Information about paediatric in-hospital antimicrobial usage and prescribing patterns to guide improvement strategies is scant. We aim to use an evaluation of the prevalence and appropriateness of antimicrobial prescription to identify antimicrobial stewardship priorities in children.
MethodsA cross-sectional point study was performed on hospitalised paediatric patients in a Spanish tertiary hospital, assessing the prevalence of antimicrobial prescription (PAP) and appropriateness of antimicrobial prescription (AAP). AAP was defined as a correct indication plus an appropriate prescribing pattern (dose, spectrum and interval). Evaluation was performed using established antimicrobial guidelines. Other factors that may have a bearing on antimicrobial prescription were also analysed.
ResultsA total of 171 patients were included. PAP was 49.7% (85/171) and AAP was 60.9% (91/161). The most common indications for antimicrobial use were antimicrobial prophylaxis (28.3%, 32/113) and pneumonia (8.2%, 8/113). Overall, 161 antimicrobials were prescribed (1.9 antimicrobials per patient): 55.3% (89/161) were empiric, 16.1% (26/161) were targeted and 28.6% (46/161) were prophylactic. Amoxicillin/clavulanate (8.2%, 14/171) and sulfamethoxazole/trimethoprim (8.2%, 14/171) were the most prescribed antimicrobials. The prescription of antifungals (11.7%, 20/171) and antivirals (1.8%, 3/171) was analysed. Major causes of inappropriate antibiotic use were prolonged prescriptions (21.7%, 35/161) and use of agents with an excessively broad coverage spectrum (21.1%, 34/161). PAP and AAP varied between wards and antimicrobials.
ConclusionsMeasurement of PAP and AAP offers valuable information for detecting priorities in hospital settings and monitoring antimicrobial usage prior to the development of antimicrobial stewardship programmes. In our setting, the main areas for improvement are duration of therapy and proper use of broad-spectrum antimicrobials.
La información sobre el uso hospitalario de antimicrobianos en pediatría para orientar estrategias de mejora es escasa. Proponemos utilizar la evaluación de prevalencia y adecuación de la prescripción antimicrobiana para identificar prioridades en programas de optimización de uso de antimicrobianos en niños.
MétodosSe realizó un estudio de corte transversal en niños hospitalizados en un centro terciario español evaluando la prevalencia de prescripción antimicrobiana (PPA) y la proporción de adecuación en prescripción antimicrobiana (PAA). Se definió la PAA como una correcta indicación más un apropiado patrón de prescripción del antimicrobiano (dosis, espectro e intervalo) según guías establecidas. Se analizaron también otros factores con influencia potencial en prescripción.
ResultadosSe incluyeron 171 pacientes, obteniendo una PPA=49,7% (85/171) y PAA=60,9% (91/161). Profilaxis antimicrobiana (28,3%, 32/113) y neumonía (8,2%, 8/113) fueron las indicaciones más frecuentes. Se realizaron 161 prescripciones antimicrobianas (1,9 antimicrobianos por paciente): 55,3% (89/161) empíricas; 16,1% (26/161) dirigidas y 28,6% (46/161) profilácticas. Amoxicilina/ácido clavulánico (8,2%, 14/171) y trimetoprim/sulfametoxazol (8,2%, 14/171) fueron los antimicrobianos más prescritos. Se analizó la prescripción antifúngica (11,7%, 20/171) y antiviral (1,8%, 3/171). Las principales causas de uso inapropiado de antibióticos fueron el uso prolongado (21,7%, 35/161) y espectros de cobertura demasiado amplios (21,1%, 34/161). La PPA y PAA variaron según área de hospitalización y antimicrobianos.
ConclusionesLa PPA y PAA ofrecen información valiosa para detectar prioridades en hospitales previamente al desarrollo de programas de optimización de uso de antimicrobianos y monitorizar el uso de antimicrobianos. En nuestro centro la duración del tratamiento y el espectro antimicrobiano excesivo fueron las principales áreas a mejorar.
Inappropriate use of antimicrobials is linked to increasing antibiotic resistance due to important ecological effects.1,2 It represents a major public health problem worldwide generating increasing morbidity, mortality and hospitalisation rates in recent years.1–4 Resources invested in development of new antimicrobials will decrease, so proper use of those available must be a priority.5–7 Antimicrobial stewardship (AS) programmes that have been successfully implemented in adults have also been developed for paediatric populations.3,7–11 Although the key principles for paediatric AS have been recently defined, paediatric patients have particular characteristics that hinder establishment of such programmes, as well as assessment of their impact.3
Benchmarking studies of antimicrobial use are fundamental to implement these programmes identifying opportunities for improvement,12 with corresponding impact interventions.1,13 In this study, we aim to describe the prevalence of antimicrobial prescribing in a tertiary paediatric hospital and assess the appropriateness of antimicrobial use.
