Introduction
In the year 2003, the aids pandemic was responsible for more than three million deaths, and five million people are calculated to have contracted the virus during this period. This takes the number of infected people throughout the world to 42 million, with 20 million deaths registered since the origin of the pandemic was identified1. The geographical and economic differences in this disease are obvious, with more than 95% of cases and deaths by aids occurring in the third world (70% in Africa), especially among young adults, with a gradual increase in women. It is dramatic to see how, in sub-Saharian Africa, the epidemic continues to spread and, in many countries, the high percentage of people infected with aids has devastating effects on families and on the productive economy. The explosion of this epidemic in developing countries has raised the need to take urgent preventive measures and provide expanded access to antiretroviral therapy. However, in some parts of the world, these measures, although essential, are probably insufficient to curb the epidemic, so that the development of a vaccine is the only available possibility of controlling it.
Therefore, the development of an efficacious HIV vaccine is not only an area of aids research which has yet to be resolved but also an urgent need for developing countries. This awareness has led to a considerable increase in financing the search for an aids vaccine. To obtain an effective HIV vaccine represents a major challenge and priority scientific objective for public and private institutions, governments and NGOs2-4 (table 1). This chapter analyses prototype vaccines being developed and the results obtained, the scientific difficulties in developing a vaccine, especially with regard to the mechanisms of viral escape from the immune response. Furthermore, as this research is set in the social, economic and healthcare context of the aids pandemic, current controversies surrounding clinical trials with the different types of vaccine5 are addressed in this review.
Difficulties in obtaining an HIV vaccine
In order to develop a vaccine, it is necessary to know the genes of the pathogen involved in the induction of a specific immune response, and to use experimental models to test the efficacy of the virus. Despite the fact that, in the past, there have been important advances in the control of infectious diseases by vaccination, many of the mechanisms used by the pathogen to take over cell machinery are unknown. The same applies to mechanisms of escape from the immune system and ways of boosting an immune response capable of eliminating the infected cell. In scientific terms, obtaining an efficacious vaccine capable of preventing HIV infection faces a series of challenges:
Characterization of the major immunodeficiency determinants
The immune response begins with the recognition by CD4 lymphocytes via their receptor (TcR) of foreign antigens in the class-II HLA groove presented by cells specialized in antigen processing. Antigen recognition activates the different effector mechanisms of the immune system: cytokine and chemokine synthesis, production of antibodies by B lymphocytes, activation of CD4 lymphocytes and generation of cytotoxic CD8 lymphocytes. These last events represent the main mechanism involved in killing of virus-infected cells and to initiate this process cytotoxic lymphocytes must recognize the antigenic determinants of the virus lodged in the class-I HLA groove of infected cells6. As a consequence of the polymorphism of the HLA system both in antigen presenting cells and target cells different peptides (that are anchored in class II and class I HLA molecules respectively) are selected according to individual HLA haplotypes. In many viruses there are "major immunodominant determinants" (epitopes) which induce a potent response by the immune system. The efficacy of this response depends on two characteristics: first, they correspond to epitopes or domains of the viral proteins which are conserved among the different isolates, even in the context of highly variable viruses; second, these major determinants are capable of binding to the grooves of most HLA haplotypes. The existence and identification of these major immunogenic determinants is crucial when developing a vaccine, since they make up viral targets par excellence by being "universal" in two senses: as epitopes conserved in the viral protein among different isolates and as epitopes susceptible to antigenic presentation by most subjects regardless of their HLA haplotype. However, in the case of HIV, no similar dominant epitopes have been found to date, which represents a very important limitation when designing a vaccine. The presence of these major immunogenicity determinants in viruses with tremendous genetic variability, such as HIV-1, makes it practically impossible to define these epitopes empirically and experimentally. Nevertheless, bioinformatics can provide us with predictive methods which make identification easier7.
The definition of surrogate protection parameters
The objective of a vaccine is to induce an efficacious, memory-type immune response which allows the immune system to react against the infectious agent by preventing its spread. For this objective to be reached, it is essential to know which immunological effectors are efficacious in the control of the infection in order to define a series of surrogate immunological parameters which enable us to evaluate whether a vaccine preparation is efficient or not. The intense immune response of HIV-infected patients is reported to encompass practically all the effector mechanisms of the immune system. This response is relatively wide, since it is developed against numerous epitopes, and practically all the viral proteins, both structural and regulatory, are recognized as foreign antigens. However, controversy still surrounds the role of "protection" played by each of these components of the antiviral response. Below, we describe the type of immune response generated against HIV infection.
