In the present update of the guidelines, a starting combination antiretroviral treatment (cART) is recommended in symptomatic patients, in pregnant women, in serodiscordant couples with a high risk of transmission, in patients co-infected with hepatitis B virus requiring treatment, and in patients with HIV-related nephropathy. Guidelines on cART are included in the event of a concurrent diagnosis of HIV infection with an AIDS-defining event. In asymptomatic naïve patients, cART is recommended if the CD4+ lymphocyte count is <500cells/μL; if the CD4+ lymphocyte count is >500cells/μL, cART can be delayed, although it may be considered in patients with liver cirrhosis, chronic infection due to hepatitis C virus, high cardiovascular risk, plasma viral load (PVL) >105copies/mL, CD4+ lymphocyte percentage <14%, cognitive impairment, and age >55 years. cART in naïve patients requires a combination of 3 drugs, and its aim is to achieve undetectable PVL. Treatment adherence plays a key role in sustaining a favorable response. cART can, and should be, changed if virological failure occurs, in order to return to undetectable PVL. Approaches to cART in acute HIV infection, in women, in pregnancy, in tuberculosis, and post-exposure prophylaxis are also examined.
En esta actualización se recomienda iniciar el tratamiento antirretroviral (TAR) en los pacientes sintomáticos, en las embarazadas, en las parejas serodiscordantes con alto riesgo de transmisión, en la hepatitis B que requiera tratamiento y en la nefropatía relacionada con el VIH. En caso de diagnóstico simultáneo de infección por el VIH y un evento definitorio de sida, se incluyen directrices sobre el inicio del TAR. En los pacientes asintomáticos se recomienda iniciar el TAR cuando la cifra de linfocitos CD4+ sea inferior a 500células/μl. Si esta es superior a 500células/μl se aconseja diferir el tratamiento en general, pero puede considerarse en los pacientes con cirrosis hepática, hepatitis crónica por virus C, riesgo cardiovascular elevado, carga viral plasmática (CVP)>105copias/ml, proporción de CD4+ inferior al 14%, deterioro neurocognitivo y edad superior a 55años. El TAR inicial requiere la combinación de 3fármacos y su objetivo es conseguir CVP indetectable. La adherencia juega un papel fundamental en la duración de la respuesta. En caso de fracaso virológico, se debe y se puede lograr de nuevo CVP indetectable. Se comentan los criterios de TAR en la infección aguda, en la mujer, en el embarazo, en la tuberculosis y en la profilaxis postexposición.
Combined antiretroviral treatment (cART) is evolving so quickly that GeSIDA and the Spanish National Plan on AIDS update their Treatment Guidelines annually. A panel of experts has revised the advances published or reported at scientific meetings in the last year and updated them. The present document is an executive summary of the current guidelines.1
After more than 20 years of experience with cART, a series of general principles on which this treatment is based have been defined. Decisions on treatment take into account the patient's clinical situation, CD4+ lymphocyte count, and plasma viral load (PVL). The goal of cART is to achieve a PVL <50copies/mL or under the detection threshold. In order to achieve this goal, we need 3 different drugs and a high level of adherence to treatment, this being essential to sustain a strong virologic response. By inhibiting viral replication, it is possible to achieve variable restoration of the immune system, depending on previous damage. Several negative aspects of cART have also been defined; these include adverse effects, frequent drug–drug interactions, and the presence of resistance mutations when therapeutic drug levels are not achieved. By taking these principles into account and selecting appropriate drugs, it is possible to design multiple treatment combinations. Clinicians should know patient characteristics in order to provide the most suitable treatment and make recommendations on preventing transmission of HIV.
How to monitor cARTThe parameters needed to monitor cART are set out below.
CD4+lymphocyte count. CD4+ lymphocyte count is the main marker of the risk of opportunistic infections and indicates when to start cART.
Recommendation:
- 1.
CD4+ lymphocyte count should be monitored frequently, as it is the most important parameter in determining when to initiate cART (A-I).
Plasma HIV RNA (viral load) testing. PVL is a secondary determinant of when to initiate cART, and its fast and sustained suppression is a marker of the effectiveness of cART.
Recommendations:
- 1.
PVL should be measured jointly with the CD4+ lymphocyte count (A-II).
- 2.
PVL is the most important indicator of response to antiretroviral therapy and is the main parameter for evaluating, defining, and addressing failures in cART (B-I).
- 3.
PVL should be measured using a technique with a detection threshold of at least 50copies/mL. The same method should always be used (A-I).
- 4.
If a decision is to be taken based on the result of PVL testing, then this result should be confirmed beforehand using another test (A-II).
Drug-resistance testing. Viral genome mutations are the consequence of rapid HIV-1 turnover and error-prone reverse transcriptase. The appearance of resistant mutations is associated with virologic failure. Resistance mutations can be either primary or secondary to virologic failure.
Recommendations:
- 1.
Genotypic resistance assay is recommended in clinical practice at diagnosis, when starting cART (if more than 1 year from the previous determination), in pregnant women with a detectable viral load, in virologic failure, and in post-exposure prophylaxis (index patient) (B-II).
- 2.
Genotyping of the viral integrase gene is not recommended in naïve patients (C-III).
- 3.
HIV subtype should be determined, mainly in immigrant patients or if there is quick clinical progression (CIII).
Plasma drug levels. Plasma concentrations of antiretroviral drugs correlate with effectiveness and toxicity; therefore, ascertaining drug levels may be useful for optimizing drug doses.
Recommendations:
- 1.
Measurement of plasma levels of antiretroviral drugs is not routinely recommended for the management of patients with HIV-1 infection (C-III).
- 2.
Measurement of plasma levels of antiretroviral drugs may be helpful in the management of specific clinical situations such as drug–drug interactions, transplant, severe underweight or obesity, pregnancy, and kidney or liver failure (C-III).
HLA-B*5701 screening. The presence of the HLA-B*5701 allele is related to a hypersensitivity reaction to abacavir (ABC), a life-threatening multiorgan clinical syndrome observed during the first 6 weeks of treatment.
Recommendations:
- 1.
