Cellulosimicrobium cellulans (formerly Oerskovia xanthineolytica), a nocardia-like bacillus widely distributed in the environment, is rarely associated with human infections.1 Cases reported so far in the literature are mostly related with the presence of foreign bodies in immunocompromised patients.2 We describe a case of long-term recurrent catheter infection by C. cellulans.
A 59-year-old woman with rectal adenocarcinoma and lung metastases, treated with bevazizumab through a Port-a-Cath™, presented to the emergency department with a 24-h history of fever. On examination, her temperature was 37.8°C and diffuse abdominal pain, related to current treatment, was observed. Inflammatory signs were not observed in catheter's insertion. C-reactive protein (CRP) levels were elevated (63.4mg/L) with normal blood cell count. A chest X-ray showed no other findings except those related with patient's basal condition. After admission, two sets of aerobic and anaerobic BD BACTEC Plus Blood Culture Bottles (Becton Dickinson, Sparks, MD) were obtained and antipyretics were initiated. Twenty-four hours later, both blood cultures (BC) were positive for C. cellulans. Differential quantitative BC were subsequently obtained and an intravenous vancomycin regimen (15mg/kg/12h) was initiated. On day +3 after admission, a differential time to positivity (DTP) between both BC was recorded, demonstrating a catheter-related bloodstream infection caused by C. cellulans. Antimicrobial therapy was immediately switched to vancomycin plus imipenem and conservative management of the catheter with antibiotic lock therapy was carried out. Lock therapy consisted of a 14-day course of instillations (5mL of solution) with vancomycin (2mg/mL solution) plus heparin (20IU/mL solution). On day +5, the patient was discharged considering her clinical improvement. Lock therapy was completed after 14 days and control-BC were negative. However, 15 months later she returned to the emergency department with a 5-day history of fever, and was diagnosed of a device-related sepsis. Biochemical tests showed elevated levels of CRP (327mg/L) and procalcitonin (2.17μg/L), which suggested a more severe infectious process than the precedent. C. cellulans grew again from both the peripheral-BC and the catheter-BC, exhibiting the same antimicrobial susceptibility pattern than the previous isolate. Vancomycin regimen (15mg/kg/12h) was initiated, catheter's removal was carried out, and the infection was finally eradicated.
In both episodes, Gram staining of positive BC showed Gram-positive filamentous rods. BC aliquots were plated onto blood agar, which showed growth of smooth, yellow colonies after overnight incubation. Identification of the isolate was primarily carried out using MALDI-TOF (Brucker Daltonics, Leipzig, Germany; score>2). Furthermore, identification was confirmed by API®Coryne (bioMérieux, Marcy-l’Étoile, France; code=7572727) and 16S rRNA gene amplification and sequencing3 (100% identity according to GenBank database). The isolates exhibited in vitro resistance to rifampicin, gentamicin, clindamycin, tetracycline and ciprofloxacin, and susceptibility to vancomycin, following EUCAST disk diffusion criteria for Corynebacterium.