Humoral response
HIV infection induces an intense antibody response against practically all the regulatory and structural proteins of HIV8. Some of these antibodies have neutralizing capacity in vitro9 and in in vivo adoptive immunotherapy experiments9,10. However, the production of antibodies with neutralizing capacity is scarce and viral escape from these antibodies is rapid11. Furthermore, in the immunization models developed to date, high levels of neutralizing antibodies are not consistently obtained and their presence is not systematically associated with protection. These data raise severe concerns about the role of the humoral response in the control of HIV infection12,13. Nevertheless, almost all preventive vaccines induce neutralizing antibodies and their role as a surrogate protection marker is clearly demonstrated in other diseases. Therefore, "a priori", a preventive HIV vaccine should induce broad-spectrum neutralizing antibodies14 and this is one of the huge challenges currently facing the development of an aids vaccine. Recent studies which define the location of neutralization epitopes, antibody structure and mechanism of action15 represent an important advance to define the characteristics that a given vaccine must have to induce neutralizing antibodies.
Cellular response
Most studies agree that the combined response of CD4 and CD8 is probably the most important protective mechanism against HIV16. Study of the cellular response has shown that in seropositive patients there is a clonal expansion of CD4 and CD8 lymphocytes which are active against HIV. This expansion is particularly intense in patients with primary infection and correlates with the control of viral replication17,18. There are also reports showing an intense CD4 and CD8 response in some patients during immune reconstitution after antiretroviral therapy, especially in those with a good immunological status before starting therapy19,20. The same phenomenon has been described in patients with structured treatment interruptions who spontaneously control viral replication21. Although it is difficult to draw conclusions as to a cause-effect relationship between the appearance of a specific type of immune response and the control of viral replication, all the data seem to suggest that both helper and cytotoxic immune responses are essential to contain viral replication in the early stages of the disease, when the immune system is relatively undamaged. The most conclusive experimental data on the role of the cellular response in the control of viral replication come from studies in which selective depletion of CD8 lymphocytes in macaques infected with SIV leads to a huge increase in viremia and accelerated evolution to aids22.
Immune response in mucosa
HIV transmission occurs mainly via the mucosa. The large quantity of CD4+ lymphocytes in genito-rectal lymphoid tissue represent a major reservoir for the replication of HIV or SIV, even when the infection is contracted intravenously. The gut-associated lymphoid tissue system (GALT) is set up by activated B and T lymphocytes and dendritic cells which migrate through the lymphatic system and bloodstream to distant lymph nodes where they become resident. Thus, the induction of a strong immune response in mucosa is probably a necessary requirement for a vaccine to be efficient against HIV.
Viral escape mechanisms
Each family of viruses develops different escape mechanisms to avoid elimination by the immune system. A vaccine must face the same escape mechanisms and, to be successful, must induce a series of immune responses capable of overcoming them.
Genetic variability
The rate of variability of HIV is due to the high error rate of reverse transcriptase (one substitution per 103-104 nucleotides and round of copy). In consequence there is wide intersubtype and intrasubtype variability but the immunological relevance for vaccine design of this genetic disparity is a matter of debate. Several investigations have shown that genetic differences among HIV subtypes do not correlate with immunotypes. In fact, several genetic subtypes could share common protective epitopes and more than one immunotype can be found in the same genetic subtype. In general, neutralizing antibodies seem to be more strain-specific, whereas cellular immune responses have a broader spectrum. This lack of fidelity generates a high diversity in viral proteins which allows escape from the control of specific immune response. Therefore, HIV shares with other RNA viruses a common escape mechanism related to their high variability that allow the virus finding holes in the immune repertoire. Together with the variability generated by the high error rate of reverse transcriptase other mechanisms such as genetic recombination, which produces new subtypes and "mosaic" viruses among different subtypes, are also involved in the generation of HIV variants. Several molecular epidemiology studies have stressed the rapid dissemination of HIV variants and have described the distribution of several subtypes or recombinant viruses in different parts of the world. This could be an obstacle to the development of a universal vaccine23.