Screening for HLA-B*5701 should be performed at diagnosis or before starting an ABC-containing regimen (A-I).
- 2.
If HLA-B*5701 screening is positive, ABC should not be prescribed (A-I).
- 3.
Negative tests do not completely rule out a future hypersensitivity reaction; therefore, the patient should be informed about this possibility (A-I).
HIV-1 co-receptor tropism assays. Tropism assay is useful when prescribing maraviroc.
Recommendations:
- 1.
A tropism assay should be performed whenever the use of a CCR5 inhibitor is being considered (A-I).
- 2.
Tropism assay is also recommended during virologic failure when salvage therapy is being considered (A-III).
Acute HIV infection is symptomatic in more than half of all cases, although symptoms are rarely recognized because they are similar to those of a common viral infection.
Progression to AIDS is faster in patients with characteristics such as symptom type, viral load, CD4+ lymphocyte count, and viral tropism. Taking these parameters into account, we should choose whether or not to start cART. At present, starting cART during acute infection is controversial, as the potential long-term benefit is unknown.
Recommendations:
- 1.
cART is recommended immediately in acute HIV-infection in the following cases: (i) neurological involvement (e.g., meningitis, encephalitis, Guillain–Barré syndrome) or involvement of any other organ or system (e.g., hepatitis, myocarditis, thrombocytopenia); (ii) the acute symptoms last for more than 7 days; (iii) an immunosuppression-related event is diagnosed; (iv) in severe immunosuppression (CD4+ lymphocyte count <350cells/μL); or (v) if the patient has non-R5 viral tropism or a PVL >100,000copies/mL 3 months after infection (B-II). In the case of patients with asymptomatic recent or acute infection, ART should be started if the criteria set out in (iv) or (v) are met (B-II).
- 2.
Starting treatment should be considered when there is a high risk of HIV-1 transmission (A-II).
- 3.
cART should also be initiated in relevant indications independently of the CD4+ lymphocyte count (see section on chronic HIV infection), as well as in pregnant women who become infected with HIV during pregnancy (apply the same level and strength of recommendation).
- 4.
Drug-resistance testing and co-receptor tropism assay should be performed at diagnosis (of an acute or recent HIV infection), regardless of whether cART is initiated (B-II).
- 5.
If cART is to be initiated, the same drug regimens recommended in chronic infection should be used. Raltegravir (RAL) and 2 nucleoside reverse transcriptase inhibitors (NRTI) (preferably tenofovir/emtricitabine [TDF/FTC]) would be the drugs of choice, owing to the faster reduction in PVL and higher concentration in genital secretions, which may help reduce transmission of HIV (B-III).
- 6.
If a resistance testing result is not available, a boosted protease inhibitor (PI)-based regimen is preferred until this result is available (A-II).
- 7.
cART is a lifelong therapy (A-I).
- 8.
If acute infection is not treated, patients should be evaluated as having chronic infection (4–6-month schedule) (A-I).
The objectives of cART are to reduce morbidity and mortality, improve quality of life, restore and preserve the immune system, ensure optimal and persistent suppression of PVL, and prevent HIV transmission.
Recommendations:
- 1.
cART should be initiated depending on clinical manifestations, CD4+ lymphocyte count, PVL, and comorbidities (A-II).
- 2.
cART should be initiated if a symptomatic infection (clinical B or C event, CDC 2003 classification) is diagnosed (A-I).
- 3.
In asymptomatic patients, initiation of cART is based on CD4+ lymphocyte count, PVL, and specific comorbidities and characteristics (Table 1):
- •
cART is recommended if CD4+ lymphocyte counts are <500cells/μL (A-I).
- •
In patients whose CD4+ lymphocyte count is >500cells/μL, the benefit of starting or deferring cART is still unknown; however, cART should be recommended for patients with liver cirrhosis, chronic hepatitis C, PVL>105copies/mL, CD4+ percentage <14%, age over 55 years, high cardiovascular risk, and neurocognitive disorders (C-III).
Table 1.cART indication for asymptomatic patients with chronic HIV infection.a
acART will be always recommended, regardless of CD4+ lymphocyte count, in pregnant women, in serodiscordant couples with a high transmission risk, in patients co-infected with hepatitis B virus requiring treatment, and in patients with HIV-related nephropathy.
cSome experts recommend starting cART in this CD4+ lymphocyte stratum, whereas others only recommend it in specific situations, namely, liver cirrhosis, chronic hepatitis C, plasma viral load >105copies/mL, CD4+ percentage <14%, age >55 years, high cardiovascular risk, and neurocognitive impairment.
- •
- 4.
Starting cART is recommended regardless of the CD4+ lymphocyte count in the following cases:
- •
Serodiscordant couples if there is a high risk of sexual transmission in order to reduce it (A-I). cART should not substitute other measures to prevent HIV-1 transmission in any case (A-II).
- •
Pregnant women (A-I).
- •
HIV-associated nephropathy (A-II).
- •
Patients with hepatitis B co-infection requiring treatment (A-II).
- •
In spite of the above recommendations, initiation of cART should always be assessed on a case-by-case basis. Before initiation, PVL and CD4+ lymphocyte counts should be confirmed and clinicians should prepare patients to accept the treatment, adjust cART to patient lifestyle, comorbidities, provide information on drug–drug interactions, and assess the risk of poor adherence (A-III).
