Post-exposure prophylaxis (PEP) can be a secondary measure to prevent infection by human immunodeficiency virus (HIV) when primary prevention has failed. PEP is advised for people with sporadic and exceptional risk exposure to HIV.
This consensus document about occupational and non-occupational PEP recommendations aims to be a technical document for healthcare professionals. Its main objective is to facilitate the appropriate use of PEP. To this end, some recommendations have been established to assess the risk of transmission in different types of exposure, situations where PEP should be recommended, special circumstances to take into account, antiretroviral (ARV) guidelines including start and end of the treatment, early monitoring of tolerance and adherence to the treatment, subsequent monitoring of people exposed, independently of having received PEP or not, and need of psychological support.
This document is intended for all professionals who work in clinical practice in the field of HIV infection.
La Profilaxis Post-Exposición (PPE) puede ser una medida secundaria para prevenir la infección por el virus de la inmunodeficiencia humana (VIH) cuando la prevención primaria ha fallado. La PPE se aconseja en personas con una exposición de riesgo al VIH esporádica y excepcional.
Este Documento de Consenso sobre las recomendaciones de las PPE ocupacional y no ocupacional pretende ser un documento técnico para los profesionales sanitarios. Su principal objetivo es facilitar el uso apropiado de la PPE. Para ello, se han elaborado unas recomendaciones para la valoración del riesgo de transmisión en los diferentes tipos de exposición, de las situaciones en las que debe recomendarse la PPE, de las circunstancias especiales a tener en cuenta, de las pautas de antirretrovirales (ARV) con su inicio y duración, del seguimiento precoz de la tolerancia y adherencia del tratamiento, del seguimiento posterior de las personas expuestas independientemente de que hayan recibido PPE o no, y de la necesidad del apoyo psicológico.
El Documento va dirigido a todos aquellos profesionales que trabajan en la práctica clínica en el ámbito de la infección por VIH.
There is a risk of HIV, HCV and HBV transmission in occupational exposure and it differs depending on the fluid the professional has been exposed to. For HIV, prospective studies performed in healthcare workers have estimated that the average risk of transmission after a percutaneous exposure to blood is 0.3% (CI 95%: 0.2–0.5%). The risk of transmission after exposure to other fluids or tissues has not been quantified, but it is probably considerably lower than that of contact with blood.
The average incidence of seroconversion after a percutaneous exposure to a positive HCV source is 1.8% (range: 0–7%).
Accidental infection by HBV constitutes a well-established occupational risk for healthcare professionals if they are not vaccinated against this virus. The risk for unvaccinated workers to acquire an HBV infection in a percutaneous accident depends on the marker levels of the person from which the blood or corporal fluid comes from. Studies performed in healthcare workers with percutaneous exposure to HVB-contaminated blood show that the risk of transmission is lower than 30% if the source patient is HBs Ag positive and HBe Ag positive and lower than 6% if he/she is HBe Ag negative.
Post-exposure prophylaxis is a biomedical prevention strategy.1 Without a doubt, the best way of preventing occupational transmission is avoiding exposure. To this end, each institution should foster resource allocation to:
- (a)
Teach and train workers in universal precautions. Including vaccination against HBV (Article 8 of the Royal Decree 664/1997 about protection of workers against risks related to exposure to biological agents at work) and the appropriate management of residues, which should be followed in every risk situation of contact with potentially contaminated liquids like blood or other fluid or tissue contaminated with blood, semen, vaginal discharge, cerebrospinal, synovial, pleural, peritoneal, pericardial or amniotic fluid.
- (b)
Have the necessary materials available to act as a barrier (gloves, masks, white coats and protective glasses), as well as containers for disposable potentially contaminated material and security equipment (set up in various Autonomous Communities through transposition of the Council Directive 2010/32/EU of 10 May 2010 implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector).
- (c)
Guarantee counselling and 24-h assistance with access to serological diagnosis, preferably in less than 2h and always before 72h for HIV and as soon as possible and always before 96h for HBV and HCV (see algorithms of action).
- (d)
Facilitate the access to medication in necessary cases within established time limits.
- (e)
Establish the appropriate monitoring protocols.
- (f)
Have professionals available working in attention and monitoring of occupational exposure (OE) cases (Labour Risk Prevention Services, Infection Units and Emergency Rooms).
- (g)
Establish centralized notification rules to create a registry through Labour Risk Prevention Services (LRPS) and assess the efficacy of the interventions.
The worker must receive urgent assistance, for which he/she will immediately go to the labour health service during working hours and to the emergency services outside working hours, where he/she will be given a report explaining the assistance received, including injuries suffered. They must inform the immediate superior so that he/she can fill the accident report, which will be sent to a mutual insurance company, independently of being treated at the labour health service or the emergency room.
For skin wounds (punctures, cuts) and spatters to damaged skin, it is recommended to:
- (a)
wash with water and soap;
- (b)
let blood flow;
- (c)
disinfect the wound with an antiseptic (povidone-iodine, chlorhexidine gluconate);
- (d)
cover with a waterproof bandage.
For spatters to mucosa (connective tissue, etc.) it is recommended to wash with abundant water or physiological saline.
Caustic agents must never be applied. It is not recommended to “press”, because it induces hyperaemia, which may increase the risk to acquire the infection.
Assessment of risk of HIV occupational transmissionThe risk of transmission after an OE depends on multiple factors like serological state of the worker, type of exposure, quantity of the virus existing in the inoculum and virological state of the source, as well as time elapsed since exposure.
Various studies prove that exposure to large viral loads is associated to a higher risk of transmission of the infection. Therefore, in periods of high viraemia, like acute infection stages or advanced stages of the disease, if the source is not receiving ART the risk of transmission is higher.
On the other hand, exposure to corporal fluids of patients with HIV infection and undetectable viral load does not completely eliminate the risk of transmission. However, it makes it very improbable, being essential to monitor and assess the PEP.