Mutations in the viral epitopes recognized by CTLs
One central aspect of HIV infection which is not totally understood is the reason why viral replication is not controlled despite the potent immune responses elicited in primary infection. Although several explanations have been put forward, the most widely reported is viral escape through mutations in the viral epitopes recognized by the different effector mechanisms of the immune system24. Escape from CTL response is due to ad hoc mutations of the viral epitopes which interact with the groove of the HLA molecules. It has been shown that mutations in critical residues generate viral escape in both animal models and patients with primary infection and this event results in loss in the CTL response and parallel increase in viremia25,26. However, in the chronic phase of infection, there is no clear correlation between the presence of specific CTL and the elimination or persistence of a given viral variant27. In addition to merely quantitative data, functional analysis have shown qualitative differences between CTL from progressors and non-progressors, such as the expression of perforins28, production of cytokines and chemokines, and reduced activity of the T-cell receptor against viral epitopes presented in the HLA complex23. These data suggest that qualitative aspects of CTL may be also important in the control of viral replication. A general strategy for maximizing the efficacy of a vaccine would be to obtain a cytotoxic response against a large number of epitopes from several proteins. However, recent studies suggest that a more targeted approach can be more effective. Thus, CTL against non-structural proteins (Tat, Nef) are more efficient in controlling infection but more prone to viral escape and do not last as long as CTL against the structural proteins Gag and Pol29. For a sterilizing vaccine, the objective would be to induce an intense CTL response against early proteins, whereas immunization against structural proteins would generate a response which would control viral replication thus attenuating infection. Another problem which may be a serious genetic resistance barrier is the adaptation of the virus to the HLA haplotype of the infected patient. In this situation peptides from viral mutants generated would reduce their affinity to HLA thus decreasing recognition by TcR and generating a greater resistance to CTL response30.
Biochemical characteristics of the viral envelope and escape from antibody action
The structure of the viral envelope in its native form hides the domains of interaction with viral coreceptors due to the trimeric structure and folding of the protein (oligomeric exclusion and entropic masking)31. Exposure of these conserved epitopes which are identified by neutralizing antibodies takes place at the moment of interaction between the viral and cellular membranes, a setting in which antibodies efficacy is lower given their low accessibility to neutralization epitopes. A second, more classic escape mechanism is epitopic mutation in the hypervariable regions found in the external domain of the viral envelope. Nevertheless, recent studies show that escape from these antibodies does not always require mutation in amino acid residues but takes place by glycosylation of the residues and formation of carbohydrate structures on viral gp120 known as "glycan shields" that represent authentic barriers to the action of neutralizing antibodies32. One of the most spectacular studies published during the last year shows how, during evolution in a specific patient, the viral envelopes gradually become resistant to all types of neutralization by neutralizing antibodies via accumulation of the previously mentioned escape mechanisms32.
Early establishment of infection
Both in animal models and in patients with primary infection through sexual contact the establishment of HIV infection is a very rapid process33. In a few hours, the lymphoid cells of the rectal and vaginal submucosa become infected and, in seven days, the infection spreads to systemic lymph nodes where it reaches viral and proviral loads similar to levels found in chronic infection34. The speed at which these reservoirs appear, before a specific immune response is triggered, represents a major obstacle to the control of viral replication since once established HIV infection will "persists" in lymphocytes despite immune response35.
Latency and reactivation
HIV can infect target cells in a latent form. In this situation no viral proteins are expressed on the membrane of infected cells thus allowing escape from immune surveillance. Furthermore, reactivation-reinfection processes take place in lymphoid organs, which provide an ideal cellular microenvironment for the process of infection: dendritic cells express in their membrane a lectin (DC-SIGN) which interacts with the virions and lymphocytes and enhances HIV infection36. Antigenic recognition by lymphocytes and the presence of cytokines in this microenvironment in turn increase infection of target cells and promote viral replication. As confirmation of these data, HIV-specific lymphocyte clones have been shown to be infected at higher proportion, which implies a preferential immunosuppression of the HIV-specific responses37. It must be stressed that the continuous generation of new, latently infected cells from the active viral replication compartment generates a "continuous archive" of changes produced in the virus throughout the disease, by including treatment-resistant mutated genomes and variants of the immune escape. The latent compartment is therefore not static and in some way HIV stores its "history" in latently infected cells32 thus contributing to viral diversity as a mechanism of escape from antiretroviral therapy and vaccines.