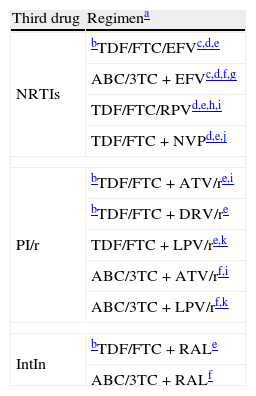
Initial optionsThe preferred cART for naïve patients consists of a combination of at least 3 drugs including 2 NRTIs plus a boosted PI or a non-nucleoside reverse transcriptase inhibitor (NNRTI) or an integrase inhibitor (IntIn) (Table 2). When choosing an NNRTI, boosted PI, or IntIn, clinicians should take the following into consideration:
Initial antiretroviral treatment combinations.
| Third drug | Regimena |
| NRTIs | bTDF/FTC/EFVc,d,e |
| ABC/3TC+EFVc,d,f,g | |
| TDF/FTC/RPVd,e,h,i | |
| TDF/FTC+NVPd,e,j | |
| PI/r | bTDF/FTC+ATV/re,i |
| bTDF/FTC+DRV/re | |
| TDF/FTC+LPV/re,k | |
| ABC/3TC+ATV/rf,i | |
| ABC/3TC+LPV/rf,k | |
| IntIn | bTDF/FTC+RALe |
| ABC/3TC+RALf | |
ABC, abacavir; ATV/r, boosted atazanavir; DRV/r, boosted darunavir; EFV, efavirenz; FTC, emtricitabine; 3TC, lamivudine; LPV/r, boosted lopinavir; NVP, nevirapine; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir.
The numbered comments (see below) address issues affecting the specific regimen, although they do not intend to be exhaustive guidelines on the precautions to be taken when using the drugs. More information can be obtained from the main text of the document (see above) and from the Summary of Product Characteristics of the individual drugs.
Ordered by third drug. The use of co-formulated drugs is recommended. As data regarding the therapeutic equivalence of FTC and 3TC are lacking, the use of one or the other in the selected regimens depends basically on available experience of use in combination with the other drugs.
To be avoided in women wishing to become pregnant and in patients with unstable neuropsychiatric impairments. To be used with precaution in patients performing dangerous activities if they present somnolence, dizziness, and/or impaired concentration.
TDF should be used with precaution in patients with risk factors for kidney failure. Not indicated if GFR <30mL/min). The combination of PI/r and TDF considerably increases the risk of nephrotoxicity.
In ACTG 5202, ABC/3TC was associated with a higher frequency of virologic failure than TDF/FTC in patients with PVL >100,000copies/mL.
Greater risk of virological failure than with TDF/FTC/EFV in patients with a PVL >100,000copies/mL. The EMA has approved use of RPV only in patients with PVL <100,000copies/mL.
As initial treatment, a combination of 2 NRTIs+1 NNRTI, or 2 NRTIs+1 boosted PI, or 2 NRTIs+1 IntIn can be used (see below for the agents of choice) (A-I).
Nucleoside reverse transcriptase inhibitorsPreferred combinations:
- 1.
The NRTIs of choice for initial treatment are TDF/FTC or abacavir/lamivudine (ABC/3TC) (A-I).
- 2.
If possible, a co-formulated fixed-dose combination of NRTIs is recommended (A-I).
- 3.
TDF/FTC should be used with caution in patients with kidney dysfunction (B-II).
- 4.
ABC/3TC may be used with caution in patients with a PVL >100,000copies/mL, especially when the third drug is an NNRTI (A-I).
Efavirenz, nevirapine, and rilpivirine are authorized for treatment in naïve patients, although rilpivirine should only be administered to patients with a PVL <100,000copies/mL.
Recommendations:
- 1.
Efavirenz (EFV) is generally preferred to nevirapine (NVP). EFV has been studied in more trials, and more experience has been accumulated (C-III).
- 2.
EFV is contraindicated during the first trimester of pregnancy, so other options should be recommended in women who do not use effective contraception. It is also to be avoided in patients performing dangerous tasks if they experience somnolence, dizziness, and/or impaired concentration (B-III).
- 3.
NVP is contraindicated in women with a CD4+ lymphocyte count >250cells/μL and in men with a CD4+ lymphocyte count >400cells/μL (A-II).
- 4.
Rilpivirine (RPV) is only recommended in patients with a PVL <100,000copies/mL (A-I), since the risk of virological failure is greater with RPV than with EFV in a patient with >100,000copies/mL.
Different studies have shown that boosted PIs (lopinavir [LPV/r], saquinavir [SQV/r], fosamprenavir [FPV/r], atazanavir [ATV/r], and darunavir [DRV/r]) offer certain advantages (efficacy and genetic barrier) over a non-boosted PI. However, side effects are more frequent with boosted PIs.
Recommendation:
- 1.
ATV/r QD, DRV/r QD, and LPV/r BID or QD are the preferred PIs for naïve patients (A-I).
IntIn prevent viral integrase from binding the reactive edges of viral DNA to the cell DNA.
Recommendation:
- 1.
RAL can be used in naïve patients BID when combined with TDF/FTC or ABC/3TC (A-I).
These drugs prevent the entry of HIV into the cell by blocking the CCR5 co-receptor. They are exclusively active on R5-tropic viruses.
Recommendation:
- 1.
Maraviroc (MVC) should only be used when a regimen containing an NNRTI, PI, or IntIn is not possible. HIV should be R5-tropic. This recommendation is based on the outcomes of the MERIT study (C-I).
Several clinical trials have studied the appropriate time to initiate cART in patients with an AIDS-defining opportunistic infection.
Recommendations:
- 1.
Patients with a concurrent diagnosis of HIV infection and an AIDS-defining opportunistic infection should start cART as soon as possible (within the first month or, ideally, in the first 2 weeks) (A-I).
- 2.
Patients with cryptococcal or tuberculous meningitis who are on treatment should undergo close monitoring, as these conditions are difficult-to-treat opportunistic infections that may be worsened by the potentially harmful consequences of immune restoration (B-II).
We define simplification of cART as the switch from a suppressive cART regimen that achieved viral suppression to another easier (“lighter”) regimen (reducing pill burden or dose frequency) that remains suppressive. The main goals of simplification are to improve quality of life, improve adherence, and prevent or reverse specific adverse effects.
Recommendations:
- 1.
Simplification should never mean a loss of optimal virologic control and can only be offered to patients who have not experienced virologic failure (A-I).
- 2.
The best candidates to undergo simplification are patients under prolonged virologic suppression (≥6 months) with good adherence (>90%) (B-II).
- 3.