Types of occupational exposurePercutaneous exposures are most efficient in HIV transmission than mucosae. CDC estimates that the average risk of HIV seroconversion after a percutaneous OE is approximately 0.3% (CI 95%: 0.2–0.5%), which means three infections of every 1000 incidents. After exposure of mucosae, the risk is reduced to 0.09% (CI 95%: 0.006–0.5%), being even lower when contact is with damaged skin.
Regarding the factors related to the accident, this will depend on puncture's depth:
- (1)
Superficial accidental inoculation: erosion.
- (2)
Intermediate depth: appearance of blood.
- (3)
Deep accidental inoculation.
The deeper, the higher the risk of transmission on type of material used:
- (1)
Hollow needle: higher risk than with suture needles, because the latter only have a thin layer of fluid in the needle's surface.
- (2)
Full shank needle or surgical knife.
The risk increases in association with the increase of the needle's diameter, barrier factors (use of gloves decreases the injected volume by 50%), type and condition of the epithelium or the surface exposed (undamaged, healthy skin is an excellent barrier and presents low or no risk).
Finally, the risk is directly related to the type of fluid to which the worker has been exposed to, being visible blood on the device the one with higher risk. Other fluids with high infection potential, which require a precise assessment, are blood, semen and vaginal discharge. Infection potential of CSF is unknown; serous and amniotic fluids present an unknown infection potential. Vomits, faeces, saliva, sweat, tears, urine and sputum do not have a significant risk, except if they contain visible blood.
Characteristics of the sourceIt is essential to known the serological state of the source patient by contacting his/her doctor. If this state is unknown or cannot be known, a complete serological survey must be performed under previous request of verbal or written consent, which consists on, HBV: requesting HBs antigen (Ag). HCV: if it is positive, consider measuring the viral load. HIV: if it positive, measure the viral load.
It is recommended to have serological results of HIV at hand preferably within 2h after exposure. Techniques available today guarantee results with excellent sensitivity and specificity in less than 30min, and give essential information about the need to start or continue PEP. However, the administration of PEP should not be postponed until receiving the serological results of the source case and should be cancelled if these are negative.
There is no published data of any case of occupational transmission during the window period of the HIV infection.
If the source patient has a known HIV infection, it is essential to get to know the viral load, ART type (if he/she receives it), as well as his/her pharmacological history and the patient's reasons to change the treatment (resistances or intolerance).
If the source's serological state cannot be known, it will be considered as high risk.
Characteristics of the workerA complete serology of the exposed person should be performed after exposure, to determine his/her serological status against HIV, HCV and HBV (anti-HBs, anti-HBc, HBs Ag) except if he/she is known to be positive. Moreover, a basic clinical analysis including a haemogram and renal and hepatic function will be performed. For the serology of HIV, it is recommended to perform a fourth generation test (including the detection of antibodies and P24 antigen).
Previous considerations to post-exposure prophylaxisBefore considering PEP use, it is necessary to take into account if the person exposed visits the healthcare service within 72h after the exposure and to gather as much information about the source person. Besides performing PEP or not, all proceedings and interventions must be clearly registered.
If PEP is appropriate, it is recommended to start it as soon as possible after the OE, preferably within the first 24h and always within the first 72h. It is not recommended to start PEP later than 72h after the OE.
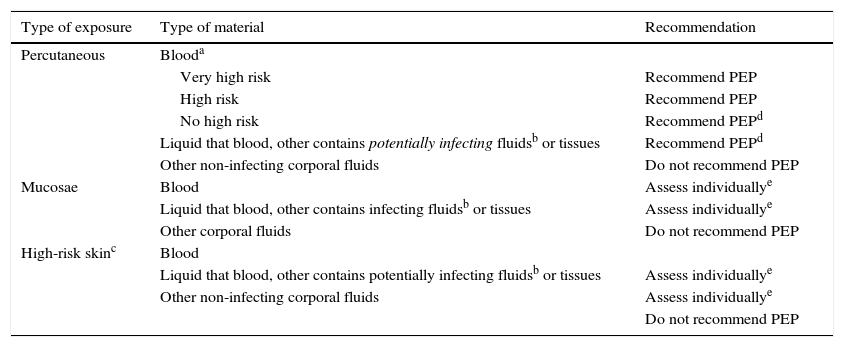
General recommendations for OPEP are gathered in Table 1. In general, it is recommended to perform PEP when the risk of transmission is high; when the risk is not high, each case should be assessed individually, and when the risk is negligible or null, it is not recommended.
OPEP general recommendations.
| Type of exposure | Type of material | Recommendation |
|---|---|---|
| Percutaneous | Blooda | |
| Very high risk | Recommend PEP | |
| High risk | Recommend PEP | |
| No high risk | Recommend PEPd | |
| Liquid that blood, other contains potentially infecting fluidsb or tissues | Recommend PEPd | |
| Other non-infecting corporal fluids | Do not recommend PEP | |
| Mucosae | Blood | Assess individuallye |
| Liquid that blood, other contains infecting fluidsb or tissues | Assess individuallye | |
| Other corporal fluids | Do not recommend PEP | |
| High-risk skinc | Blood | |
| Liquid that blood, other contains potentially infecting fluidsb or tissues | Assess individuallye | |
| Other non-infecting corporal fluids | Assess individuallye | |
| Do not recommend PEP |
Very high risk is defined as accident with a very large volume of blood (deep puncture with a needle used in vascular access of the patient) and with a high HIV viral load.
High risk is defined as accident with large volume of blood or accident with blood that contains a high HIV viral load.
No high risk: accident without exposure to a large volume of blood or without exposure to blood with a high HIV viral load (puncture with suture needle of a patient with low or undetectable viral load).
Includes semen, vaginal discharge, CSF and synovial, pleural, peritoneal, pericardial and amniotic fluids.
High-risk cutaneous contacts when it is with high HIV viral load liquids, when contact is very prolonged, the area is large or there are spots of damaged skin.
Recommendations
- (1)
It is necessary to know the serological state of the source patient against HIV. Results should be known preferably within the first 2h. Strong recommendation, high quality evidence.