MethodsSettingThis was a cross-sectional descriptive study performed in a Spanish tertiary paediatric hospital with 256 hospitalisation beds distributed among different hospital areas: neonatology and neonatal intensive care unit (ICU), paediatric ICU, various medical and surgical wards, children with complex chronic conditions unit, departments responsible for five different types of solid organ transplantation and a haematology–oncology department that performs haematopoietic stem cell transplantation.
EthicsThe study was approved as part of an initiative from the “Hospital Infection Commission” as a measure of hospital quality of care. All collected data were anonymised and the need of obtaining a written inform consent was waived.
Study designThe use of antimicrobials was evaluated from November 11th to December 4th, 2013, following a similar approach to that first developed by the European Surveillance of Antimicrobial Consumption group (ESAC) using a point prevalence survey methodology.8,14,15 Following agreement, each ward was fully evaluated during one full day. Data were collected randomly from different departments on different days, without previous notifying to the prescribers about the scheduled evaluation day. For surgical specialties, Monday was avoided to have reliable data on antibiotic surgical prophylaxis.
Patients between 0 days and 18 years of age with active antimicrobial prescription on the assessment day starting at 10:00h were included, excluding patients admitted to the ward on that day. Data were gathered using a predesigned codified survey format, protecting patient's confidentiality. Patients without antimicrobial prescription were counted for the analysis (total bed occupancy per ward), but their data were not recorded. All systemic antimicrobials were included: antibiotics, antifungals, antivirals and antituberculosis drugs.
Variables and definitionsData recorded for each included patient were: age, ward, infectious syndrome, type of admission (scheduled or emergency), risk factors for infection and multi-resistant infection, prescribed antimicrobials detailing type of indication as well as dose, interval and spectrum. Prescribed treatment was evaluated by a Paediatric Infectious Diseases specialist trained in proper antimicrobial use and experienced in AS. Prevalence of antimicrobial prescription (PAP) was calculated for each department and for the entire centre, dividing the total of patients receiving treatment by the total bed occupancy at the time of evaluation and for each evaluated drug. When calculated for each ward and the entire centre, a patient receiving more than one antimicrobial, was counted as one in the PAP numerator. When calculated for each evaluated drug, each patient receiving the antimicrobial was counted as one in the PAP numerator. No aggregate data were used for measuring antimicrobial consumption. Treatment indication and adequacy of prescription pattern were assessed, following reference clinical practice guidelines published by the Spanish, European and American Paediatric Infectious Diseases Societies (Consensus Documents from the Spanish Society for Paediatric Infectious Diseases and Infectious Disease Society of America guidelines) adapted to the antimicrobial sensitivity patterns in our environment. Prescription was classified as “adequate” when it had a proper dose, interval and antimicrobial spectrum adjusted for patient's weight/age and infectious syndrome and when treatment length did not exceed the recommended one. Appropriateness of antimicrobial prescribing proportion (AAP) was defined as the total amount of treatments with a correct indication plus an adequate prescribing pattern (correct dose, interval, spectrum and length) divided by the total of prescribed antimicrobials.
Factors that may impact upon antimicrobial prescription were registered. Infectious syndrome was defined as the patient's diagnosis that prompted antimicrobial prescription. Risk factors for infection were defined as clinical features or device utilisation that increase a patient's susceptibility to infections. Risk factors for multi-resistant infection were defined as prior conditions that predispose to infection by multi-resistant microorganisms, and included prior antibiotic use, hospital admission within 3 months of current episode, prior admission to intensive care/reanimation unit and previous infection or colonisation by multi-drug resistant organisms on any occasion.
StatisticsStatistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA), calculating confidence intervals using the Wilson score. Data are represented as numbers (percentage) with 95% confidence intervals (CI) for categorical variables, and mean±standard deviation (SD) for the numerical variables.
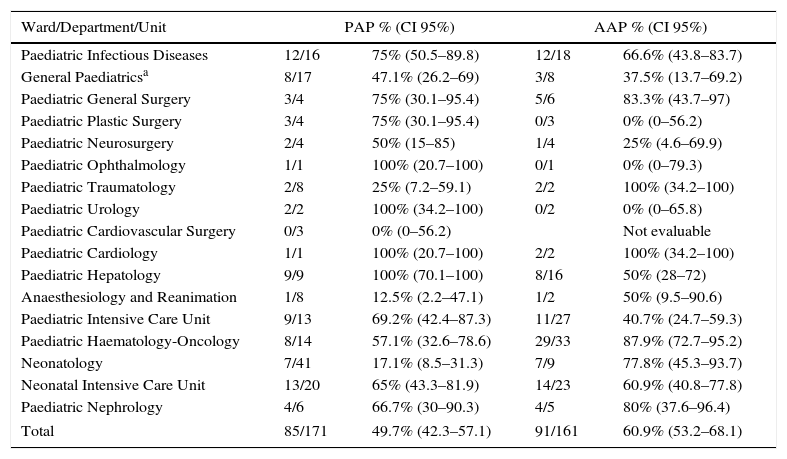
ResultsTotal bed occupancy was 171 patients with a mean (±SD) age 4.3±5.2 years. Overall, 85 patients (49.7%; 95% CI: 42.3–57.1) were receiving one or more antimicrobials, with PAP and AAP by ward summarised in Table 1. Reasons for admission were medical urgency for 58 patients (68.2%), scheduled medical admission for 12 (14.1%), scheduled surgical admissions for 11 (12.9%), and surgical emergency in 4 (4.7%) cases.