Prototype HIV vaccines. Experimental results
Attenuated viruses
Attenuated virus vaccines are without doubt the most efficacious because the germ carries out a limited series of replication cycles and simulates a low-level infection which induces the whole spectrum of antiviral response in a physiological setting. In the case of lentiviruses, one of the most spectacular findings was that which showed that a defective Nef-deleted SIV virus induced a protective response against the challenge with highly pathogenic aggressive viable viruses38. These experimental data had a natural correlate in the "Sydney Cohort", made up of 14 patients infected through blood transfusion from a seropositive donor and who, after 12 years of infection, had an excellent clinical and immunological status. The cloning and characterization of the virus in these patients and the donor showed that it presented deletions in the Nef gene and in critical regulatory sequences of the LTR region39. These findings led to the proposal of attenuated HIV vaccines similar to defective SIV mutants. However, it must be stressed that attenuated vaccines are usually used against viruses which do not persist or, alternatively, the attenuated virus used as vaccine is not capable of persisting in the host. This is not the case for Nef-defective viruses which not only infect, but also replicate and persist in the host, with the risk of drifting towards more aggressive variants in the vaccinated subject. The first alarming data came from vaccination of newborn macaques in which, in contrast with was found in adults, the innocuous virus rapidly induced aggressive infection and death by immunodeficiency40. Furthermore, prolonged follow-up of patients from the Sydney cohort enabled us to observe an immunological deterioration and blips of viremia in some subjects41. Similarly, some adult macaques vaccinated with the defective SIV virus developed aids from the virus they had been vaccinated with, which suffered reversions of the mutant phenotype42. Therefore, the use of vaccines from defective viruses has been ruled out, and this approach has been explicitly excluded in guidelines and recommendations of regulatory agencies.
Inactivated viruses
Inactivated viruses have scarcely been used as preventive vaccines. On the contrary, this is the most widely used model in therapeutic vaccines of which Remune® is the prototype. These viral preparations are composed of complete virions or particles whose envelope has been eliminated, which are then inactivated using different chemical methods and administered in conjunction with Freund's incomplete adjuvant43.
Viral proteins
The first HIV vaccines were based on the hepatitis B immunization model. They were composed of recombinant proteins gp 120 and gp 160 produced by genetic engineering or using vaccinia virus as expression vectors. In pre-clinical studies and in phase I and phase II clinical trials, the preparation was safe and induced antibody synthesis against the viral envelope44, but these antibodies were incapable of neutralizing wild-type variants "in vitro"45. In spite of the evidence against the efficacy of this prototype, phase III trials were continued (see below).
Other trials have used the regulatory protein tat in toxoid form, which has provided good protection results in macaque studies, although its role remains controversial46.
Viral peptides
Peptide vaccines have little immunogenic capacity, since, in many cases, the antibodies do not recognize the primary structure of the aminoacid sequence, but rather secondary and tertiary structures in the target proteins which are not simulated by the peptides. Therefore, peptides are generally used in combination with other vaccine preparations such as viral vectors or DNA in order to induce complementary immunization47. The advantages of these combinations are low toxicity, the possibility of preparing peptide "cocktails" which cover a wide range of viral isolates in proteins presenting high variability, and the use of "mixed peptides" which, by including T and B immunodominant epitopes induce cellular and humoral responses.
Bacterial and viral vectors (live-attenuated)
These systems use viruses or bacteria into whose genome HIV genes are inserted in such a way that their proteins are expressed during the course of replication of the vectors in the host cell. The most developed models are those which use poxvirus (Vaccinia, Canarypox, Modified Ankara Virus/MVA)48 and adenovirus49. Other experimental approaches use bacteria (BCG, Salmonella)50 and RNA viruses (coronavirus, VSV, SFV, reovirus, poliovirus, influenza) including also lentivirus51. Some of these systems are limited by the risk that exogenous genetic information from the vector can be integrated in the host genome. The advantage of these viral and bacterial systems lies in the possibility of inserting several viral genes in their genomes and their capacity to express high levels of viral proteins. Strong antigen expression can in turn induce a potent and prolonged immune stimulation, particularly of cellular immune responses, against these proteins. The vaccine prototypes currently being developed include the genes gag, pol, env and nef in different combinations48,49, different priming-boosting strategies and vaccine doses. These types of preparation have failed as preventive vaccines in animal models, since they have not achieved protective immunity, probably due to the fact that the humoral response induced against HIV proteins is erratic and of reduced potency. They do, however, induce a potent cellular response which makes viral load stabilize at low levels48,49. In the most optimistic scenario it has been suggested that this response could be enough to "attenuate" the infection and transforming vaccinated patients who become infected into "long-term survivors". New vectors, such as BCG, Salmonella and Poliovirus are expected to induce greater humoral and cellular immunity in the mucosa by means of oral administration, thus improving the efficacy of these vaccines.