A regimen containing a boosted PI plus 2 NRTIs in a patient who has not experienced virologic failure can substitute the PI with EFV, NVP, RPV, or ATV and simplify to QD regimens such as EFV+TDF+3TC (or FTC), RPV+TDF+FTC, EFV+ddI+3TC (or FTC), ATV/r+TDF/FTC, or ATV+ABC/3TC (A-I).
- 4.
The switch to a single-pill regimen can improve adherence (A-I).
- 5.
It is not recommended to simplify a PI to ABC if the patient has received suboptimal NRTI-based regimens (A-I).
- 6.
Simplification to ABC plus TDF+3TC or TDF+ddI (didanosine) is contraindicated (A-II).
- 7.
Simplification to NVP or RAL may provide some metabolic advantages for patients with a high cardiovascular risk (B-I).
- 8.
Switching from enfuvirtide to RAL in virologically suppressed patients has proven to be safe and effective (A-I).
- 9.
Monotherapy with DRV/r (QD) or LPV/r (BID) should be used only in cases of toxicity or intolerance to NRTIs. Furthermore, this strategy should be restricted to patients who fulfill the following conditions: (i) no previous failure with PI; (ii) PVL <50copies/mL for at least 6 months; and (iii) excellent adherence to treatment (C-I). Patients with a nadir CD4+ lymphocyte count <100cells/μL may have a greater risk of failure with this regimen; therefore, its indication in this setting should be evaluated very carefully.
- 10.
Other possible simplifications should be performed in the context of a clinical trial and not in clinical practice (A-III).
Virologic failure is defined as detection of PVL >50copies/mL in 2 consecutive blood samples (or if it is detectable 24 weeks after starting therapy).
Factors related to virologic failure may be associated with the patient, the therapeutic regimen, and the virus. Adherence is the most important predictor of virologic response. Drug-related factors include the potency of the drug, insufficient plasma drug levels due to absorption problems, or drug–drug interactions. Among the virus-related factors, resistance is the most relevant.
When considering a new cART regimen after virologic failure, it is important to know the resistance mutation pattern and previous treatment history. Two scenarios are possible: early virologic failure (first or second failure and few resistance mutations) and advanced failure (additional mutations to ≥3 drug families).
Changing cART in early virologic failure- 1.
A regimen in a patient experiencing virological failure requires a prompt switch to avoid cumulative mutations that enable the subsequent cART to achieve an undetectable viral load (A-III).
- 2.
The new cART regimen should contain 3 fully active agents chosen based on the results of drug-resistance testing or their equivalents if only partially active drugs are being used (A-I).
- 3.
The goal of new cART is to achieve an undetectable PVL (<50copies/mL) (A-I).
- 4.
Drug-resistance testing and a tropism assay should be performed in order to tailor the best alternative regimen (A-II). Drug-resistance testing should be performed while the patient is still taking the failing regimen or as soon as he/she stops taking it. Previously detected mutations should be taken into account if the patient has undergone genotypic tests (“cumulative genotype”) (A-II).
- 5.
When choosing a new cART regimen, we should analyze the causes of virologic failure (e.g., inadequate adherence, drug–drug interactions), previous drug history, previous toxicity, and resistance mutations (B-II).
- 6.
A first-generation NNRTI-based regimen that has failed should be switched to a PI-based regimen, because a regimen containing etravirine (ETR) is less effective and not a preferred choice in this setting (A-I).
- 7.
DRV/r (600/100mg BID) has proven to be superior to LPV/r BID and is the preferred boosted PI in a salvage regimen. This superiority can be shown statistically when protease has ≥1 primary mutations or when the fold-change of LPV/r is >10 (A-I). DRV/r 800/100mg QD can be used in patients who have not previously taken PI and/or do not present specific DRV mutations (A-I).
- 8.
Thymidine analogs should be avoided for salvage regimens if other options are available (A-I).
- 1.
The new cART regimen should be convenient, well tolerated, and with the lowest toxicity possible (C-II).
- 2.
The use of TPV/r is restricted to cases in which the estimated residual activity is clearly superior to that of DRV/r BID and etravirine is not required (combined use of etravirine and TPV/r is contraindicated) (A-II).
- 3.
Structured interruptions of cART aiming to increase the efficacy of the salvage regimen should not be made (A-I).
- 4.
Patients with virologic failure and no other treatment options should not stop cART, even when they are receiving a regimen with proven resistance. At this point, we should seek a regimen based on drugs that reduce viral fitness without adding mutations (e.g., 3TC or FTC or TDF). CD4+ lymphocyte count and viral load should be closely monitored (B-II).
- 5.
Dealing with patients with advanced virologic failure is complex. Clinicians or virologists with experience in resistance mutations and cART salvage regimens should be consulted, since they may have access to restricted drugs (i.e., drugs that are still in clinical trials) in order to provide a cART regimen with a chance of success (C-III).
Adherence is the patient's ability to commit to the choice of cART, as well as to initiating and taking it in order to achieve suitable suppression of viral replication.
Recommendations:
- 1.
The patient should be prepared and clinicians should identify and correct causes that limit adherence before starting cART. If the patient is not ready, deferring cART is a better option (A-III).
- 2.
The first check-up should be within the first 2–4 weeks after starting cART so that factors limiting adherence can be corrected (B-III).
- 3.
Suitable adherence should be monitored and reinforced at every visit (A-III).
- 4.
Adherence should be assessed by a multidisciplinary team involving not only clinicians and nursing staff, but also psychologists and pharmacy staff (B-III).
- 5.
No matter what the clinical setting, adherence should be periodically monitored in order to detect individual lack of adherence and identify its magnitude. Such an approach could help to determine the causes of the problems detected (e.g., treatment abandonment or vacations, loss to follow-up) and to create specific strategies for the patient and for the smooth running of the clinical team (B-III).
- 6.
In patients with irregular adherence, it is preferable to use a PI-based regimen rather than an NNRTI-based regimen in order to avoid resistance mutations (B-III).
- 7.
Co-formulated and QD fixed-dose regimens make cART and long-term adherence easier. Using a single-pill regimen is the most efficient strategy to prevent selective drug adherence (A-III).
Adverse effects of antiretroviral agents may appear either at the beginning of treatment or in the mid-long term.