Prevalence of antimicrobial prescription and appropriateness of antimicrobial prescribing proportion by ward/department/unit of origin.
| Ward/Department/Unit | PAP % (CI 95%) | AAP % (CI 95%) | ||
|---|---|---|---|---|
| Paediatric Infectious Diseases | 12/16 | 75% (50.5–89.8) | 12/18 | 66.6% (43.8–83.7) |
| General Paediatricsa | 8/17 | 47.1% (26.2–69) | 3/8 | 37.5% (13.7–69.2) |
| Paediatric General Surgery | 3/4 | 75% (30.1–95.4) | 5/6 | 83.3% (43.7–97) |
| Paediatric Plastic Surgery | 3/4 | 75% (30.1–95.4) | 0/3 | 0% (0–56.2) |
| Paediatric Neurosurgery | 2/4 | 50% (15–85) | 1/4 | 25% (4.6–69.9) |
| Paediatric Ophthalmology | 1/1 | 100% (20.7–100) | 0/1 | 0% (0–79.3) |
| Paediatric Traumatology | 2/8 | 25% (7.2–59.1) | 2/2 | 100% (34.2–100) |
| Paediatric Urology | 2/2 | 100% (34.2–100) | 0/2 | 0% (0–65.8) |
| Paediatric Cardiovascular Surgery | 0/3 | 0% (0–56.2) | Not evaluable | |
| Paediatric Cardiology | 1/1 | 100% (20.7–100) | 2/2 | 100% (34.2–100) |
| Paediatric Hepatology | 9/9 | 100% (70.1–100) | 8/16 | 50% (28–72) |
| Anaesthesiology and Reanimation | 1/8 | 12.5% (2.2–47.1) | 1/2 | 50% (9.5–90.6) |
| Paediatric Intensive Care Unit | 9/13 | 69.2% (42.4–87.3) | 11/27 | 40.7% (24.7–59.3) |
| Paediatric Haematology-Oncology | 8/14 | 57.1% (32.6–78.6) | 29/33 | 87.9% (72.7–95.2) |