- (2)
If the source patient has a known HIV infection, it is essential to get to know the viral load, ART type (if he/she receives it), as well as his/her pharmacological history and the patient's reasons to change the treatment (resistances, intolerance or toxicity). Strong recommendation, high quality evidence. If the source patient's serological state cannot be known, it will be considered as high risk. Strong recommendation, moderate quality evidence.
- (3)
PEP must start as soon as possible after OE to HIV, preferably within the first 24h and always within the first 72h. Strong recommendation, moderate quality evidence. In percutaneous OE to blood with high or very high risk it is recommended to perform PEP. Strong recommendation, moderate quality evidence.
- (4)
It is recommended to perform PEP in percutaneous OE to blood with lower risk or to other potentially infecting body fluids. However if the source's viral load is negligible it can be considered not to perform PEP. Strong recommendation, low quality evidence.
- (5)
It is recommended to perform PEP in OE of mucosae or skin with high risk to blood or other potentially infecting body fluids. However, if the source's viral load is negligible it can be considered not to perform PEP. Weak recommendation, low quality evidence.
- (6)
In OE to non-infecting body fluids it is not recommended to perform PEP. Strong recommendation, low quality evidence.
- (7)
PEP should be cancelled in case the source patient is confirmed to be HIV negative. Strong recommendation, low quality evidence.
HIV shares transmission routes with HBV and HCV. Therefore, the assessment of the situation should be performed considering both types of hepatitis. HCV is not transmitted effectively through occupational exposure, as it has been previously said, the average seroconversion incidence after a contact with an HIV positive patient's blood is 1.8% and transmission through mucous membranes is very rare. HBV, as it has been discussed before, is frequently transmitted if the exposed worker is not vaccinated.
Assessment of the risk exposure is the same as for HIV regarding the way and type of exposure. It is essential to assess appropriately the presence of infection in the source patient. If the serological state is unknown, a blood drawn should be performed to make a serology under previous request of informed consent and be able to access the results in the shortest time. Systematic determination of HBV or HCV viral load is not necessary. It is only advised in case that the patient is in advanced immunosuppression state or has other diseases associated with the possibility of a false negative result of the serology. If the patient does not give its consent for the performance of serological determinations, being this unknown or impossible to be performed, the source should be considered as infected.
Moreover, the sensitivity of the exposed worker should be assessed, determining the serology of HCV, of HBV if he/she is not vaccinated and of anti-HBs if he is vaccinated and this was unknown before or he/she is immunosuppressed. The worker is considered susceptible of HBV infection when he/she is not vaccinated and, when vaccinated, he/she presents antiHBs <10mIU/ml.
There is no effective prophylaxis against HCV, because viral kinetics shows that there must be a previous established infection so that the treatment can be effective. Therefore, monitoring of these patients is important, to be able to diagnose a possible HCV acute infection as soon as possible, in which case the treatment can be more efficient.
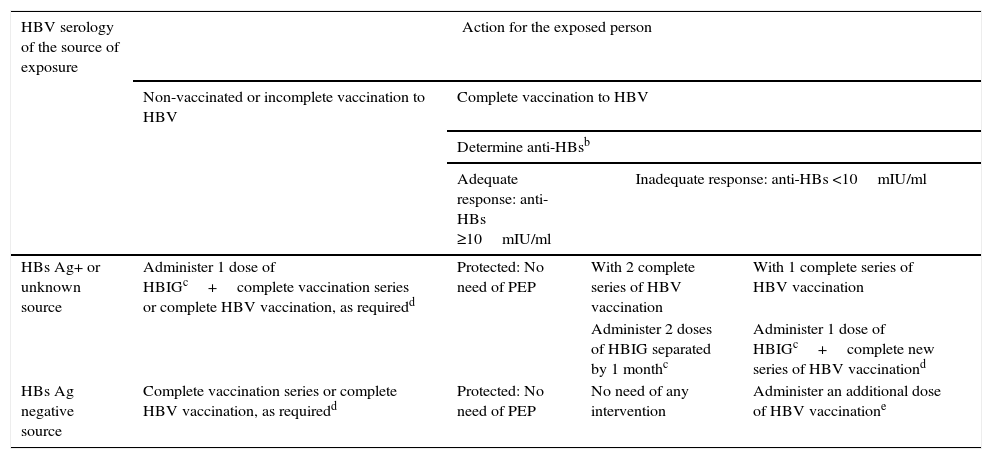
Table 2 shows the protocol for action and PEP against HBV.
Occupational and non-occupational post-exposure prophylaxis (PEP) against HBV.a
| HBV serology of the source of exposure | Action for the exposed person | |||
|---|---|---|---|---|
| Non-vaccinated or incomplete vaccination to HBV | Complete vaccination to HBV | |||
| Determine anti-HBsb | ||||
| Adequate response: anti-HBs ≥10mIU/ml | Inadequate response: anti-HBs <10mIU/ml | |||
| HBs Ag+ or unknown source | Administer 1 dose of HBIGc+complete vaccination series or complete HBV vaccination, as requiredd | Protected: No need of PEP | With 2 complete series of HBV vaccination | With 1 complete series of HBV vaccination |
| Administer 2 doses of HBIG separated by 1 monthc | Administer 1 dose of HBIGc+complete new series of HBV vaccinationd | |||
| HBs Ag negative source | Complete vaccination series or complete HBV vaccination, as requiredd | Protected: No need of PEP | No need of any intervention | Administer an additional dose of HBV vaccinatione |
Percutaneous, mucosa or damaged skin exposure to blood, fluids or corporal tissues with visible blood, other potentially infecting corporal fluids (vaginal discharge, semen and cerebrospinal, synovial, pleural, pericardial, peritoneal and amniotic fluids) and laboratory samples containing virus, sexual exposure and victim of assault or sexual abuse.
HBIG: Hepatitis B immunoglobulin; dose of 0.06ml/kg (12–20IU/kg) intramuscular. It should be administered as soon as possible after exposure, preferably within the first 24h. Its efficiency has not been proved if administered later than 7 days after exposure.
Recommendations
Exposure to HBV and/or HCV
- (1)
In the case of HBV, the actions and PEP depend on the situation of the source patient as well as of the exposed person (strong recommendation, moderate quality evidence).
