruses. Health authorities monitor data on resistance prevalence in circulating influenza strains and update it for clinicians.190 Two classes of antivirals are currently available for treatment of influenza infection: M2 inhibitors (amantadine and rimantadine), and neuraminidase inhibitors (oseltamivir and zanamivir).186 In addition, there are some investigational drugs for influenza infection such as intravenous zanamivir, parenteral peramivir, and inhaled laninamivir.191
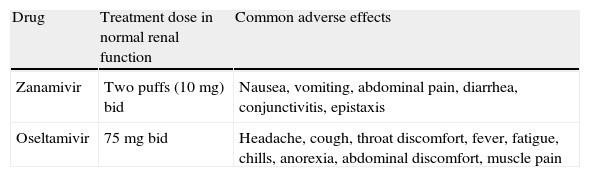
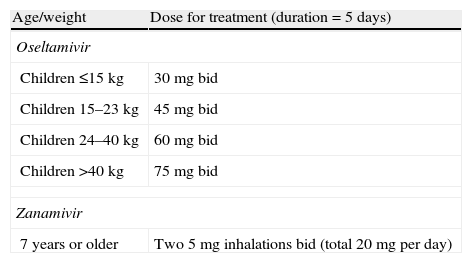
In general, most seasonal A (H3N2), influenza A (H1N1)pdm and influenza B viruses are resistant to M2 inhibitors, that are no longer recommended.74,147 Neuraminidase inhibitors generally remain active against most circulating strains of influenza, and are the antiviral drugs currently used for treating influenza infections.74,147 However, oseltamivir-resistant strains of influenza A (H1N1)pdm have been isolated over the course of therapy among patients with severe immunosuppression such as stem cell or organ transplantation recipients.192–194 Influenza resistance is generally due to a mutation, in the H275Y position of the neuraminidase protein, which confers resistance to oseltamivir but not to zanamivir. Thus inhaled zanamivir is the treatment of choice for patients with oseltamivir resistance.1,2,74,147 Approved antiviral neuraminidase inhibitors are usually well tolerated, with the most common adverse effects being gastrointestinal symptoms.169 The recommended doses and adverse effects of oseltamivir and zanamivir are shown in Table 6. Although oseltamivir dose adjustment is not needed in hepatic impairment, oseltamivir doses should be modified in renal insufficiency according to the recommendations included in this document.
Recommended doses and adverse effects of neuraminidase inhibitors for treating influenza.
| Drug | Treatment dose in normal renal function | Common adverse effects |
| Zanamivir | Two puffs (10mg) bid | Nausea, vomiting, abdominal pain, diarrhea, conjunctivitis, epistaxis |
| Oseltamivir | 75mg bid | Headache, cough, throat discomfort, fever, fatigue, chills, anorexia, abdominal discomfort, muscle pain |
bid, twice daily.
However, approved antiviral neuraminidase inhibitors have some drawbacks. Inhaled zanamivir requires the patient to inspire deeply and may induce bronchospasms, and it is poorly tolerated by patients with underlying lung disease.147,169 There are no current data on the safety, tolerability, or efficacy of inhaled zanamivir in lung transplant recipients. Nebulization of the commercially available preparation of zanamivir has been reported to cause ventilator dysfunction and even death, and should not be used195 (EIII).
In addition, oral oseltamivir bioavailability may be altered in critically ill patients and in those with gastrointestinal dysfunction.196 Higher doses of oseltamivir have been suggested in SOT recipients with influenza infection. The aim of raising the doses of oseltamivir to 150mg twice daily adjusted for creatinine clearance is to increase oral bioavailability and improve antiviral efficacy. Although some SOT recipients with severe influenza infection have been treated successfully and safely with high doses of oseltamivir,197 proven benefits of this strategy are lacking (BIII).
Intravenous antiviral therapies, when available, should be considered in SOT recipients severely ill despite receiving oral therapy, in patients in whom oral absorption is a concern, or in those recipients with suspected or confirmed oseltamivir resistance198–203 (AIII). Nevertheless, it should be noted that studies evaluating the efficacy of intravenous zanamivir in critically ill patients with influenza have encountered mixed results.201–203 In the same regard, there are few data about the clinical effectiveness of intravenous peramivir.199,200,203 Ongoing studies will provide information concerning the appropriate use of new antiviral drugs.