| Neonatology | 7/41 | 17.1% (8.5–31.3) | 7/9 | 77.8% (45.3–93.7) |
| Neonatal Intensive Care Unit | 13/20 | 65% (43.3–81.9) | 14/23 | 60.9% (40.8–77.8) |
| Paediatric Nephrology | 4/6 | 66.7% (30–90.3) | 4/5 | 80% (37.6–96.4) |
| Total | 85/171 | 49.7% (42.3–57.1) | 91/161 | 60.9% (53.2–68.1) |
PAP: prevalence of antimicrobial prescription (total number of patients receiving 1 or more antimicrobials/total bed occupancy).
AAP: appropriateness of antimicrobial prescribing proportion (total number of appropriate antimicrobial prescriptions/total number of prescribed antimicrobials).
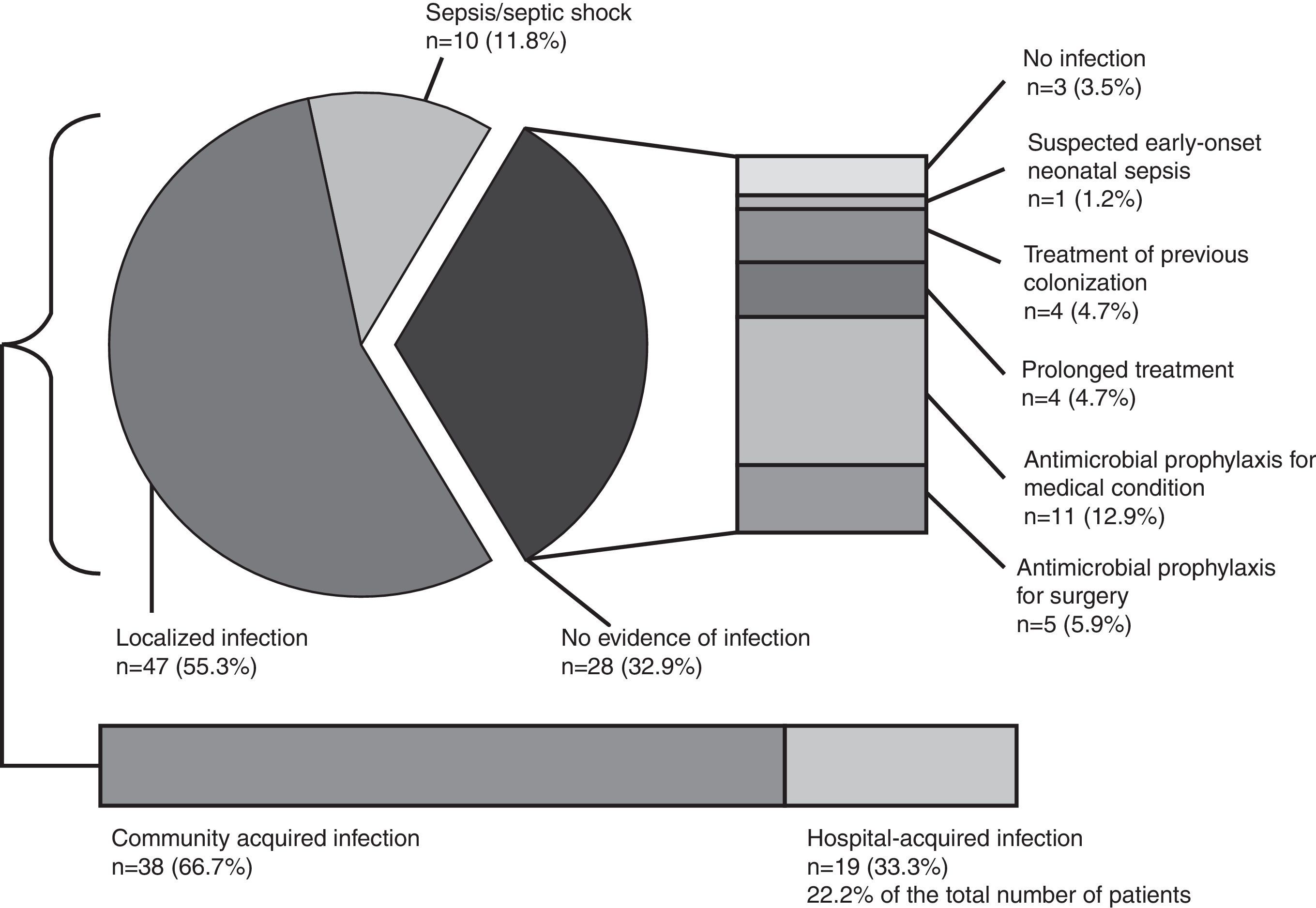
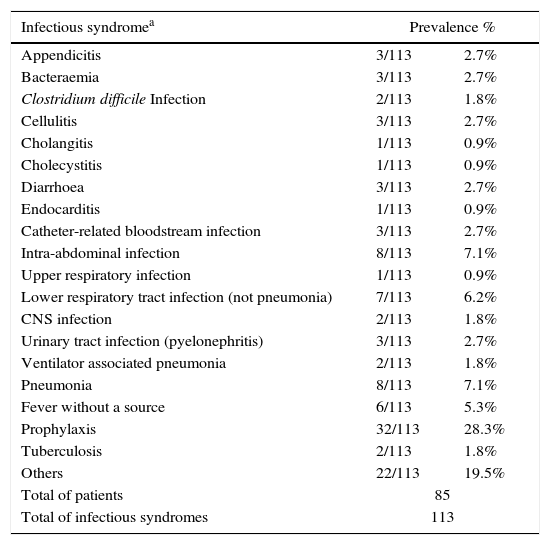
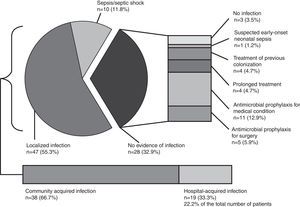
Globally, 113 infectious syndromes justifying treatment with antimicrobials were identified (Table 2). Severity of infection and infection source (community or hospital acquired) are shown in Fig. 1. Prevalence of risk factors for the development of infection and multi-resistant infection among patients receiving antimicrobials are summarised in Table 2. Temporary central venous catheters and previous admission to ICU or a post-surgical reanimation unit were the most prevalent.
Infectious syndromes and risk factors for infection and multi-resistant infection in patients with antimicrobial treatment.