- (2)
If the vaccination guideline against HBV is correct, monitoring should not be performed, except for possible legal implications. In these cases, a serological study against HBV should be performed at the start and at 6 months (strong recommendation, low quality evidence).
- (3)
In the case of HCV, there is no effective PEP, so an early diagnosis of a possible acute infection of the exposed person should be guaranteed to be able to treat him/her as soon as possible (strong recommendation, very low quality evidence).
Risk and basic fundamentals of non-occupational percutaneous transmission are similar to those of occupational transmission, except for the frequent difficulty to identify the source of exposure that characterizes (NOE). Therefore, this section will focus on sexual transmission.
Non-occupational exposure (NOE) is defined as the accidental contact by sexual or percutaneous means to blood and/or other potentially HIV infected biological fluids outside the occupational or perinatal field.
Assessment of the risk of non-occupational transmission of HIVHIV transmission can only take place by exposure to potentially infecting fluids. The highest risk of transmission is through blood or fluids containing visible blood. Semen, vaginal discharge, cerebrospinal, pleural, pericardial, peritoneal and amniotic fluids, and human milk are also considered potentially infecting. Urine, faeces, saliva, vomit, nasal discharge, tears, sweat and sputum are not considered infecting as long as they do not contain visible blood.
The probability of HIV transmission will basically depend on the type of exposure, the source person's state, the amount of virus in the inoculum and the exposed individual.
Taking all these factors into account, different risk degrees can be established. These risk degrees can be modified by the aforementioned concurrent factors. When referring to sexual relations, these are considered as potential risk when a condom has not been used or has been used inappropriately. The type of exposure is described from the exposed point of view.
- 1.
Considerable risk, if the following three conditions are met:
- •
exposure of anus, vagina, eyes, mouth or other mucosa membranes, damaged skin or percutaneous contact;
- •
with potentially infecting fluids;
- •
and an HIV positive source.
In these cases it is recommended that the person exposed receive NOPEP
- •
- 2.
Risk to be assessed individually, if the following three conditions are met:
- •
exposure of anus, vagina, eyes, mouth or other mucosa membranes, damaged skin or percutaneous contact;
- •
with potentially infecting fluids;
- •
and a source with unknown HIV state.
The probability of getting an HIV infection when the source's state is unknown, can be estimated by multiplying the probability of the person being positive and the probability of transmission.
As it has been said, in NOE it is not always possible to know if the source of exposure has an HIV infection. This is the reason why NOPEP is recommended if the source has a high probability of being infected by HIV:
- 1.
men who have sex with men (MSM),
- 2.
intravenous drug user (IDU),
- 3.
sex worker,
- 4.
sex offender,
- 5.
background of ingress in correctional facilities or
- 6.
an individual coming from a country with an HIV prevalence higher than 1% (Haiti, Bahamas, Jamaica, Belize, Trinity and Tobago, Estonia, Russia, Thailand and Sub-Saharan Africa).
- 1.
- •
- 3.
Negligible risk:
- •
Any type of exposure to fluids not considered potentially infecting, independently of the source's state relating HIV.
- •
Any type of exposure with any type of fluid if the source is HIV negative.
- •
In these cases it is not recommended to perform NOPEP.
- •
- 4.
No considerable risk:
- •
Kisses.
- •
Bite without interruption.
- •
Superficial scratch with a sharp object, including needles abandoned on the street.
- •
Infecting fluids on undamaged skin (0%).
- •
In these exposures it is not recommended to perform NOPEP.
- •
Currently, exposure by sexual means is, with a big difference, the most frequent cause of NOE, being receptive anal intercourse the exposure with highest risk. Other high risk exposures are blood transfusions (currently the risk is almost inexistent because of the systematic screening of HIV serology of all patients), followed by the shared used of syringes between IDU and percutaneous exposure with needle.
Regarding sexual intercourse, sexual exposure is considered to be of risk if it has been practiced without a condom or by break or misuse of the condom. The risk of infection increases considerably in case of traumatic sexual intercourse, violent ones, in existence of genital injuries and/or STI, bleeding or menstruation during the intercourse or in existence of a high HIV viral load in blood and/or fluids.
Risk assessment depending on the contagion route (sexual, intravenous): Measurement of risk of transmission depends on the prevalence of an HIV infection in the population to which the source person belongs to and on the estimated risk of the type of exposure.
Together with the aforementioned circumstances, it is necessary to take into account those circumstances that imply an increase of the risk of transmission. These are:
- –
Infectivity of the source person: acute HIV infection, advanced disease with low levels of CD4+ (<350cells/ml) or AIDS events if the source is not receiving ART, as well as the existence of an HIV viral load >5000cop/ml.
- –
Existence of bleeding, menstruation, bleeding ulcers or STI.
Previously, we have talked about other circumstances in which it is recommended to assess NOPEP (MSM, IDU, etc.). All of this justifies that the assessment of the risk should be individualized.
Previous considerations to post-exposure prophylaxisBefore considering PEP use, it is necessary to take into account if the person exposed visits the healthcare service within 72h after the exposure, to gather as much information about the source person and to assess the capacity of performing a clinical monitoring of the person exposed. Independently of performing PEP or not, all proceedings and interventions must be clearly registered.
After the accidental exposure, exposed wounds and skin should be washed with water and soap. On the other hand, after an accidental puncture, the puncture area should never be pressed.
Also, people who suffer an accidental exposure should be assessed as soon as possible to evaluate if PEP is appropriate.
As in OPEP, if NOPEP is appropriate, it is recommended to start it as soon as possible after the NOE, preferably within the first 24h and always within the first 72h. It is not recommended to start PEP later than 72h after the NOE.