DNA vaccines
The observation that "naked DNA" is capable of inducing an immune response against several viruses and in different animal models broke new ground in the development of vaccines52. In infection models with SIV and SHIV, it has been observed that, as with microbial vectors, immunization with DNA is capable of inducing an immune response which, although it does not protect against infection, can often attenuate viral replication and clinical symptoms53. The main limitation of DNA vectors is that the intensity of the immune response induced is low, therefore they are generally used in combination with viral vectors. A disadvantage of this type of vaccine is the potential long-term secondary effects owing to chromosomal integration processes.
New adjuvants
Adjuvants are preparations which boost the immune response of vaccine antigens by different mechanisms. Traditional adjuvants, such as Freund's, are bacterial lysates which, by inducing a non-specific inflammatory response, "recruit" immune cells at the injection site. Others, such as ISCOM or liposomes improve the presentation of antigens. Recent studies have demonstrated the efficacy of interleukins, especially these activating Th1 responses (interleukins 2 and 12) or chemokines, in boosting the response induced by attenuated-vectors or naked-DNA vaccines54.
Vaccination by a combination of vectors
Successive inoculation with an interval of some weeks using two different vectors expressing the same HIV antigen (prime/booster) has been shown to induce a stronger cellular immune response against HIV antigens than when the same vector is administered in two doses. These procedures, which boost specific CD8 T cell induction, were developed in the murine malaria system by showing that this increase correlates with protection against the pathogen55. One of the best systems reported is based on recombinant poxviruses, especially the attenuated vaccinia virus Ankara (MVA). This vector must be administered at the second immunization (booster), whereas in the first inoculation (priming), DNA, capsids and other viral protein-expressing vectors can be used indiscriminately. The most promising prime/booster combinations include DNA/pox, SFV/pox, and Adeno/pox.
State of the art in the development of an HIV vaccine
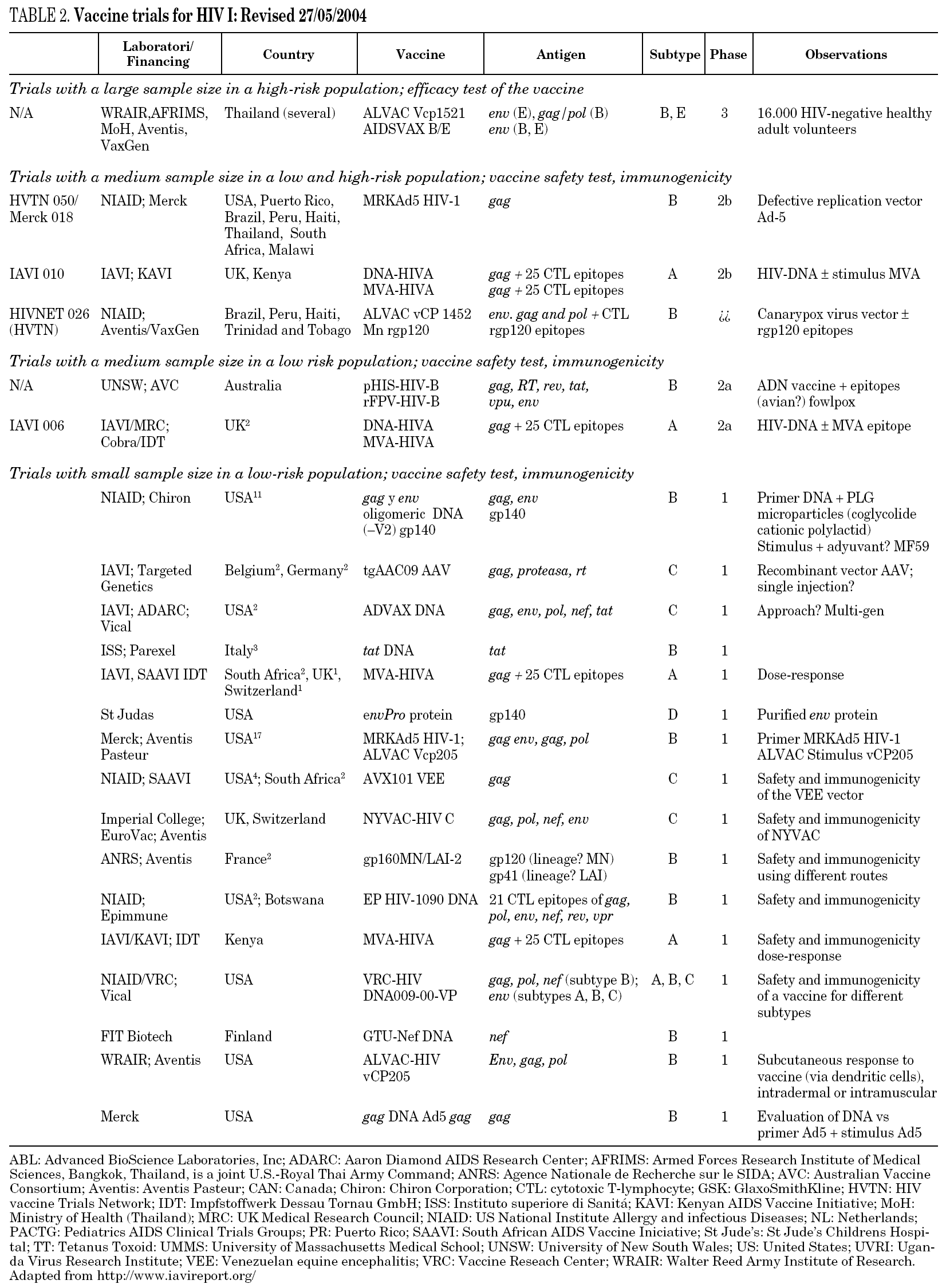
There is currently no available preventive HIV vaccine. In fact, previously described strategies have failed because no one single animal has been protected from infection in any experimental model. Table 2 gives details of trials in progress and those which are expected to enter the clinical phase in the next few years.