| Infectious syndromea | Prevalence % | |
|---|---|---|
| Appendicitis | 3/113 | 2.7% |
| Bacteraemia | 3/113 | 2.7% |
| Clostridium difficile Infection | 2/113 | 1.8% |
| Cellulitis | 3/113 | 2.7% |
| Cholangitis | 1/113 | 0.9% |
| Cholecystitis | 1/113 | 0.9% |
| Diarrhoea | 3/113 | 2.7% |
| Endocarditis | 1/113 | 0.9% |
| Catheter-related bloodstream infection | 3/113 | 2.7% |
| Intra-abdominal infection | 8/113 | 7.1% |
| Upper respiratory infection | 1/113 | 0.9% |
| Lower respiratory tract infection (not pneumonia) | 7/113 | 6.2% |
| CNS infection | 2/113 | 1.8% |
| Urinary tract infection (pyelonephritis) | 3/113 | 2.7% |
| Ventilator associated pneumonia | 2/113 | 1.8% |
| Pneumonia | 8/113 | 7.1% |
| Fever without a source | 6/113 | 5.3% |
| Prophylaxis | 32/113 | 28.3% |
| Tuberculosis | 2/113 | 1.8% |
| Others | 22/113 | 19.5% |
| Total of patients | 85 | |
| Total of infectious syndromes | 113 | |
| Risk factors for infectiona | Prevalence % | |
|---|---|---|
| Urinary catheterisation | 7/109 | 6.4% |
| Temporary central venous catheterisation | 25/109 | 22.9% |
| Permanent central venous catheterisation | 8/109 | 7.3% |
| Surgical drains | 4/109 | 3.7% |
| Mechanical ventilation | 12/109 | 11% |
| Recent surgery (<7 days) | 11/109 | 10.1% |
| Neutropenia | 5/109 | 4.6% |
| Immunosuppression | 20/109 | 18.4% |
| Prosthetic material use | 1/109 | 0.9% |
| Multiple trauma | 0/109 | 0% |
| Others | 16/109 | 14.7% |
| Total of patients | 85 | |
| Total of risk factors for infection | 109 | |
| Total of patients with risk factors for infectionb | 58/85 | 68.2% |
| Risk factor for multi-resistant infectiona | Prevalence % | |
|---|---|---|
| Previous antibiotic treatment | 18/65 | 27.7% |
| Previous hospital admission in the last 3 months | 12/65 | 18.5% |
| Previous admission in ARU/ICUc | 30/65 | 46.2% |
| Previous multi-resistant colonisation/infection | 5/65 | 7.7% |
| Total of patients | 85 | |
| Total of risk factors for multi-resistant infection | 65 | |
| Total of patients with risk factors for multi-resistant infectionb | 51/85 | 60% |
Severity of infection and infection source. Distribution of the diverse causes of infection along with the severity of the infection. In the absence of infection, other factors that condition antimicrobial use (antimicrobial prophylaxis, suspected infection and previous colonisation) are detailed (n=number of patients, prevalence in %).
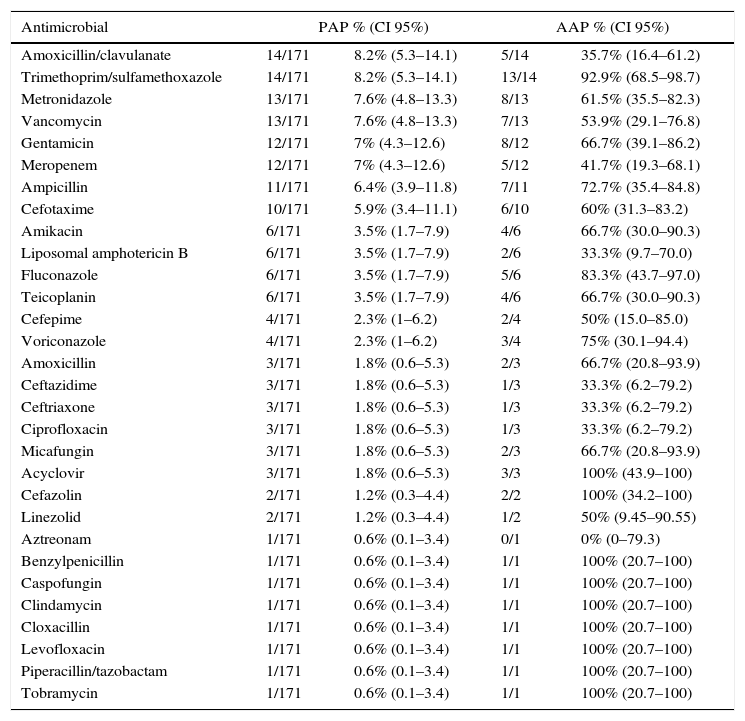
We registered 161 prescriptions for different antimicrobials (antimicrobial prescription per patient ratio=1.9:1). PAP and AAP per antimicrobial prescribed are summarised in Table 3. The most prevalent prescribed antimicrobials were amoxicillin/clavulanate and sulfamethoxazole/trimethoprim, each prescribed in 14 patients (8.2%). Antifungals were prescribed in 20 patients (11.7%); and antivirals (acyclovir) in 3 patients (1.8%) (Table 3).