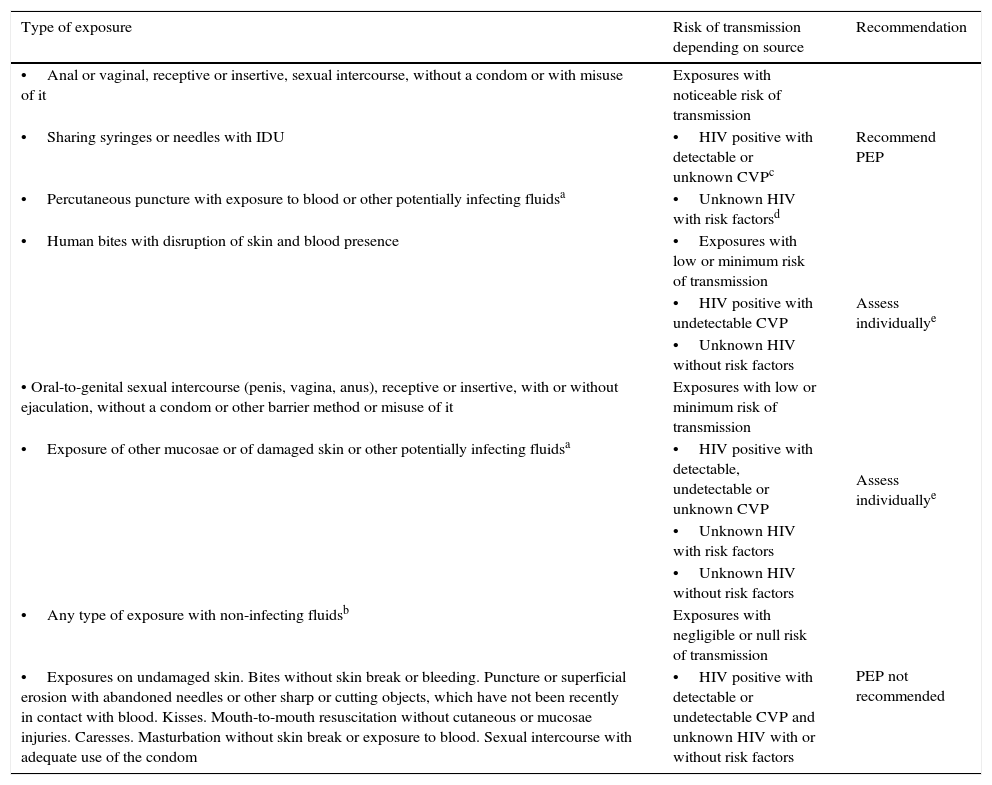
General recommendations about NOPEP are gathered in Table 3. In general it is recommended to perform PEP when the risk of transmission is noticeable, when the risk is low or minimum each case should be assessed individually and when the risk is negligible or null it is not recommended.
NOPEP recommendations.
| Type of exposure | Risk of transmission depending on source | Recommendation |
|---|---|---|
| •Anal or vaginal, receptive or insertive, sexual intercourse, without a condom or with misuse of it | Exposures with noticeable risk of transmission | |
| •Sharing syringes or needles with IDU | •HIV positive with detectable or unknown CVPc | Recommend PEP |
| •Percutaneous puncture with exposure to blood or other potentially infecting fluidsa | •Unknown HIV with risk factorsd | |
| •Human bites with disruption of skin and blood presence | •Exposures with low or minimum risk of transmission | |
| •HIV positive with undetectable CVP | Assess individuallye | |
| •Unknown HIV without risk factors | ||
| • Oral-to-genital sexual intercourse (penis, vagina, anus), receptive or insertive, with or without ejaculation, without a condom or other barrier method or misuse of it | Exposures with low or minimum risk of transmission | Assess individuallye |
| •Exposure of other mucosae or of damaged skin or other potentially infecting fluidsa | •HIV positive with detectable, undetectable or unknown CVP | |
| •Unknown HIV with risk factors | ||
| •Unknown HIV without risk factors | ||
| •Any type of exposure with non-infecting fluidsb | Exposures with negligible or null risk of transmission | PEP not recommended |
| •Exposures on undamaged skin. Bites without skin break or bleeding. Puncture or superficial erosion with abandoned needles or other sharp or cutting objects, which have not been recently in contact with blood. Kisses. Mouth-to-mouth resuscitation without cutaneous or mucosae injuries. Caresses. Masturbation without skin break or exposure to blood. Sexual intercourse with adequate use of the condom | •HIV positive with detectable or undetectable CVP and unknown HIV with or without risk factors |
Blood, fluids containing visible blood, semen, vaginal discharge, cerebrospinal, pleural, pericardial, peritoneal, synovial and amniotic fluids and human milk.
Urine, faeces, saliva, vomits, nasal discharge, tears, sweat and sputum, if they do not contain visible blood.
MSM, IDU, sex worker, sexual abuser, background of ingress in correctional facilities and people coming from countries with an HIV prevalence higher than 1% (Haiti, Bahamas, Jamaica, Belize, Trinity and Tobago, Estonia, Russia, Thailand and Sub-Saharan Africa).
Assess each case individually. Generally it is recommended to start PEP if the source is HIV+ with detectable or unknown CVP, or if he/she is HIV unknown with risk factors. If the source is HIV+ with undetectable CVP or if he/she is HIV unknown without risk factors, it can be considered not to perform PEP, because the risk of transmission is very low.
Recommendations
- (1)
People who suffer an accidental exposure should be assessed as soon as possible to evaluate if PEP is advised. Strong recommendation, moderate quality evidence.
- (2)
After accidental exposure, exposed wounds and skin should be washed with water and soap. On the other hand, after an accidental puncture, the puncture area should never be pressed. Strong recommendation, moderate quality evidence.
- (3)
In case of an HIV exposure, independently of performing PEP or not, all proceedings and interventions should be clearly registered. Strong recommendation, moderate quality evidence.
- (4)
It is recommended to start NOPEP as soon as possible after the exposure, preferably within the first 24h and always within the first 72h. Strong recommendation, moderate quality evidence.
- (5)
It is recommended to perform NOPEP in exposures with considerable risk. Strong recommendation, low quality evidence.