Pregnant women with influenza infection have increased risk of complications and poor neonatal outcomes.204,205 At present, there are scarce data on safety and efficacy for antiviral drugs for the treatment of influenza infection during pregnancy and the reported experience mainly involves oseltamivir.206 Data from limited studies do not indicate that oseltamivir is a human teratogen.206 During the 2009 influenza A pandemic, women who received delayed treatment with neuraminidase inhibitors were more likely to develop severe disease.204,205 Accordingly, antiviral treatment should be offered as soon as possible to pregnant transplant recipients with suspected or documented influenza considering the risk-benefit balance (AII).
What other therapeutic measures should be adopted in solid-organ transplant recipients with influenza infection?Recommendations- 49.
Drugs containing salicylates should be avoided in children and adolescents with influenza infection because of the risk of Reye's syndrome (EIII).
- 50.
Immunomodulatory drugs such as statins and macrolides have not demonstrated any benefit in treating influenza infection (CIII).
- 51.
High doses of glucocorticosteroids should be avoided in patients with influenza infection because of the risk of opportunistic infections, prolonged viral shedding, severe disease and death (EII).
The most important consideration for treating children and adolescents with symptomatic influenza infection is to avoid drugs containing salicylates because of the increased risk of Reye's syndrome.207
There are no clinical data demonstrating beneficial effects of immunomodulatory adjunctive therapies such as corticosteroids, statins and macrolides in SOT recipients with influenza infection. The use of corticosteroids is not recommended for this reason and also because of the risk of opportunistic infections and prolonged viral shedding.198 In addition, in severely ill nonimmunocompromised patients with influenza, the use of glucocorticoids has been shown to increase the risk of severe disease and death208–210 (EII). Studies evaluating the benefit of statins in influenza infections have given mixed results: some supporting efficacy in terms of reducing morbidity and mortality in patients with influenza,211 but not others.210 Experimental studies have shown inhibitory effects of macrolides on influenza virus replication improving survival,212 however no evidences of benefits have been established in patients with influenza infection.210
In SOT recipients with influenza infection, the serum levels of immunosuppressive drugs should be promptly determined and dose adjustment should be performed whenever necessary. In patients requiring ICU admission the current SEMICYUC/SEIMC recommendations should be followed.213
When should antibiotic treatment be administered to SOT recipients with influenza infection?Recommendations- 52.
Secondary/concomitant bacterial infections in SOT recipients with influenza should be suspected in patients with pneumonia, shock, purulent sputum and leukocytosis, especially in diabetic patients, thus antibiotic treatment should be administered in this setting (AIII).
Antibiotics should be administered when bacterial pneumonia is suspected in SOT recipients who have influenza. The most common cause of pulmonary infiltrates during influenza infection is viral pneumonia.164–166 However, between 4% and 17% of SOT recipients with influenza have secondary/concomitant bacterial infection.1,2,74 Furthermore, a report of autopsies of patients who died from influenza A (H1N1)pdm09 infection found that 29% of cases had evidence of bacterial infection.214
A study of hospitalized patients with influenza infection comparing patients with viral pneumonia with those with secondary bacterial pneumonia found that patients diagnosed with secondary bacterial pneumonia were more likely to have chronic liver disease, purulent sputum, tachycardia, pleural effusion, leukocytosis and elevated C reactive protein.211 The presence of shock has also been associated with bacterial infection in immunocompetent patients with influenza.215,216 Moreover, having diabetes mellitus and shock were risk factors for secondary/concomitant pulmonary infection in SOT recipients with influenza A (H1N1)pdm infection.2
These findings may suggest that among non-severe SOT recipients with influenza, non-diabetic patients can be managed without antibacterial therapy in the absence of symptoms associated with secondary bacterial infection. This practice reduces the number of patients exposed to antibiotics and antimicrobial overuse.
How should neuraminidase inhibitors be used in solid-organ transplant recipients with renal function impairment?Recommendations- 53.