Prevalence of antimicrobial prescription and appropriateness of antimicrobial prescribing proportion by antimicrobial.
| Antimicrobial | PAP % (CI 95%) | AAP % (CI 95%) | ||
|---|---|---|---|---|
| Amoxicillin/clavulanate | 14/171 | 8.2% (5.3–14.1) | 5/14 | 35.7% (16.4–61.2) |
| Trimethoprim/sulfamethoxazole | 14/171 | 8.2% (5.3–14.1) | 13/14 | 92.9% (68.5–98.7) |
| Metronidazole | 13/171 | 7.6% (4.8–13.3) | 8/13 | 61.5% (35.5–82.3) |
| Vancomycin | 13/171 | 7.6% (4.8–13.3) | 7/13 | 53.9% (29.1–76.8) |
| Gentamicin | 12/171 | 7% (4.3–12.6) | 8/12 | 66.7% (39.1–86.2) |
| Meropenem | 12/171 | 7% (4.3–12.6) | 5/12 | 41.7% (19.3–68.1) |
| Ampicillin | 11/171 | 6.4% (3.9–11.8) | 7/11 | 72.7% (35.4–84.8) |
| Cefotaxime | 10/171 | 5.9% (3.4–11.1) | 6/10 | 60% (31.3–83.2) |
| Amikacin | 6/171 | 3.5% (1.7–7.9) | 4/6 | 66.7% (30.0–90.3) |
| Liposomal amphotericin B | 6/171 | 3.5% (1.7–7.9) | 2/6 | 33.3% (9.7–70.0) |
| Fluconazole | 6/171 | 3.5% (1.7–7.9) | 5/6 | 83.3% (43.7–97.0) |
| Teicoplanin | 6/171 | 3.5% (1.7–7.9) | 4/6 | 66.7% (30.0–90.3) |
| Cefepime | 4/171 | 2.3% (1–6.2) | 2/4 | 50% (15.0–85.0) |
| Voriconazole | 4/171 | 2.3% (1–6.2) | 3/4 | 75% (30.1–94.4) |
| Amoxicillin | 3/171 | 1.8% (0.6–5.3) | 2/3 | 66.7% (20.8–93.9) |
| Ceftazidime | 3/171 | 1.8% (0.6–5.3) | 1/3 | 33.3% (6.2–79.2) |
| Ceftriaxone | 3/171 | 1.8% (0.6–5.3) | 1/3 | 33.3% (6.2–79.2) |
| Ciprofloxacin | 3/171 | 1.8% (0.6–5.3) | 1/3 | 33.3% (6.2–79.2) |
| Micafungin | 3/171 | 1.8% (0.6–5.3) | 2/3 | 66.7% (20.8–93.9) |
| Acyclovir | 3/171 | 1.8% (0.6–5.3) | 3/3 | 100% (43.9–100) |
| Cefazolin | 2/171 | 1.2% (0.3–4.4) | 2/2 | 100% (34.2–100) |
| Linezolid | 2/171 | 1.2% (0.3–4.4) | 1/2 | 50% (9.45–90.55) |
| Aztreonam | 1/171 | 0.6% (0.1–3.4) | 0/1 | 0% (0–79.3) |
| Benzylpenicillin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Caspofungin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Clindamycin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Cloxacillin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Levofloxacin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Piperacillin/tazobactam | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
| Tobramycin | 1/171 | 0.6% (0.1–3.4) | 1/1 | 100% (20.7–100) |
PAP: prevalence of antimicrobial prescription (total number of patients receiving the specific antimicrobial/total bed occupancy).
AAP: appropriateness of antimicrobial prescribing proportion (total number of appropriate antimicrobial prescriptions/total number of prescribed antimicrobials).
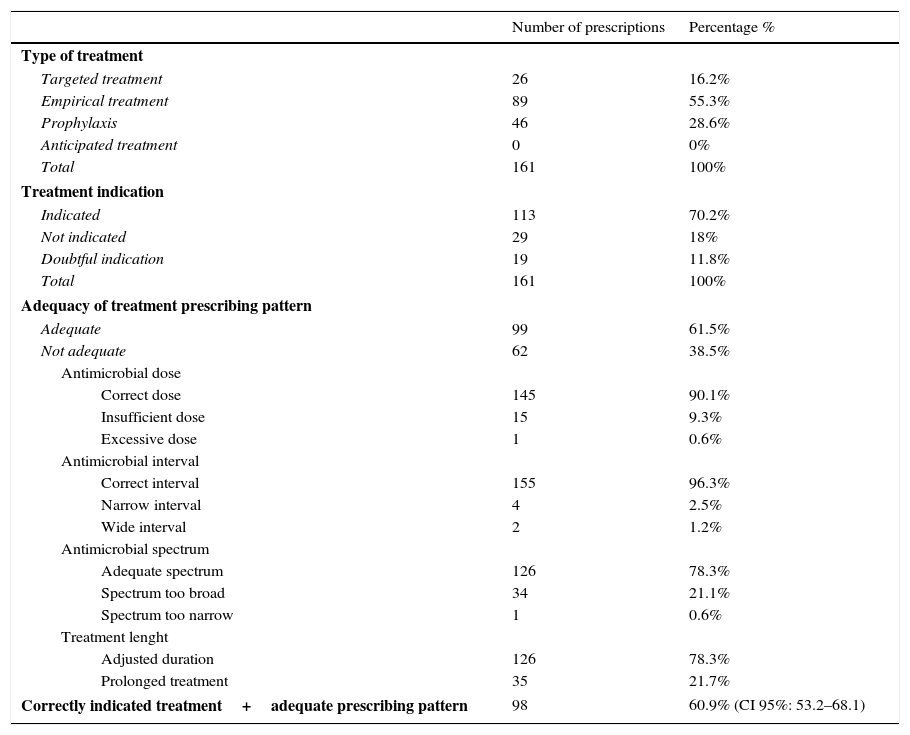
Antimicrobial treatment characteristics and evaluation of indication and prescription adequacy pattern are shown in Table 4. One hundred and thirteen (70.2%) treatments were considered indicated and 99 (61.5%) were correctly prescribed, while the remaining had some failure either in dosing, interval adjustment, spectrum or length of treatment (Table 4). There were 98 treatments which were both indicated and correctly prescribed, which represents a total AAP of 60.9%. (CI 95%: 53.2–68.1) (Table 4).