The only phase III trials carried out are based on the use of gp 120 of the recombinant and bivalent B/B in the U.S. or B/E in Thailand56. Unfortunately, the results of these studies have shown no benefit in protection from infection with an efficacy value of 3.8%57. At present, new phase III trials are about to begin in Thailand using a combination of recombinant gp 120 and a poxvirus vector (ALVAC). This trial has raised controversy over whether it should be carried out, since both the experimental results and the immune response induced by these vaccine preparations have been very limited58,59.
The most advanced pre-clinical protocols of the new vaccine prototypes include that being developed by Aventis Pasteur in Uganda, which uses a Canarypox vector for the expression of viral structural proteins60. Also in Uganda, January 2003 saw the start of a phase I trial combining DNA + MVA (strain A)61. A similar phase I clinical trial sponsored by IAVI and KAVI is being carried out in Kenya. Unfortunately, it seems unlikely these assays go on due to the low proportion of individuals in which a relevant immune response has been elicited with the vaccine preparations tested. In Europe, through the EUROVACS initiative a clinical phase I trial using poxviral-based vaccine NYVAV has been completed by Juin 2004. Another phase I trial based on the combination DNA/NYVAC expressing gp120, gag, pol and nef of subtype C was launched also in Juin 2004. A comparative assay between NYVAC-C and MVA-C is planned in 2005. This latter immunogen will be generated in Spain at the National Centre of Biotechnology. Complete and updated information on the situation of existing vaccines and clinical trials is available at: www.hvtn.org/trials.
Current scientific and ethical aspects
Obtaining an aids vaccine has become a global enterprise2-5. This is very positive because it has increased social awareness of the disease and financial support. It is also important to note that priority given to vaccine research and the development of new approaches appears at a time when we know much more about the pathogenesis of aids than ten years ago.
Nevertheless, it must be remembered that demonstrating the usefulness of a vaccine is a long and expensive process. Therefore, a critical aspect of the problem is to define strategies and criteria for the different phases in vaccine development: type of vaccine, objectives of the vaccine, experimental design, animal models, pre-clinical and laboratory analysis, phase I and II trials and requirements and infrastructure for phase III trials. Given the healthcare, social and political priorities, this subject is sometimes affected by serious concerns outside the scientific world. These include the social pressure from international organizations and countries devastated by the epidemic and financial pressure from the pharmaceutical companies. Although some of these motives are understandable, given the severity of the situation, these attitudes can also distort the scientific process. Below, we summarize the key questions in the search for an "aids vaccine".