Inhaled zanamivir has very limited systemic bioavailability and dose adjustment is not required in patients with renal function impairment (AIII).
- 54.
A dosage reduction of oseltamivir is recommended only for patients with a glomerular filtration rate under 30mL/min (AIII).
- 55.
It is recommended to administer 75mg of oral oseltamivir for the first dose in all patients with renal function impairment and then adjust the doses according to renal function (AIII).
- 56.
Oseltamivir does not affect pharmacokinetics of cyclosporine, mycophenolate or tacrolimus (AII).
- 57.
A 30mg dose of oseltamivir given once weekly in chronic ambulatory peritoneal dialysis or after alternate sessions in hemodialysis patients provides sufficient exposure to oseltamivir to allow safe and effective treatment and prophylaxis for influenza infection (AII).
- 58.
A regimen of either 30mg daily or 75mg every 48h is recommended for patients on continuous renal replacement therapies (AII).
Patients with chronic kidney diseases and in particular those with end-stage renal disease who developed influenza infection have an increased risk of morbidity and mortality.217 Neuraminidase inhibitors are drugs that are mainly eliminated by renal clearance,218 thus neuraminidase inhibitor dosage should be adjusted in cases of renal failure.
Estimation of renal function in chronic kidney diseaseThere are various glomerular filtration rate (GFR) estimating equations that provide more accurate estimates of measured GFR than serum creatinine alone.219 The Modification of Diet in Renal Disease (MDRD) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) are the most frequently used GFR estimating equations. In order to stratify chronic kidney diseases, estimated GFR is the key point.220 Patients are classified as shown in Table 7.
Kidney Disease outcomes Quality Initiative (K/DOQI) stages of Chronic Kidney Disease.
| Stage 1 | GFR >90mL/min per 1.73m2 with albuminuria |
| Stage 2 | GFR 60–89mL/min per 1.73m2 |
| Stage 3a | GFR 45–59mL/min per 1.73m2 |
| Stage 3b | GFR 30–44mL/min per 1.73m2 |
| Stage 4 | GFR 15–29mL/min per 1.73m2 |
| Stage 5 | GFR <15mL/min per 1.73m2 or treatment by dialysis |
Inhaled zanamivir has very limited systemic bioavailability and dose adjustment is not required in patients with renal function impairment. Oseltamivir phosphate is orally administered and is rapidly metabolized to oseltamivir carboxylate, the active form. Oseltamivir is primarily excreted through the kidney and therefore patients with impaired renal function experience increased systemic exposure to oseltamivir increasing the severity of renal impairment.221 When compared with patients with normal renal function, the area under the curve of oseltamivir carboxylate serum levels were increased by 2-, 3- and 10-fold in patients who had mild (Stage 2 CKD), moderate (Stage 3 CKD) and severe (Stages 4 and 5 CKD) renal impairment, respectively.222
As exposure to higher concentrations of oseltamivir that has not been associated with reduced tolerability,223 a dosage reduction is recommended only for patients with a GFR under 30mL/min. Tubular secretion can overcome the reduced GFR to some extent in cases of moderate renal failure.224 As the therapeutic margin of oseltamivir carboxylate is quite large, administration of a slightly higher dosage (i.e. about 50% higher) than the reduced dosage calculated according to the GFR has been recently recommended.221 Regardless of renal function, it is recommended to administer 75mg of oseltamivir for the first dose in all cases, adjusting the dose according to renal function (Table 8).221 Nevertheless, it has been suggested that further investigation on circulating oseltamivir carboxylate concentrations in cases of renal function impairment is warranted in order to validate such approach.221
Dosage of olseltamivir in patients with renal impairment.
| Stage of Chronic Kidney Disease | GFR (mL/min) | Treatment | Prophylaxis |
| 75 mg first dose in all cases, then: | |||
| 1–2 | >60 | 75mg bid | 75mg od |
| 3 | 30–60 | 75mg bid | 75mg od |
| 4 | 15–30 | 45mg od | 45mg q2d |
| 5 | <15 | 75mg sd | 75mg sd |
bid, twice daily; od, once daily; q2d, every other day; sd, single dose.