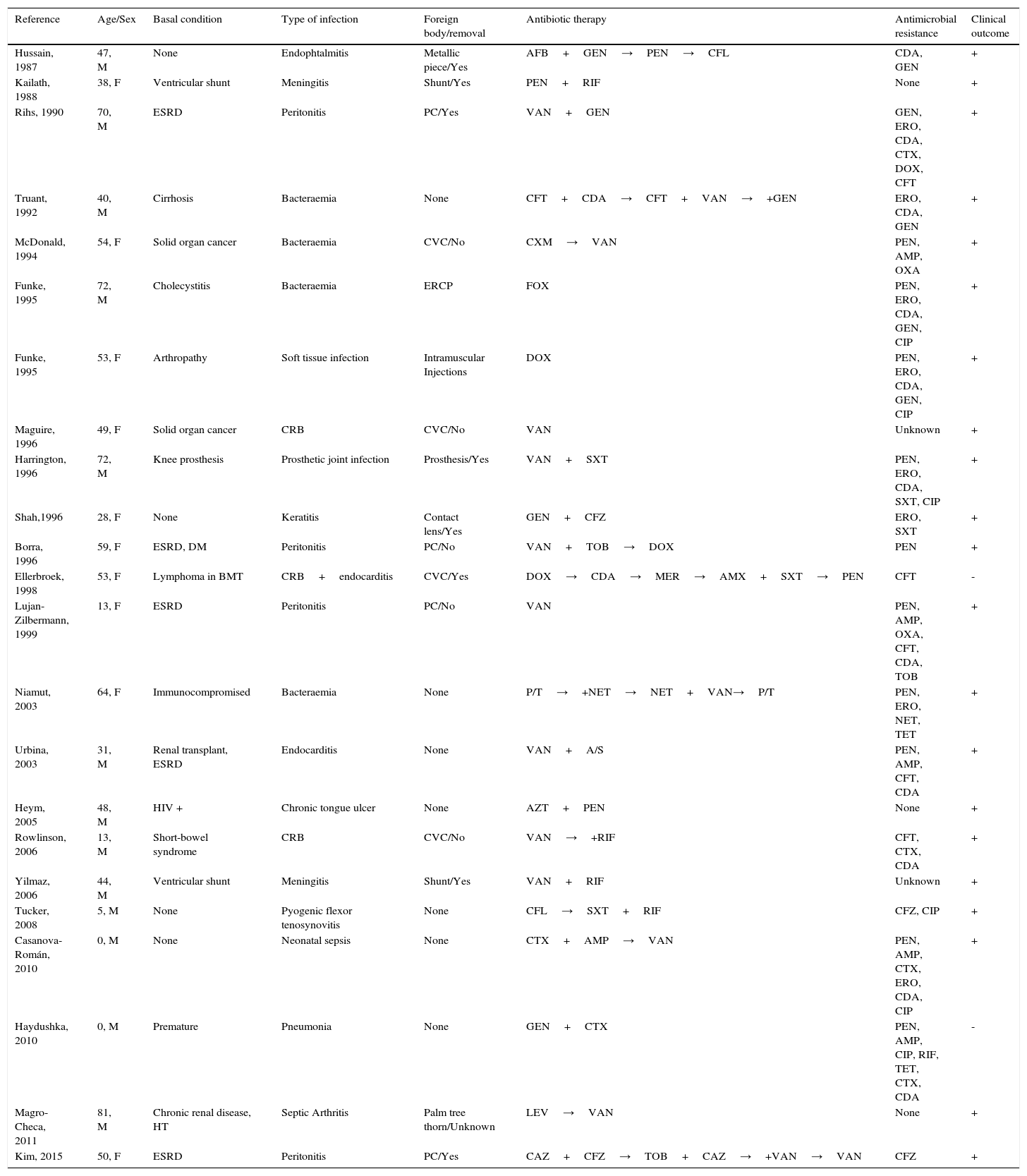
Table 1 summarizes the 23 cases of C. cellulans infection reported so far in the literature. As shown, most patients had some kind of immunosuppression or severe underlying condition, in particular end-stage renal disease (ESRD) (n=5; 22%). Focus of infection was mostly related to the presence of a foreign body (n=16; 70%), which was finally removed in the majority of cases (n=11; 69%) for complete recovery. Antibiotic susceptibility testing showed universal susceptibility to vancomycin, which was the antibiotic of choice in most cases. All cases in which it was not necessary to remove the foreign body, vancomycin was included as a part of the antibiotic regimen.
Clinical and microbiological characteristics of the 22 cases of C. cellulans infections reported up to date.
| Reference | Age/Sex | Basal condition | Type of infection | Foreign body/removal | Antibiotic therapy | Antimicrobial resistance | Clinical outcome |
|---|---|---|---|---|---|---|---|
| Hussain, 1987 | 47, M | None | Endophtalmitis | Metallic piece/Yes | AFB+GEN→PEN→CFL | CDA, GEN | + |
| Kailath, 1988 | 38, F | Ventricular shunt | Meningitis | Shunt/Yes | PEN+RIF | None | + |
| Rihs, 1990 | 70, M | ESRD | Peritonitis | PC/Yes | VAN+GEN | GEN, ERO, CDA, CTX, DOX, CFT | + |
| Truant, 1992 | 40, M | Cirrhosis | Bacteraemia | None | CFT+CDA→CFT+VAN→+GEN | ERO, CDA, GEN | + |
| McDonald, 1994 | 54, F | Solid organ cancer | Bacteraemia | CVC/No | CXM→VAN | PEN, AMP, OXA | + |
| Funke, 1995 | 72, M | Cholecystitis | Bacteraemia | ERCP | FOX | PEN, ERO, CDA, GEN, CIP | + |
| Funke, 1995 | 53, F | Arthropathy | Soft tissue infection | Intramuscular Injections | DOX | PEN, ERO, CDA, GEN, CIP | + |
| Maguire, 1996 | 49, F | Solid organ cancer | CRB | CVC/No | VAN | Unknown | + |
| Harrington, 1996 | 72, M | Knee prosthesis | Prosthetic joint infection | Prosthesis/Yes | VAN+SXT | PEN, ERO, CDA, SXT, CIP | + |
| Shah,1996 | 28, F | None | Keratitis | Contact lens/Yes | GEN+CFZ | ERO, SXT | + |
| Borra, 1996 | 59, F | ESRD, DM | Peritonitis | PC/No | VAN+TOB→DOX | PEN | + |
| Ellerbroek, 1998 | 53, F | Lymphoma in BMT | CRB+endocarditis | CVC/Yes | DOX→CDA→MER→AMX+SXT→PEN | CFT | - |
| Lujan-Zilbermann, 1999 | 13, F | ESRD | Peritonitis | PC/No | VAN | PEN, AMP, OXA, CFT, CDA, TOB | + |
| Niamut, 2003 | 64, F | Immunocompromised | Bacteraemia | None | P/T→+NET→NET+VAN→P/T | PEN, ERO, NET, TET | + |
| Urbina, 2003 | 31, M | Renal transplant, ESRD | Endocarditis | None | VAN+A/S | PEN, AMP, CFT, CDA | + |
| Heym, 2005 | 48, M | HIV + | Chronic tongue ulcer | None | AZT+PEN | None | + |