Is an aids vaccine possible and what can we expect from it?
Some scientists doubt that an efficacious aids vaccine can be found62. The reason is the difficulty in obtaining what has been defined as "sterilizing immunity" against retroviruses. If we analyze the mechanisms of action of vaccines, in most cases they do not achieve "sterilizing immunity", since they do not prevent infection but rather the persistence of the microorganism and development of disease: the germ infects, but the immune response prevents it from spreading and destroying new infected cells, thus helping to eradicate the infection. In the case of SIV and HIV infection, we know that, after the first inoculum, infection takes place in a short period of time and an important reservoir of cells from the lymphoid system become infected. In some of these initially infected cells, the virus replicates actively, but in others it remains in a state of latency as an integrated provirus in the genome of the host cell. Therefore, despite the immune response induced by vaccines, the virus can "persist" in the reservoirs from where it can replicate continuously.
What should the final objectives of an aids vaccine be?
A particularly controversial area is the "final objective" of the vaccine: some people argue that if it is not possible to induce "sterilizing immunity" to prevent infection, will it be enough to have an immune response capable of controlling the level of viral replication to sufficiently low levels that allow the immune system to escape from huge destruction. The objective would not be so much to prevent infection but to attenuate it, in such a way that the infected patients become "long-term survivors" capable of living with the virus. Another area of debate is the level of protection which must be "reached" by an aids vaccine. In contrast with the high efficacy of protection in most vaccines (above 90% of vaccinated patients) different authors propose that a partial protection rate of 25-40% should be considered as "sufficient".
This reduction in the final objectives to be attained by an HIV vaccine is arguable. On one hand, it is doubtful that "attenuation of the infection" will be a definitive phenomenon in the medium-long term. Both in animal models and in isolated cases in which an infection has been caused by a defective virus, this virus increases its virulence in the long-term. On the other hand, although it is true that the establishment of a low viral load after primary infection is a good prognostic factor in the medium term, this does not guarantee that patients who present low levels of viral load after vaccination will behave as long-term survivors.
The fact that some scientists set the "sufficient" efficacy of a vaccine at 30-40% protection level can also be criticized. This could, perhaps, be considered a realistic stance and, even in specific prevalence rates in specific risk groups, a vaccine of this type could be efficacious, but we do not know its real impact on the evolution of the epidemic in the medium term. We must not forget that one of the mechanisms of vaccine efficacy is due to the "population or epidemiological impact" in the decrease in the prevalence of the infection and the consequent reduced possibility of transmitting the germ among the general population. This epidemiological impact of the vaccine would not exist with the proposed protection rates.
Universal vaccine or à la carte vaccine: the problem of variability
Some authors suggest that variability among subtypes represents an important obstacle for the development of a universal vaccine and that "ad hoc" vaccines should be manufactured based on the subtypes circulating in each region23. However, the new vaccine prototypes use other viral genes (env, nef, gag, pol, tat) as targets which have a much lower variability than the envelope. In fact, different studies show that the immune response induced by vaccination against a specific HIV subtype is capable of acting against other subtypes63,64.
How, when and where is the efficacy of the different vaccines to be evaluated?
The efficacy of an aids vaccine must be evaluated in populations with a high rate of infection in order to obtain significant differences between the control group and the vaccinated group in the shortest time possible. This means that almost all the trials are carried out in Africa and southeast Asia, where annual seroconversion rate in the most affected areas is approximately 1% of the population1.
Carrying out trials in developing countries raises a series of ethical issues:
1. It is essential that the studies comply with all ethical requirements and that patients' rights are guaranteed65.
2. The vaccines tried must have satisfied the scientific and medical requisites of potency and safety which are necessary in any medicine tried on humans.
3. Vaccine trials need a wide-ranging follow-up infrastructure which can guarantee patient follow-up. Therefore, it is essential to develop healthcare structures and reference centers with the following objectives: recruitment and follow-up of volunteers, extraction, freeze and storage of blood according to standard procedures, and assessment of immunological parameters such as lymphoid populations, cytokine production and neutralizing antibodies. If this requisite is not met, the analysis of results could be skewed and/or incomplete, thus making it impossible to draw conclusions66.