Reference 221.
It has been demonstrated that oseltamivir does not affect the pharmacokinetics of the main immunosuppressive drugs. A recent crossover study performed in 19 adult renal allograft recipients showed that oseltamivir did not affect pharmacokinetics of cyclosporine, mycophenolate or tacrolimus.225 No data are available regarding simultaneous administration of neuraminidase inhibitors and mammalian target of rapamycin (mTOR) inhibitors.
Use of neuraminidase inhibitors in patients under renal replacement therapiesOseltamivir carboxylate pharmacokinetics in patients on hemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD) differs markedly from patients with intact renal function.226 The interindividual pharmacokinetic variability of oseltamivir carboxylate measured in both HD and CAPD patients exceeded that observed in healthy subjects with normal renal function.222 A dosing regimen of 30mg after finishing every other HD session, or 30mg weekly after a CAPD exchange, is expected to be effective and well tolerated.
In a small study of critically ill patients during the 2009 influenza pandemic, oseltamivir was well absorbed in this population.196 The usual dose of 75mg twice daily resulted in plasma concentrations of the active carboxylate metabolite that were comparable to those observed among ambulatory patients in clinical trials. In this study, oseltamivir carboxylate clearance during continuous replacement therapy (CRRT) was significantly lower than that in patients with normal renal function. The area under the curve of serum concentration was almost 6-fold higher in CRRT patients than in those with normal renal function. A regimen of either 30mg daily or 75mg every 48h was recommended for patients on CRRT.23,221 Oseltamivir dosage recommendations for patients on HD, CAPD or CRRT are summarized in Table 9.
Oseltamivir doses for patients on renal replacement therapies and other extracorporeal therapies.
| Type of procedure | Dose for treatment(limited data for these recommendations) | Dose for prophylaxis(limited data for these recommendations) |
| 75 mg first dose in all cases, then | ||
| Hemodialysis | 30mg after every other hemodialysis session* | 30mg after every other hemodyalisis session* |
| Peritoneal dialysis | 30mg weekly* | 30mg weekly* |
| Continuous renal replacement therapy | 45mg od | 45mg q2d |
| Extracorporeal membrane oxygenation | Standard treatment | |
od, once daily; q2d, every other day.
References 221,226.
Oseltamivir dose adjustment for patients who develop acute kidney failure over chronic kidney disease is not well characterized and more studies are warranted. As the therapeutic window of oseltamivir is large, extreme adjustments of oseltamivir doses are not recommended.
What are the special recommendations regarding influenza in SOT pediatric recipients?Recommendations- 59.
Influenza vaccine is approved for solid organ transplant infant recipients and recommended for children older than 6 months of age (AII).
- 60.
The pediatric solid organ transplant recipients of 6–12 months of age should be a priority group for vaccination, given the paucity of data on the use of antiviral agents in infants younger than 12 months of age (AIII).
- 61.
In children younger than 9 years of age with no previous influenza vaccination, the administration of two doses of influenza vaccine, 1 month apart (AII), is recommended.
- 62.
All household and close contacts of pediatric solid organ transplant recipients should also be vaccinated (AII).
- 63.
The adherence to the infection control measures (use of masks, compliance with respiratory isolation) may be difficult in very young children. Viral shedding may be more prolonged and copious in young children compared to adults. Thus, the young solid organ transplant recipient is expected to shed virus for an even more prolonged period, and be more likely than adults to spread the virus to close contacts and to the environment (AI).
- 64.
The use of antiviral agents in the treatment of influenza infection in children is similar to that in adults (AI).
- 65.
Adequate antiviral therapy should be initiated as early as possible in all pediatric solid organ transplant recipients with suspected influenza infection (AII) independently of the severity of illness and duration of symptoms (AIII) and without waiting for diagnostic results.
- 66.
There is concern regarding the safety of the use of oseltamivir in infants younger than 1 year of age due to data extracted from animal studies at doses higher than those used in children. While more clinical data become available, we recommend the use of oseltamivir in children under 1 year of age with dosing based on the body weight (CIII).