Characteristics of the prescribed antimicrobial treatment and evaluation of indication and adequacy of treatment prescribing pattern.
| Number of prescriptions | Percentage % | |
|---|---|---|
| Type of treatment | ||
| Targeted treatment | 26 | 16.2% |
| Empirical treatment | 89 | 55.3% |
| Prophylaxis | 46 | 28.6% |
| Anticipated treatment | 0 | 0% |
| Total | 161 | 100% |
| Treatment indication | ||
| Indicated | 113 | 70.2% |
| Not indicated | 29 | 18% |
| Doubtful indication | 19 | 11.8% |
| Total | 161 | 100% |
| Adequacy of treatment prescribing pattern | ||
| Adequate | 99 | 61.5% |
| Not adequate | 62 | 38.5% |
| Antimicrobial dose | ||
| Correct dose | 145 | 90.1% |
| Insufficient dose | 15 | 9.3% |
| Excessive dose | 1 | 0.6% |
| Antimicrobial interval | ||
| Correct interval | 155 | 96.3% |
| Narrow interval | 4 | 2.5% |
| Wide interval | 2 | 1.2% |
| Antimicrobial spectrum | ||
| Adequate spectrum | 126 | 78.3% |
| Spectrum too broad | 34 | 21.1% |
| Spectrum too narrow | 1 | 0.6% |
| Treatment lenght | ||
| Adjusted duration | 126 | 78.3% |
| Prolonged treatment | 35 | 21.7% |
| Correctly indicated treatment+adequate prescribing pattern | 98 | 60.9% (CI 95%: 53.2–68.1) |
An extensive understanding of antimicrobial usage in paediatric settings is necessary prior to the development of AS. The intention of this study was to improve such understanding, in view of the limited existence of antimicrobial hospital-quality indicators of antibiotic prescription studies in children, mostly focused on the prevalence of prescription16–19 and few assessing appropriateness of prescription.20,21 Evaluating antimicrobial consumption by ward, our total PAP surpasses the medium value for paediatric patients in Spain registered by the EPINE group report (PAP=36%; CI 95%: 34.1–38.1),22 as well as those reported in 5 Spanish hospitals participants of the Antibiotic Resistance and Prescribing in European Children Project (ARPEC) (PAP=37.7%; CI 95%: 32.1–43.3).16 PAP by ward showed a high variability, being more reliable in wards with higher bed occupancy (neonatology, infectious diseases, general paediatrics and haematology–oncology). When comparing paediatric and neonatal intensive care unit data with EPINE (Paediatric ICU PAP=65.3%; Neonatal ICU PAP=37.9%) and ARPEC (Paediatric ICU PAP=70.4%; Neonatal ICU PAP=41.6%),16,22 we found a higher PAP in our Neonatal ICU. Lower PAP was found in the haematology–oncology ward compared with EPINE data (70.4%), but similar to ARPEC results (63.5%), where tertiary hospitals such as ours are studied.16,22 All surgical wards, except paediatric traumatology, had a PAP over 50%; similar to the European and Spanish data.16,22 However, these comparative assessments should be interpreted with caution for two main reasons: (1) The limited sample size for some wards produces wide confidence intervals that in some cases overlap with the EPINE and ARPEC values; and (2) our data were gathered in November and December, whilst the EPINE study is performed yearly in spring, which could influence the differences between PAP values as well as the observed infectious syndromes.
Our total AAP was similar to other reports,20 but below our required target. Paediatric and Neonatal ICU, Paediatric Hepatology and General Paediatrics were documented as the priority wards to improve antimicrobial prescription. As far as we know, at the moment there is no reference benchmark.
Evaluating consumption by antimicrobial, the most frequently prescribed were amoxicillin/clavulanate and trimethoprim/sulfamethoxazole (mostly used as prophylaxis), differing from other series. When assessed by antimicrobial group, cephalosporins had the highest PAP, similar to previous reviews,20,23 with our centre mainly opting for third generation cephalosporins. For other ecological strategic antibiotics, carbapenems obtained a high PAP (7%), but PAP as high as 11.4% have been previously reported.20 Few studies have provided data on antifungal and antiviral use in the paediatric population, but we obtained a higher PAP for antifungals than previous reports.20 Unfortunately, we only obtained data from one antiviral (acyclovir), but the increasing use of antivirals in paediatric situations demands more studies of this therapeutic area.