Recommendations:
- 1.
Acute adverse events should be monitored during the first 2–4 weeks, especially in those patients with comorbidities or taking other drugs that may interact with cART and have clinical consequences. There should be close patient–clinician contact during these weeks. cART (or any other treatment) should be modified according to the drug interactions or severe adverse effects (A-III).
- 2.
Agents that might worsen preexisting diseases should be avoided (A-III).
- 3.
Fasting glycemia and plasma lipid levels (total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides) should be monitored at each check-up (A-II).
- 4.
Assessment of cardiovascular risk is recommended at least once a year (B-III).
- 5.
We recommend performing a basic urine analysis with proteinuria and renal glomerular filtration rate (calculated based on the MDRD equation or Cockcroft–Gault formula) at the first visit and then annually (if no risk factors for nephrotoxicity are detected) or every 6 months (if risk factors are detected at baseline) (B-III). In patients on cART, this analysis should be performed at every visit (A-III), particularly if they are taking TDF (A-II). If the glomerular filtration rate is <50mL/min or proteinuria is detected, TDF and IDV should be avoided, and the NRTI dose should be adjusted, except that of ABC (A-III). TDF is not recommended in patients with acute renal failure or if renal failure is TDF-related (A-III).
- 6.
In patients at risk of osteoporosis (post-menopausal women, smokers, low body mass index, age over 50, lack of vitamin D, hepatitis C, renal failure, diabetes, CD4+ lymphocyte count <250cells/μL, or chronic corticosteroid intake), a bone mineral density DXA scan is recommended at initiation of treatment and periodically thereafter (B-III).
- 7.
A bone metabolic study is recommended (including 25-OH vitamin D) in patients with bone density loss, osteoporosis, or frequent stress fractures (B-III).
Interactions between cART agents and other drugs may have serious clinical consequences. The most relevant consequences are PK interactions, especially those related to drug metabolism. The most important metabolic system is cytochrome P450 (CYP), its principal isoenzyme being CYP3A4. PIs, NNRTIs, and other drugs are inhibitors or inducers of CYP isoenzymes.
Recommendations:
- 1.
All concomitant medication or natural and alternative medicinal products should be assessed in order to ascertain potential interactions (B-III).
- 2.
Contraindications should be taken into account, and dose adjustments should be made when necessary (B-III).
- 3.
Monitoring drug plasma levels should be considered when 2 or more drugs with potentially relevant interactions are administered in order to avoid toxicity or inefficacy (B-II).
Chronic liver disease due to viral infection is the main relevant comorbidity among HIV-infected patients in Spain. It commonly progresses to end-stage liver disease and favors cART-induced liver toxicity.
cART and natural progression of chronic hepatitis C and BRecommendations:
- 1.
In patients co-infected with the hepatitis C virus (HCV), we recommend starting cART regardless of the CD4+ lymphocyte count. This decision should be taken on a case-by-case basis bearing in mind virological and histological factors and patient commitment (B-II).
- 2.
In patients co-infected with the hepatitis B virus (HBV) fulfilling hepatitis treatment criteria, a cART regimen containing TDF should be started regardless of the CD4+ lymphocyte count (B-II).
- 3.
In patients co-infected with HBV not requiring hepatitis treatment, a regimen including TDF should be preferred when starting cART (B-II).
Liver toxicity has been reported with every cART drug, although incidence and pathogenic mechanisms vary. The real incidence of liver toxicity is difficult to assess.
Recommendations:
- 1.
No cART agent is contraindicated in co-infection with HCV or HBV if liver function is preserved (B-II); however, drugs with the least potential liver toxicity should be prioritized (C-III).
- 2.
cART should be discontinued in the event of symptomatic hepatitis. In asymptomatic cases, it should be withdrawn if hepatitis is related to mitochondrial toxicity or hypersensitivity reaction or when transaminase increases by >10-fold the upper limit of normal (B-III).
- 3.
Switches in cART should be considered in patients with asymptomatic hepatitis whose transaminase levels increase by 5- to 10-fold the upper limit of normal, taking into account the clinical, virological, and immunological situation of the patient and exposure to cART (B-III).
Chronic liver disease can alter the absorption and metabolism of cART agents, thus increasing their toxicity or modifying antiviral potency.
Recommendations:
- 1.
Liver function and fibrosis should be assessed in every co-infected patient with hepatotropic viruses, as these can determine the cART chosen and administered. E efficacy and toxicity should also be monitored (C-III).
- 2.
In In chronic hepatitis with no hepatic failure or with only mild hepatocellular damage (Child A), cART agents can be used at the usual doses, although patients should be closely monitored if there is a risk of toxicity (B-II).
- 3.
In chronic liver disease with signs of hepatocellular failure, cART doses should be adjusted. If possible, plasma drug levels should be determined (B-III). The therapeutic range of PI is greater than that of EFV in this setting (B-II). FPV/r (in doses adjusted to Child stage C) and RAL (which needs no dose adjustment) can be considered preferred options in these patients (B-II).
- 4.
cART should be discontinued in the event of severe acute hepatitis and reintroduced once the disease has resolved (B-III).
The recommended treatment for chronic HCV is pegylated interferon and ribavirin. HCV protease inhibitors can be added for patients infected by HCV genotype 1.
Recommendations:
- 1.
cART and HCV treatment should not be initiated simultaneously (B-III).
- 2.
When treating both diseases (HIV-1 and HCV), the patient should be closely monitored to detect adverse effects early (B-III).
- 3.
Ribavirin should not be combined with ddI or ZDV (A-I).
- 4.
If pegylated interferon and efavirenz are administered simultaneously, central nervous system adverse effects should be monitored closely (B-III).
- 5.
Telaprevir can be administered to a patient requiring cART who has significant interactions with a regimen containing TDF, 3TC, FTC, ATV/r, RAL, ETR, RPV (with monitoring of the QT interval and increased surveillance of plasma bilirubin levels), or EFV, although the dose of telaprevir should be increased to 1125mg/8h when co-administered with EFV (B-I).
- 6.