- (6)
It is recommended to assess individually the need of NOPEP in exposures with low or minimum risk. In general it is recommended to start NOPEP if the source is HIV+ with detectable or unknown CVP or if he/she is HIV unknown with risk factors. If the source is HIV+ with undetectable CVP or if he/she is HIV unknown without risk factors, it can be considered not to perform NOPEP because the risk of transmission is very low. Weak recommendation, low quality evidence.
- (7)
It is not recommended to perform NOPEP in exposures with low or negligible risk. Strong recommendation, low quality evidence.
- (8)
NOPEP should be cancelled in case of confirmation that the source person is HIV negative. Strong recommendation, low quality evidence.
Assessment of risk of non-occupational percutaneous exposure is similar to that of occupational exposure, except for the frequent difficulty to identify the exposure source that characterizes NOE. Moreover, the risk of transmission of HBV and HCV by sexual means should be considered in NOE.
Regarding HBV, it is important to know the source's state and the state of immunity of the exposed subject and vaccinate or use anti-HBV gamma globulin when necessary. In patients non-immunized against HBV or who do not know their immunological state, the decision to vaccinate should not be postponed until receiving the anti-HBs serology (Table 2).
More frequently, cases of HCV transmission after sexual intercourse are described, especially in MSM. Moreover, there is no effective prophylaxis against HCV, because viral kinetics shows that for the treatment to be efficient, there should be an established infection. Therefore, monitoring of these patients is so important to be able to diagnose a possible acute HCV infection as soon as possible, in which case the treatment can be more efficient.
Recommendations
- (1)
In the case of HBV, actions depend on the situation of the source patient as well as of the exposed person (strong recommendation, moderate quality evidence).
- (2)
If HBV vaccination guideline is appropriate, monitoring should not be performed, except for possible legal implications. In these cases, a serological study against HBV should be performed at the beginning and at 6 months (strong recommendation, low quality evidence).
- (3)
Regarding HCV, currently there is no effective PEP measure, so recommendations should be aimed at an early diagnosis of a possible acute infection of the person exposed, to be able to treat him/her as soon as possible (strong recommendation, low quality evidence).
After exposure by sexual means, the possibility of transmission of other STI (like syphilis, gonococcus and chlamydia) should be considered. This depends on the prevalence in the community, the type of exposure, the existence of a traumatism and the number of source people.
Recommendations
- (1)
After an exposition by sexual means, apart from HIV, other STI should be called off, performing the appropriate diagnosis proceedings (strong recommendation, high quality evidence).
- (2)
In the case of sexual abuse, it is recommended to start a triple empirical therapy (ceftriaxone plus metronidazole plus azithromycin plus doxycycline) (strong recommendation, moderate quality evidence).
PEP against HIV is recommended at any point of pregnancy, in case there has been a significant exposure. ART risks for this population should be taken into account, especially during the first trimester of pregnancy. Therefore, up-to-date guides about ART during pregnancy should always be consulted. Regarding HBV prophylaxis, the protocol of action should be the same as in the rest of the population.
Recommendation
- (1)
In the case of risk exposure to HIV in a pregnant woman, the same PEP recommendations as for non-pregnant women should be followed (strong recommendation, low quality evidence).
In any sexual abuse case medical and legal connotations should be taken into account and apply the established protocols.
It is estimated that the risk of HIV sexual transmission might be higher in rape victims with genital and/or anal lacerations produced during the raping. Although there is not much data about the prevalence of HIV infection between people with charges of rape, PEP is recommended in every case it exists. PEP guidelines and starting times should follow the same criteria as in other risk sexual intercourse.
It is recommended to start an empirical antibiotic treatment in sole dose to avoid other STI.
Recommendations
- (1)
PEP against HIV is recommended for sexual abuse victims when there is a significant exposure, defined as direct contact of semen, vaginal discharge or abuser's blood with vagina, penis, anus or mouth of the victim, even if there is no visible macroscopic damage (strong recommendation, low quality evidence).
- (2)
PEP is recommended in cases of break of the skin's integrity or mucosa membranes if they have been in contact with blood, semen or vaginal discharge of the abuser, as well as bites that show visible blood (strong recommendation, low quality evidence).
Every person who has been assessed after an occupational or non-occupational exposure, independently of performing PEP or not, should be offered a clinical and analytic monitoring plan, information and psychological support.
The following determinations should be performed at the initial moment:
- –
General clinical analysis including a complete haemogram and biochemistry (renal and hepatic profile).
- –
HIV, HBV and HCV serology.
- –
Syphilis serology and early detection of other STI in sexual exposures.
- –
Pregnancy test in women with sexual exposures.
The beginning of HIV PEP should never be postponed until receiving the results. If eventually the source is confirmed to be HIV negative, PEP will be cancelled. Monitoring of these patients lasts 24 weeks. The only exception is the performance of a new serology against HIV in 48 weeks in case of suffering an HCV infection after exposure to an HIV-HCV co-infected source. In laboratories that have a combined fourth generation determination of antigen/antibody for HIV, monitoring may be reduced to basal assessment, 6 weeks or 4 months post-exposure.
On the other hand, people who have suffered an accidental exposure should be informed about the signs and/or symptoms of acute HIV infection. In this case, a viral load of HIV will be performed, independently of the time lapsed since the accidental exposure. Patients starting PEP should also be informed about side effects of the antiretroviral treatment, about possible drug interactions and about the need of having a good adherence to the treatment.
Moreover, during the monitoring period, especially during the first 12 weeks, the person exposed should avoid a possible secondary transmission of HIV or other infections, always using a condom during sexual intercourse, not sharing syringes, avoiding pregnancy and blood or semen donations.
Recommendations
- (1)
If monitoring includes a fourth generation HIV antigen/antibody test (including detection of P24 antigen), monitoring can conclude 4 months after exposure. If there are no fourth generation tests available, monitoring usually concludes 6 months after an HIV exposure (strong recommendation, high quality evidence).
- (2)
Likewise, these patients should be informed about secondary prevention measures (barrier contraceptives use, avoiding blood or other donations, tissues or semen, pregnancy and lactation) to prevent secondary transmission, especially during the first 6–12 weeks after exposure (strong recommendation, high quality evidence).