Our low AAP in broad-spectrum antimicrobials such as meropenem (41.7%), vancomycin (53.9%), and amphotericin B (33.3%) are essential targets to improve; as well as one of our most prescribed antimicrobials: Amoxicillin/clavulanate, AAP (35.7%).
In our study, antibiotic prophylaxis (28.3%) was the most frequent justification for prescribing antimicrobials, mostly in transplant and surgical patients, differing from other series where respiratory infection was the most prevalent diagnosis.20,23 Patient characteristics leading to antimicrobial use are important. In our centre, a larger proportion of patients (68.2%) had at least one risk factor for the development of infectious complications as a marker of high complexity attendance. Regarding severity of disease, which increases antimicrobial use,24 the majority of our patients had localised infections, with higher prevalence of sepsis compared with other studies.25 Strategies for improvement should also include the control of modifiable risk factors and targeting objectives according to each ward complexity.
In a further analysis, we noticed that wards with PAP over 50% (haematology–oncology, nephrology and paediatric surgery), which in our hospital have detailed protocols for infection antimicrobial management, obtained AAP of 80–87.9%. It seems that PAP is susceptible to other factors that may affect it (case-mix complexity, epidemics, diagnostic techniques accuracy, antimicrobial availability), while AAP can always be improved, so getting values close to 100% should be targeted.
From our data, we identified several objectives: lowering our general PAP together with the PAP of units with high antibiotic pressure index (as in both ICUs), maintaining a sustained improvement of AAP in all wards and improving AAP in strategic antimicrobials.
In our study, the most prevalent mistake in prescription was excessive treatment length, followed by inadequate spectrum selection and suboptimal dosing. Our cross-sectional design, allowed treatment length evaluation only when it exceeded recommended duration, meant we were unable to predict whether any other treatments would follow same trends and probably underestimate its real value.
Paediatric patients have peculiar prescribing characteristics, differing in many aspects from adults,3,12 so studies going beyond an evaluation of the prevalence of prescription are required. Not many studies assess prescription indication and adequacy, while complications and adverse events related to antimicrobials mostly result from failures at this level.15 More than one third of antimicrobial treatments in adults do not correctly follow evidence-based guidelines.15 Similar or worse results in paediatrics are expectable since lack of pharmacokinetic and pharmacodynamics studies hinder the establishment of optimal dosing and intervals for antimicrobial prescription adjusted to different age groups.3 In addition, in paediatrics a “proactive” attitude towards antimicrobial prescription is common, with aggressive treatment of infection and less focus on searching for the causative microbiological agent, resulting in treatment of “suspected infection” or “risk of infection” based in age group and patient symptoms. This understandable approach increases the chances of failure in indication and favours empirical treatments with broader antimicrobial spectrum than necessary.11
Moreover, the variables that can be used to reliably compare the results obtained in different paediatric studies for future benchmarking are discussible.12 Antimicrobial use aggregate data tools, like defined daily dose (DDD), prescribed daily doses (PDD) and days of treatment (DOT)2,26 are suitable for adults, but have considerable limitations for paediatrics. DDD and PDD are difficult to validate in children because optimal dose changes with weight or body surface area. DOT seems to be a viable option,27 but debatable from our perspective, as the optimal treatment duration for children infections varies widely and are under constant review.
In this regard, PAP and AAP offer simple and comprehensive information, and useful manner to monitor paediatric antimicrobial usage. The greatest challenge remains to determine and standardise “what is appropriate”. Our study uses a local definition which limits the generalisability of the results for benchmarking. Appropriateness evaluation criteria and clinical data to be linked with in the analysis have been previously described, but these need to be evaluated in comparison with a gold standard, usually locally adapted guidelines taking into account clinical and microbiological data when possible.28,29 Despite the inherent difficulties in developing universal guidelines applicable to all settings, creating centre-adapted objectives on PAP and AAP seems plausible. Studies evaluating prescribing appropriateness and intervening on it, either through post-prescribing reviews30 or active counselling and education interventions,13 have shown impact in improving the quality of prescribing. A proposed approach could be to perform multicentre studies evaluating PAP and AAP adjusted to case-mix trying to standardise the methodology to appropriateness assessment and use them to set specific targets for both markers that can be compared also with other proposed AS impact evaluation strategies.
The main limitations of our study are related to its cross-sectional design. Prospective studies assessing detailed treatment length and additional variables like infection seasonality, microbial isolation and resistance will be able to provide further insights. More paediatric AS studies evaluating PAP and AAP are needed. Both markers seem to be useful tools for describing antimicrobial usage and to identify AS priorities in hospital settings. The information obtained must be used to identify improvement opportunities, working on modifiable factors and developing new strategies for successful paediatric AS.
FundingNo specific funding was received for this article.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank Keith Veitch for the English language editing in the manuscript.