If treatment is started with boceprevir in a patient who simultaneously requires cART, the drugs that can be administered are TDF, ABC, 3TC, FTC, RAL, and ETR (A-I). In patients with an undetectable PVL and no history of virological failure with PI and in patients for whom no other therapeutic option exists, ATV/r can also be administered (C-I).
Recommendations:
- 1.
Patients co-infected with HIV and HBV requiring treatment for any infection should initiate a cART regimen containing TDF+FTC (or 3TC) as the NRTI (A-III).
- 2.
If we decide to treat HBV and defer HIV-1 treatment, drugs that do not induce resistance mutations to HIV-1 are recommended (A-III).
- 3.
Entecavir should be avoided in co-infected patients, except when HIV-1 replication is suppressed by other drugs (B-II).
- 4.
In patients co-infected with HIV-1 and HBV in whom 3TC, FTC, or TDF are to be discontinued for any reason, another agent with activity against HBV should be included (A-II).
HIV-1-infected patients have a greater risk of chronic kidney disease than the general population. The causes are HIV-1 itself, HIV-related diseases, and treatment with cART or other nephrotoxic drugs.
Recommendations:
- 1.
In HIV-1-infected patients with chronic kidney disease, it is mandatory to adjust cART drug doses in order to prevent the appearance of severe complications and potential drug–drug interactions (B-I).
- 2.
The dose of PIs, NNRTIs, and RAL does not need to be adjusted (A-I).
Standard tuberculosis treatment in HIV-1-infected patients is as effective as in the general population; therefore, recommendations are equally applicable to HIV-1-infected patients.
Initiation of cART in HIV-1-infected patients with active tuberculosisRecommendations:
- 1.
In HIV-1-infected patients receiving tuberculosis treatment, irrespective of the CD4+ lymphocyte count, cART should be started during tuberculosis therapy, as this approach can lead to an increase in survival (A-I).
- 2.
In HIV-1-infected patients with tuberculosis and CD4+ lymphocyte counts <50cells/μL, cART should be initiated at 2 weeks of treatment of tuberculosis and once tolerance to this treatment has been confirmed: this approach reduces the risk of death and development of AIDS (A-I).
- 3.
In HIV-1-infected patients with tuberculosis and CD4+ lymphocyte counts >50cells/μL, cART can be deferred until the induction phase of tuberculosis therapy is completed in order to reduce adverse effects and immune reconstitution inflammatory syndrome (IRIS) without compromising survival (A-I).
- 4.
Although the optimal time for initiating ART in patients with tuberculous meningitis remains unknown, the criteria set out above are recommended (A-III).
Recommendations:
- 1.
In HIV-1-infected patients with tuberculosis taking standard treatment, the preferred cART regimen is the combination TDF/FTC (at standard doses) or ABC/3TC (at standard doses)+EFV at 600mg/d (A-I).
- 2.
The recommended alternative regimens, in order of preference, are as follows: (i) TDF/FTC or ABC/3TC+NVP (at standard doses) or (ii) TDF/FTC or ABC/3TC+RAL at 800mg/12h (A-II).
- 3.
Other cART regimens, such as TDF/FTC or ABC/3TC+MVC (at 600mg/12h), should only be used in specific situations where it is not possible to use any of the previous regimens (C-III).
Recommendations:
- 1.
Neither tuberculosis treatment nor cART should be discontinued in patients with IRIS (A-III).
- 2.
Mild or moderate clinical forms of IRIS must be treated with nonsteroidal anti-inflammatory agents (A-III).
- 3.
Administering corticosteroids to treat IRIS with moderate-severe clinical manifestations improves symptoms without causing additional adverse effects (A-II).
The genomic organization of HIV-2 is similar to that of HIV-1, although it displays structural differences that significantly affect pathogenesis and sensitivity to antiretroviral drugs.
Recommendations:
- 1.
Preferred initial cART in HIV-2 infection is a combination of 2 NRTIs plus a boosted PI (C-III).
- 2.
cART regimens based on NNRTIs, MRV, or enfuvirtide are contraindicated in HIV-2 infection (B-III).
More than 50% of HIV-1-infected people are women, and the number of sexually transmitted new infections has increased in women in Europe. In Spain, the number of new infections in women has also increased, especially among the immigrant population.
Recommendations:
- 1.
The initiation and objectives of cART in women are the same as in men (A-III).
- 2.
Use of NVP in cART-naïve women is different (can be used if CD4+ <250cells/μL) owing to the higher incidence of rash and liver toxicity (A-II).
- 3.
The choice of the initial cART combination in women should take into account the wish to become pregnant or risk of becoming pregnant and the use of oral contraceptives. As these drugs interact with several antiretroviral agents, potential interactions should be borne in mind, and their use should be complemented with a barrier method (dual protection) (A-III).
Mother-to-child transmission is the cause of HIV-1 infection in children. The fetus is most vulnerable during the first weeks of pregnancy owing to drug toxicity. Therefore, every potentially pregnant woman should receive information about drug teratogenicity and tailor cART to her specific situation.
Recommendations:
- 1.
All pregnant women should undergo HIV-1 testing (A-III). If risk practices are present, the test should be repeated in the third trimester (C-III).
- 2.
In women who reach term without knowing their HIV-1 status, a rapid HIV-1 test should be performed, since an elective Cesarean section reduces the risk of transmission by 50% (A-II).
- 3.
The objective of cART is to achieve an undetectable PVL (A-II). The decision to initiate cART during the first trimester or to delay it until week 12 of gestation will depend on the CD4+ lymphocyte count, PVL, and individual conditions of each woman, such as presence of nausea or vomiting (A-III). Earlier initiation of cART may be more effective for reducing mother-to-child transmission (B-III).
- 4.
A resistance-mutation test should be performed on every pregnant cART-naïve woman or women with a detectable PVL (A-III).
- 5.
One of the drugs to be included in initial cART in pregnant women must be ZDV, which will be administered during pregnancy and to the newborn (A-I). The cART regimen must include 2 NRTIs that can cross the placenta (ZDV, 3TC, FTC, TDF, or ABC).