PEP guidelines of choice consist on the combination of two nucleosides/nucleotides reverse transcriptase inhibitors (NRTI) associated to a third ARVD of a different family.
Due to their better tolerance and once-a-day administration, the two preferential NRTI are co-formulated (1 pill a day) tenofovir/emtricitabine (TDF/FTC). As an alternative, co-formulated (1 pill two times a day) zidovudine/lamivudine (ZDV/3TC) can be used if you do not want to use TDF (e.g., for people with kidney disease). The use of abacavir is not recommended due to the risk of hypersensitivity to said drug, because it is not possible to have available HLA-B*5701 in the person exposed at the beginning of PEP.
As third drug a protease inhibitor enhanced with ritonavir (PI/r) or an integrase inhibitor (IN) can be used, because the probability of exposure to a virus resistant to these ARVD is very low. Among different PI/r available, just as in antiretroviral treatment guides, the preferential ones are darunavir/ritonavir (DRV/r, 800/100mg a day) or atazanavir/ritonavir (ATV/r 300/100mg a day) and as an alternative lopinavir/ritonavir (LPV/r 2 pills two times a day). As IN it is recommended to use raltegravir (RAL, 1 pill two times a day) due to its good tolerance, scarce drug interactions and larger experience. With other IN, like elvitegravir/cobicistat (EVG/COBI) and dolutegravir (DTG) there is not enough experience, so currently they should only be used as alternative drugs. Regarding the use of PI/r or RAL, the newest guides about PEP prefer RAL to enhance adherence and tolerance as well as due to its scarce risk of interactions, although it should be administered two times a day.
Generally, it is not recommended in preferential guidelines the use of first generation non-nucleosides reverse transcriptase inhibitors (NNRTI), efavirenz (EFV) and nevirapine (NVP), as third drug, due to its side effects and higher risk of primary resistances. Due to its better tolerance, rilpivirine (RPV) and etravirine (ETR) could be an alternative. A recent study has shown good results with RPV, but there is no experience with ETR in PEP.
When the source patient of the exposure has known or suspected resistances (previous virological failures) to one or more ARVD of the preferential guidelines recommended in PEP or in case the exposed person has possible contraindications to the use of some of them, it is recommended to consult an expert in HIV infection to select the most appropriate guideline in these cases, without this being a delay in the start of PEP (the recommended guideline can begin and the expert review and adjust it as soon as possible, preferably before 24–72h).
Recommendations
- (1)
It is recommended to use TDF/FTC with RAL as a preferential guideline, in both occupational and non-occupational PEP (strong recommendation, moderate quality evidence).
- (2)
As alternative guidelines TDF/FTC can be used with DRV/r, ATV/r, DTG, EVG/COBI or RPV, in both occupational and non-occupational PEP (weak recommendation, low quality evidence).
Even though the optimum length of PEP is unknown, based on the results of studies with animal models and occupational transmissions, a 28-day guideline is recommended.
Recommendations
- (1)
A 28-day guideline is recommended for PEP (strong recommendation, low quality evidence).
- (2)
A reassessment of adherence and toxicities is recommended in 72h after beginning PEP (strong recommendation, low quality evidence).
Once beginning PEP, a reassessment of the patient within 72h after exposure is recommended. Currently, new data about it can be obtained, as well as clarifying risks and benefits, modifying and adjusting the PEP guideline, guaranteeing an appropriate adherence and managing symptoms associated to side effects. Later on, clinical controls at least every 2 weeks are recommended until completing the PEP guideline.
Monitoring will be the same in OPEP and NOPEP. However, in the case of exposures by sexual means an early detection of other STI should be performed (syphilis, gonococcus, chlamydia).
Toxicity and interactionsART toxicity is a very important problem that conditions the adherence to it. To this we have to add the existence of possible drug interactions derived from the previous or concomitant administration of other drugs that may reduce the benefit of PEP or increase the possibility of side effects.
If a toxicity or side effect that can limit the success of PEP is observed, modification of the guideline or use of other treatments that minimize these side effects should be assessed (e.g. antiemetics).
When selecting PEP, there are different guides and webpages in which you can look up the main side effects of ARVD employed and the risk of interactions.
Psychological approachIt is recommended that the professional assess in the PEP prescription visit the need of giving psychological support depending on the level of anxiety and worries the patient has. If the anxiety level is high, there is a risk that the transmission of information is useless because the emotional state of the patient can make it more difficult for the patient to comprehend the instructions to follow, with a fundamental effect of a worse adherence to the treatment. If we confront a sexual abuse situation it is essential to refer the patient to the mental health centre of reference and monitor the adherence properly, because it has been shown to be inadequate in the context of sexual abuse.
Regarding occupational PEP, the professional should give all the necessary information to resolve doubts. Even though it is not usual, if anxiety or distress is very high and it may interfere with his/her work activities, it will be necessary to refer the patients to mental health specialists.
Regarding children and adolescents, the professional should assess the need to refer to the corresponding mental health team depending on anxiety and worries the family or child presents. The referral of the family to an entity that treats these topics and that has qualified professionals available can also be considered. In the case of proven sexual abuse, referral for psychological intervention by professionals specialized in this field will be essential. In the case of adolescents, it is important that the professional uses during interviews a close language and style, aimed at creating a climate of confidence and closeness that facilitates the obtaining of true information.
Post-exposure prophylaxis in children and adolescentsWhat should be done in case of taking care of a child or adolescent with a potential or confirmed HIV exposure is the following:
- 1.
Wash the entrance door: wound (skin or mucosa), exposed mucosa (oral, genital, ocular).
- 2.
Determining if post-exposure prophylaxis for HIV is necessary.
- 3.
Notify parents or legal guardians.
- 4.
In case of suspecting or confirming sexual abuse, notify the corresponding judicial authorities and guarantee a forensic physical examination by appropriately trained and accredited professionals.
- 5.
In case of not having the necessary capacity available, refer the child to a medical centre with the appropriate capacity for assessment and integral care
- 6.
Perform a basal serological test of HIV.
- 7.
Assess the risk of other transmissible pathogens.
- 8.
Basal analytical assessment.