Influenza infection is recognized to cause increased morbidity and mortality in healthy and immunocompromised children. Influenza infection is a common cause of visits to medical clinics and emergency departments in children younger than 5 years of age. One study estimated that annual influenza epidemic results in 50–95 clinic visits per 1000 children and 6–27 emergency department visits per 1000 children per year.227
Influenza illness more likely presents as nonspecific symptoms especially in young infants compared to older children and adults, for example sepsis-like picture or diarrhea. In a comparison of presenting symptoms in adult versus pediatric SOT recipients with influenza A (H1N1) virus infection, children were more likely to present as fever, rhinorrhea, sore throat, and headache than adults.1
Among pediatric organ transplant recipients, influenza infection has been associated with significant complications, including respiratory failure requiring mechanical ventilation and death.228
Evidence has demonstrated that oseltamivir is effective in the treatment of children with influenza infection.229 In previously published retrospective cohorts of pandemic influenza in pediatric SOT recipients, the treatment was well tolerated and the authors concluded that early initiation of therapy (antiviral treatment within 48h from symptoms onset) was associated with a lower likelihood of pneumonia, with reduced need for ICU admission and reduced need for mechanical ventilation.1,230 Dose adjustment is provided in Table 10.
Dose recommendations for children 12 months of age or older.
| Age/weight | Dose for treatment (duration=5 days) |
| Oseltamivir | |
| Children ≤15kg | 30mg bid |
| Children 15–23kg | 45mg bid |
| Children 24–40kg | 60mg bid |
| Children >40kg | 75mg bid |
| Zanamivir | |
| 7 years or older | Two 5mg inhalations bid (total 20mg per day) |
Note: Adjustments recommended for patients with a glomerular filtration rate under 30mL/min. For these patients, the treatment dose is reduced to a once daily dose.
bid, twice daily.
Non-adjuvanted influenza seasonal vaccine demonstrated seroprotection ranging from 38% to 71% in pediatric transplant recipients.231
Despite the limitations (studies with few number of patients, mainly on pediatric kidney and liver transplant recipients, performed during different immunosuppressive regimens) the use of influenza vaccine is associated with rates of seroresponse that are close to that of immunocompetent children. In addition, it does not appear to be any increase in the risk of developing vaccine associated adverse events.231–233
Influenza A (H1N1) infection has been documented as a risk factor for acute rejection and the development of obliterative bronchiolitis in pediatric lung transplant recipients.234
Conflict of interestThe authors have no conflict of interest to declare.
We thank Yolanda Meije MD, PhD, José María Aguado MD, PhD, Jesús Fortún MD, PhD and Julián de la Torre-Cisneros MD, PhD, members of GESITRA-SEIMC, for their comments of the manuscript, and Michael McConnell for critical reading.
This consensus statement proposes the following criteria to locally assess the implementation and adherence to recommendations:
- 1.
Percentage of donor – recipient transmission of influenza infection.
- 2.
Percentage of influenza infection among SOT recipients exposed to a microbiologically proven case.
- 3.
Percentage of SOT recipients that receive the influenza vaccine each season.
- 4.
Percentage of household contacts of SOT recipients that receive the influenza vaccine each season.
- 5.
Percentage of SOT recipients with flu-like symptoms in which samples for microbiological diagnostic are collected.
- 6.
Percentage of SOT recipients with pneumonia in which samples for microbiological diagnosis are collected during influenza season.
- 7.
Percentage of SOT recipients that receive antiviral treatment within the first 48h after the onset of symptoms.
- 8.
Percentage of SOT recipients with influenza infection and renal function impairment in which antiviral dose is administered according to the recommendations.
- 9.
Percentage of SOT recipients younger than 9 years of age with no previous vaccination who receive two doses of influenza vaccine, 1 month apart.
This document has been reviewed and is supported by the Spanish Society of Transplantation (SET), the National Transplant Organization (ONT) of the Ministry of Health of Spain, the Spanish Society of Nephrology (SEN), the Section of Heart Failure and Heart Transplantation of the Spanish Society of Cardiology (SEC) and the Spanish Society of Liver Transplantation (SETH).