- 6.
In general, HIV-1-infected women who are already taking cART and become pregnant must maintain their previous cART regimen only if this regimen is well tolerated and effective. Many experts recommend not withdrawing EFV after 5–6 weeks of gestation (C-III).
- 7.
Combining ddI and d4T is contraindicated owing to the risk of lactic acidosis (B-III).
- 8.
It is essential to plan to achieve an undetectable PVL before labor (week 32–34). If a suitably low viral load is not achieved (<1000copies/mL), a Cesarean section should be programmed at weeks 37–38 (A-II).
The HIV-1 epidemic continues to expand. Over the past few decades, several medical interventions have been launched to reduce transmission. Some goals have been reached with specific programs, such as risk reduction practices among illicit drug users, male circumcision, condom use, control of STDs, reducing mother-to-child transmission, and reducing transmission between members of serodiscordant couples.
Role of cART in the prevention of HIV-1 infectionRecommendations:
- 1.
cART should be used to prevent transmission of HIV-1 from an infected person to his/her heterosexual partner (A-I).
- 2.
cART should be used to prevent transmission of HIV-1 between persons with other risk practices (A-III).
Pre-exposure prophylaxis can be an adequate strategy, although a final evaluation would be necessary once the results of ongoing studies are available. Given the state of the art, no recommendations have been made in Europe. Nevertheless, the WHO and the CDC have prepared a series of recommendations on the use of pre-exposure prophylaxis with oral TDF or TDF/FTC in non-HIV-1-infected persons who have a high risk of acquiring the infection through sexual relations, mainly heterosexual persons with multiple partners, men who have sex with men, and members of serodiscordant heterosexual couples. Given the scarcity of resources, the best public health strategy available today is to offer cART to all infected patients and reserve pre-exposure prophylaxis for very specific cases.
Occupational post-exposure prophylaxisInitiation of cART after occupational exposure to HIV-1 reduces the risk of transmission. Globally, the risk of HIV-1 transmission after a percutaneous exposure to infected blood ranges from 0.24% to 0.65%.
Recommendations:
- 1.
Hospitals should have written guidelines for managing exposure to HIV-1 (whether occupational or not), have a quick serological diagnosis kit available, and ensure quick permanent access to cART for post-exposure prophylaxis (A-III).
- 2.
In order to prescribe post-exposure prophylaxis, the index patient (suspected or HIV-positive), the serologic status of the exposed health worker, and the characteristics of the exposure episode should be considered before indicating prophylaxis (A-III).
- 3.
Post-exposure prophylaxis should be initiated as soon as possible (best within 2h) and within 24–36h. Prophylaxis will be extended for 4 weeks (A-II).
- 4.
Starting post-exposure prophylaxis is not recommended after 72h post-exposure (A-III).
- 5.
Regimens for prophylaxis should contain 3 antiretroviral agents. The recommendation is for a fixed combination of TDF/FTC combined with a PI/r (A-III) or RAL (C-III).
- 6.
Should a resistant virus be suspected in the index patient, prophylaxis should include drugs without cross-resistance (A-III).
- 7.
If there is any doubt as to the convenience of post-exposure prophylaxis, an immediate first dose is recommended. Continuation should be reassessed by an HIV expert within the following 24h (A-III).
- 8.
Follow-up should include reassessment of post-exposure prophylaxis within 24–72h and monitoring of adherence and tolerability. Screening for HIV-1, HBV, HCV (in the index patient or when infection is suspected) should be performed at months 1, 3, and 6 after exposure (B-III).
Recommendations for non-occupational post-exposure prophylaxis are based on the results of observational studies carried out in women who suffered sexual assault and in men who have sex with men, as well as on information on other types of prophylaxis and data from animal experiments.
Recommendations:
- 1.
Non-occupational post-exposure prophylaxis should be given on a case-by-case basis and within an integrated medical approach (A-III).
- 2.
Non-occupational post-exposure prophylaxis should be recommended in those situations in which there is an “appreciable risk” of transmission and under the following circumstances: (i) early initiation (similar to occupational post-exposure prophylaxis), (ii) lack of contraindication to cART agents, (iii) exceptional exposure, and (iv) when there is a guarantee of clinical and laboratory monitoring (B-III).
- 3.
Antiretroviral agents, length of prophylaxis, and follow-up will be the same as those of occupational post-exposure prophylaxis (A-III).
- 4.
In the event of a sexual exposure, other STDs and pregnancy status should be evaluated (A-III).
Koldo Aguirrebengoa has done consulting work for Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories, GlaxoSmithKline and Merck; has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim Bristol-Myers Squibb, Janssen, Merck, Roche, and ViiV Healthcare and has received payment for development of educational presentations for Abbott Laboratories, Gilead Sciences and GlaxoSmithKline.
Antonio Antela has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences and Janssen-Cilag and has received financial compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme and ViiV Healthcare, as well as payments for development of educational material for Boehringer Ingelheim, Gilead Sciences and ViiV Healthcare.
José R. Arribas has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; has enjoyed grants for clinical research from Janssen; has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare and has received payment for development of educational presentations for Janssen.
Víctor Asensi has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmithKline and ViiV Healthcare; has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmithKline and ViiV Healthcare and has received payment for development of educational presentations for Bristol-Myers Squibb.
Juan Berenguer has done consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Merck, and ViiV Healthcare; has enjoyed grants for clinical research from Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Roche, and ViiV Healthcare.
José R. Blanco has done consulting work for Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, and ViiV Healthcare, as well as payments for development of educational presentations for Gilead Sciences and Bristol-Myers Squibb.
Vicente Boix has done consulting work for Abbott Laboratories, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Merck, Pfizer and ViiV Healthcare; has enjoyed clinical research grants from Gilead Sciences, GlaxoSmithKline, Janssen and Merck; has received compensation for lectures from Janssen, Bristol-Myers Squibb, Merck, Pfizer and ViiV Healthcare and has received payment for development of educational presentations for Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen and Merck.
Pere Domingo has performed consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences Janssen and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare.