- 9.
Initiate a prophylactic treatment with ARV, if it is indicated, ideally within the first 6h and always before the 72h after the exposure
- 10.
Initiate treatment or prophylaxis for other pathogens if it is indicated (see below).
- 11.
Establishing calendar and contain of the monitoring.
Basal analytical assessment:
- –
Complete blood count.
- –
Hepatic function tests.
- –
Assessing the vaccination state against HBV and tetanus.
- –
HCV serology.
- –
In case of suspecting or confirming sexual abuse perform tests to diagnose:
- •
Gonorrhoea, syphilis and chlamydia.
- •
HBV, HSV and HPV.
- •
Bacterial vaginosis and trichomoniasis.
- •
The most common situation is, with a big difference, the accidental puncture with needle of unknown origin. In these cases no antiretroviral prophylaxis should be performed. Bites happen frequently among children. However, HIV levels in saliva are very low, so the risk of HIV transmission is negligible when skin is undamaged. There are very few documented cases of possible transmission through this mechanism.
In any case, a bite that causes blood, both for the bitten person as well as for the biter, can be an indication of PEP.
Recommendation
- 1.
If a negative HIV child bites an HIV+ person or is bitten by an HIV+ person damaging the skin, he/she should receive post-exposure prophylaxis against HIV (strong recommendation, low quality evidence).
Sexual abuse by an infected adult or adolescent, even though it is statistically less frequent in adults, has a higher risk of viral transmission in children, due to their larger anatomical vulnerability, to traumatisms, a thinner vaginal and anal mucosa, cervical ectropion, etc. Therefore, the risk in the case of a confirmed penetration, may be higher than that derived from studies in adults, especially in small children and if important injuries have been caused (rips, etc.)
There are no studies that show that PEP in paediatrics is effective and the difficulty of performing these studies is understandable.
Prophylactic treatment against HIV in children and adolescentsExcept for adolescents, who can receive an identical guideline as adults, children should receive a guideline adjusted to this age, taking into account the approval state of ARVD for paediatric age recommended in adults’ PEP, as well as the availability of suspension for children who cannot swallow pills or capsules.
Prophylactic treatment against HBV in children and adolescentsIn case the child is not properly vaccinated or he/she is vaccinated but presents negative serology (anti-HBs), vaccination and immunoglobulin specifically against HBV intramuscular is recommended, if possible during the first 72h.
Recommendations
- 1.
The same diagnosis system should be followed as for adults (strong recommendation, low quality evidence).
- 2.
If it is indicated, a 28-day length of PEP treatment is recommended, preferably within the first 6h after exposure, and always within the first 72h (strong recommendation, low quality evidence).
- 3.
PEP in adolescents (>12 years old) can be performed following an identic guideline as for adults (strong recommendation, low quality evidence).
- 4.
PEP in children younger than 12 years old should consist on emtricitabine (FTC)+zidovudine (AZT)+lopinavir enhanced with ritonavir (LPV/r). In children who cannot swallow pills, these drugs will be administered in paediatric suspension (strong recommendation, low quality evidence).
This document was financed with funds from the National AIDS Plan.
Conflict of interestIn order to avoid and/or minimize potential conflicts of interest, people form the panel of experts/as have made a formal declaration of interest. In this statement the authors have received funding to participate in conferences and to conduct research and have received payments as speakers by public institutions and pharmaceutical companies. These activities do not affect the clarity of this recommended and/or grants received. Significantly regarding drugs in document speaks only active ingredient and no trademark.
Writing Committee: Rosa Polo Rodriguez. Especialista en Medicina Interna. Secretaría delPlan Nacional sobre el Sida. Madrid. Fernando Lozano. Especialista en Medicina Interna. Unidad Clínica de Enfermedades Infecciosas y Microbiología. Hospital Universitario de Valme. Sevilla. Pedro González de Castro. Especialista en Medicina del trabajo. Presidente de la Sociedad Española de Medicina del TrabajoEsperanza. MaAlonso Jiménez. DUE., Presidenta de la Federación Española de Enfermería del Trabajo FEDEET. Oscar Miró. Especialista en Medicina Interna. Servicio de Urgencias del hospital Clinic. Barcelona. Jose Ramón Blanco. Especialista en Medicina Interna. Departamento de Enfermedades Infecciosas/VIH. Hospital San Pedro CIBIR. Logroño. David Moreno. Especialista en Pediatría. Infectología Pediátrica. Hospital Regional Universitario de Málaga. Carlos Dueñas. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital Universitario de Burgos. Enriqueta Muñoz Platón. Especialista en Medicina Preventiva. Servicio de Medicina Preventiva. ComplejoHospitalario de Toledo. Marina Fernández Escribano. Especialista en Medicina del Trabajo. Servicio de Prevención de Riesgos Laborales Hospital Ramón y Cajal. Madrid. Jesús Sanz Sanz. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital Universitario de La Princesa. Madrid. Carmina Fumaz. Especialista en Psicología Clínica. Hospital de día de VIH. Hospital UnversitarioGermansTrias i Pujol. Badalona. Ignacio Santos. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital Universitario de La Princesa. Madrid. Federico García. Especialista en Microbiología y Parasitología Clínicas. Servicio de Microbiología. Complejo Hospitalario. Universitario de. Granada. MaJesús Téllez. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital Universitario Clínico San Carlos. Madrid. Raúl González Montero. Especialista en Pediatría. Infectología pediátrica. Hospital San Juan. Alicante. MaVictoria Vals Jimenez. Especialista en Medicina del Trabajo. Servicio Prevención de Riesgos Laborales. Áreade salud de Segovia. Juan Emilio Losa. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital Universitario Fundación Alcorcón. Madrid. MaLuisa Valle Robles. Especialista en Medicina del trabajo. Servicio de prevención de riesgos laborales. Area de Salud de Segovia. Jose Antonio Iribarren. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital de Donosti. San Sebastian. Enrique Ortega. Especialista en Medicina Interna. Unidad de Enfermedades Infecciosas/VIH. Hospital General Universitario. Valencia.