4. One demand by governments is that if a vaccine is efficacious, free access to the vaccine must be guaranteed to the country where the evaluation was carried out.
5. According to the ethical guidelines of UNAIDS, lifelong antiretroviral therapy must be administered to any person infected during the clinical trial67.
An important problem, now the center of social and scientific controversy, is to define the requirements a vaccine preparation must fulfill to start a phase III clinical trial. The journal "Science" has been the forum for a series of letters from prestigious scientists criticizing investment strategy in the development of an aids vaccine and the initiation of phase III clinical trials58,59. The strict scientific position defends that there are no consistent data on the efficacy of current vaccine prototypes to carry out phase III clinical trials. Consequently, such investment should be concentrated in basic research in order to get a better understanding on the mechanisms of protective immune responses and to develop new relevant animal models. Faced with this stance, a more humanist position bases the start of phase III trials on the catastrophic situation in developing countries and on the counterargument that, if there are no adequate animal models, it will be anyway necessary to carry out all the phases of the studies, including phase III, in humans to obtain a definitive response. Despite the reticence and pessimism of a large part of the scientific community, the general impression is that phase III trials will be carried out. It is important to remember the cost and effort involved in these trials, which require the follow-up of 10,000 patients for at least five years to obtain conclusive results.
Therefore, with regard to aids vaccines, we are living in difficult times in which a huge economic investment will be necessary so that the scientific community can generate, develop and evaluate all the vaccine prototypes imaginable in animal models in order to find the Holy Grail of vaccines. As a reference, in case the European Union decided to start a program of phase I and II clinical trials with a reduced number of vaccine prototypes already generated in European laboratories an investment of 1.2 billions euros in the following 10 years should be required. With this objective in mind, the development of vaccine research centers has been proposed68. These centers would combine: (i) a critical mass of investigators, (ii) their sole dedication to the development of prototype HIV vaccines, (iii) a long-term commitment by academic, governmental and private institutions, (iv) sufficient resources and (v) continuous exchange of information and collaboration with the private sector. As a consequence of this policy the main leader organizations (NIH, IAVI, ANRS, EU, Gates Foundation...) should finance vaccine development centers and would coordinate their work. The prototypes considered interesting would be prepared under the conditions of Good Manufacturing Practice for use in humans and would enter a previously defined process of pre-clinical studies and phase I, II and III clinical trials. All the prototypes would meet the minimum requirements for clinical application, which would mean not only defining these criteria but also involving the regulatory authorities (FDA, EMEA) in their development. The evaluation of prototypes also requires the definition of those immunological markers which must be used to evaluate their potential efficacy. This in turn would mean developing standardized and reproducible trials to evaluate the humoral and cellular responses to HIV and the approval of laboratories which would carry out these immunological determinations. Lastly, the necessary healthcare structures should be set up to carry out the trials in clinical phases in developing countries, and the ethical criteria to be fulfilled in these trials should be defined. Given the large number of current prototypes (table 2), the application of homogeneous evaluation criteria is the only way to reach consistent conclusions which can be extrapolated to all situations.
Nevertheless, it is important to be aware that this search is full of unanswered questions and that it can fail despite all the efforts made. As it may not be possible to develop a vaccine it may be time to convey this terrible possibility to society.
The history of vaccines is defined by the words "empiricism" and "success". No intervention has saved as many lives throughout the history of medicine as vaccines. These successes were often the fruit of the most basic empiricism. However, at present, empiricism cannot serve as the basis of success in the scientific development of an aids vaccine.
To conclude, in recent years, the development of an aids vaccine has changed radically due to different factors: the devastating growth of the epidemic, social awareness, financial investment and, in particular, a better understanding of the pathogenesis. All these new elements enable us to face this challenge rationally and with adequate resources. Only scientific effort combined with unprecedented solidarity will allow us to decide whether it is possible to find a vaccine against HIV and whether its application will be sufficient to curb the current aids pandemic.
Acknowledgments
Our laboratories are funded by redes temáticas cooperativas de investigación en sida (RIS G03/173) y de genética clínica y molecular (RIG 03/07), "Fundación para la Investigación y la Prevención del Sida en España" (FIPSE 36365/02;36344/02;12456/03;36259/01), "Programa Nacional de Salud" (SAF 2003-09209 y 0037-04-01) and Comunidad de Madrid. We are grateful to Florencia Etcheverry for her help in revising the bibliography of this manuscript.