Vicente Estrada has performed consulting work for Abbott Laboratories, Gilead Sciences and Janssen; has enjoyed grants for clinical research from Abbott Laboratories and Janssen and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Merck and ViiV Healthcare, as well as payments for development of educational presentations for Abbott Laboratories.
Federico García has done consulting work for GlaxoSmithKline Laboratories, Merck and ViiV Healthcare; has enjoyed grants for clinical research from Merck and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Merck, and ViiV Healthcare.
José M. Gatell has done consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, and ViiV Healthcare.
Félix Gutiérrez has performed consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and ViiV Healthcare; has enjoyed grants for clinical research from Merck and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck and ViiV Healthcare, as well as payments for development of educational presentations for Gilead Sciences.
Hernando Knobel has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Josep M. Llibre has done consulting work for Abbott Laboratories, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Pfizer, Roche, and ViiV Healthcare; has received compensation for lectures from Abbott, Boehringer-Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Roche, and ViiV and has received payments for development of educational presentations for Boehringer-Ingelheim, Merck, and ViiV Healthcare.
José López Aldeguer has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; has enjoyed grants for clinical research from ViiV Healthcare and Merck and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Fernando Lozano has performed consulting work for Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare.
Josep Mallolas has done consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen, Roche and ViiV Healthcare; has enjoyed grants for clinical research from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Roche, Merck, and ViiV Healthcare.
Esteban Martínez has done consulting work for Abbott Laboratories, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare and has received financial compensation for lectures from Abbott Laboratories, Boehringer -Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec and ViiV Healthcare, as well as payments for development of educational presentations for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline and ViiV Healthcare.
Celia Miralles has performed consulting work in laboratories Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare and has received financial compensation for writing manuscripts Laboratories Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences and ViiV Healthcare, as well as payments for development of educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José M. Miró has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Cubist, Gilead Sciences, Merck, Novartis, Pfizer, and Theravance; has enjoyed grants for clinical research from Cubist, Novartis, Health Research Fund (FIS) of Instituto de Salud Carlos III (Madrid), Foundation for Research and Prevention of AIDS in Spain (FIPSE, Madrid), Ministry of Health, Social Policy and Equality (MSPSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, EE. UU.) and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Cubist, GlaxoSmithKline, Gilead Sciences, Janssen, Merck, Novartis, Pfizer, Roche, Schering-Plough, Theravance and ViiV Healthcare. Dr. J.M. Miró in 2011 has received a grant (INT 10/219) from Intensification of Research Activity of the National Health System and the Department of Health of the Generalitat of Catalonia (I3 NHS and prics Programs).
Santiago Moreno has done consulting work for Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche; has enjoyed grants for clinical research from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche.
Rosario Palacios has done consulting work for Boehringer Ingelheim and has received compensation for lectures from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare and Roche.
María J. Pérez Elías has performed consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories Laboratories, Gilead Sciences, ViiV Healthcare and Janssen; has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare, as well as payments for development of educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Janssen, Merck, and ViiV Healthcare.
Juan A. Pineda has performed consulting work for Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Janssen, Merck, Pfizer, Schering-Plough and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories, Bristol -Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Schering-Plough and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, Janssen, Merck, Roche, Schering-Plough and ViiV Healthcare
Rosa Polo declares no conflicts of interest.
Antonio Rivero has done consulting work for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare; has enjoyed grants for clinical research from Abbott Laboratories Laboratories, Gilead Sciences, Merck, and ViiV Healthcare and has received compensation for lectures from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare and development payments educational presentations for Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare.
Jesús Santos has undertaken consultancy work for Boehringer Ingelheim and Janssen and has received compensation for lectures from Bristol-Myers Squibb and Gilead Sciences, as well as payments for development of educational presentations for Bristol-Myers Squibb.
Montse Tuset has enjoyed clinical research grants from Bristol-Myers Squibb, Gilead Sciences and Merck and has received compensation for lectures from Janssen, Merck, and ViiV Healthcare.
Francesc Vidal declared no conflicts of interest.
Grupo de Estudio de Sida (GESIDA) and Plan Nacional del Sida (PNS), Pere Domingo1, Rosa Polo2, Fernando Lozano3, José López Aldeguer4, Koldo Aguirrebengoa5, Vicente Estrada6, Félix Gutiérrez7, Hernando Knobel8, Josep M. Llibre9, Celia Miralles10, José M. Miró11, Antonio Rivero12, Jesús Santos13, and Montserrat Tusset11, Antonio Antela14, Víctor Asensi15, José R. Arribas16, José R. Blanco17, Vicente Boix18 Federico García19, José M. Gatell11, Josep Mallolas11, Esteban Martínez11, Santiago Moreno20, Rosario Palacios13, María J. Pérez-Elías20, Juan A. Pineda3, Francesc Vidal21 and Juan Berenguer3
1Hospital de la Santa Creu i Sant Pau, Barcelona; 2Secretaría del Plan Nacional del Sida, Ministerio de Sanidad, Servicios Sociales e Igualdad; 3Hospital Universitario Virgen de Valme, Sevilla; 4Hospital Universitario La Fe, Valencia; 5Hospital de Cruces, Bilbao; 6Hospital Clínico, Madrid; 7Hospital General Universitario, Elche; 8Hospital del Mar, Barcelona; 9Hospital Universitari Germans Trías i Pujol, Badalona; 10Hospital Xeral, Vigo; 11Hospital Clinic, Barcelona; 12Hospital Reina Sofía, Córdoba; 13Hospital Universitario Virgen de la Victoria, Málaga; 14Hospital Clínico Univ., Santiago de Compostela; 15Hospital Universitario Central de Asturias, Oviedo; 16Hospital La Paz, IdiPAZ, Madrid; 17Hospital San Pedro, Logroño; 18Hospital General Universitario, Alicante; 19Hospital Universitario San Cecilio, Granada; 20Hospital Ramón y Cajal, Madrid; 21 Hospital Universitari Joan XXIII, Tarragona, 22Hospital General Universitario Gregorio Marañón, Madrid
The Panel of Experts is presented in Appendix 1.