| Rowlinson, 2006 | 13, M | Short-bowel syndrome | CRB | CVC/No | VAN→+RIF | CFT, CTX, CDA | + |
| Yilmaz, 2006 | 44, M | Ventricular shunt | Meningitis | Shunt/Yes | VAN+RIF | Unknown | + |
| Tucker, 2008 | 5, M | None | Pyogenic flexor tenosynovitis | None | CFL→SXT+RIF | CFZ, CIP | + |
| Casanova-Román, 2010 | 0, M | None | Neonatal sepsis | None | CTX+AMP→VAN | PEN, AMP, CTX, ERO, CDA, CIP | + |
| Haydushka, 2010 | 0, M | Premature | Pneumonia | None | GEN+CTX | PEN, AMP, CIP, RIF, TET, CTX, CDA | - |
| Magro-Checa, 2011 | 81, M | Chronic renal disease, HT | Septic Arthritis | Palm tree thorn/Unknown | LEV→VAN | None | + |
| Kim, 2015 | 50, F | ESRD | Peritonitis | PC/Yes | CAZ+CFZ→TOB+CAZ→+VAN→VAN | CFZ | + |
Abbreviations: F: female, M: male, BMT: bone marrow transplantation, DM: diabetes mellitus, ESRD: end-stage renal disease, HIV: human immunodeficiency virus, CVC: central venous catheter, ERCP: endoscopic retrograde cholangiopancreatography, AFB: amphotericin B, AMP: ampicillin, AMX: amoxicillin, A/S: ampicillin-sulbactam, AZT: azithromycin, CAZ: ceftazidime, CDA: clindamycin, CIP: ciprofloxacin, CFL: cephalexin, CFT: ceftriaxone, CFZ: cefazolin, CRB: catheter-related bacteraemia, CTX: cefotaxime, CXM: cefuroxime, DOX: doxycycline, ERO: erythromycin, FOX: cefoxitin, GEN: gentamicin, HT: hypertension, LEV: levofloxacin, MER: meropenem, NET: netilmicin, OXA: oxacillin, PEN: penicillin, PC: peritoneal catheter, P/T: piperacillin-tazobactam, RIF: rifampicin, SXT: trimethoprim-sulfamethoxazole, TET: tetracycline, TOB: tobramycin, VAN: vancomycin.
The case we report here reflects not only the expected features of an infection caused by C. cellulans but also the probable long-term asymptomatic colonization of the catheter for more than a year, and the subsequent recurrence. It seems unlikely that both episodes could be independently trigger from a peripheral focus. Certain features of the microorganism such as low pathogenicity and biofilm formation could be involved in that long-term colonization. The presence of biofilm implies not only the lower effectiveness of antibiotics but also a diminished host immune response.4 Most cases of C. cellulans infections led to catheter's removal for the complete eradication of the pathogen, whereas some authors have reported fully recovery by using vancomycin therapy (alone or in combination) and conservative management of the catheter.2,5-8 However, in the case we report, this strategy was discouraged. Persistence of these microorganisms despite the use of antibiotics with in vitro activity may be partially explained by biofilm formation into the catheter's lumen.4,9 Opportunistic pathogens like C. cellulans could increasingly being encountered due to the extensive use of long-term medical devices and a high survival rate of immunocompromised patients.
Conflict of interestOn behalf of all authors, the corresponding author states that there is no conflict of interest.