The etiology of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) can influence the efficacy of Public Health preventive strategies. This study aimed to determine the high-risk papillomavirus (HR-HPV) prevalence in CIN2+ cases in unvaccinated women in Galicia (Spain), the expected impact of bivalent vaccination, and the distribution of HPV 16 in squamous lesions.
Material and methodsNinety-four histologically confirmed cases of CIN2+ (2009–2010) were retrospectively studied: 23 CIN2, 58 CIN3− squamous carcinoma in situ (CIN3-CIS), 5 adenocarcinoma in situ (AIS), and 8 invasive squamous cervical cancer (SCC). Linear Array HPV Genotyping Test (Roche Diagnostics, Mannheim, Germany) was performed on the cervical specimens. Bivalent vaccination impact was calculated, based on regional vaccination coverage data, local HR-HPV prevalence, and reported efficacy (direct and cross-protection) of the vaccine.
ResultsHR-HPV prevalence was 96.8%. The most frequent genotypes were HPV 16 (48.8–58.2%) and HPV 31 (9.3%–12.1%), considering single infections or single-multiple infections, respectively (hierarchical attribution). In squamous lesions, HPV 16 prevalence in women younger than 45 years of age increased in severe lesions (CIN3-CIS/SCC, OR 4.2), and was higher than in older women (OR 5.5). The vaccine could reduce the cumulative incidence of CIN2+ by 50.6% (direct protection), or by 62.7% (direct and cross-protection).
ConclusionHPV vaccination could have a great impact in women younger than 45 years of age due to the high prevalence of HPV 16 in their lesions.
La etiología de la neoplasia intraepitelial cervical de grado 2 o peor (CIN2+) influirá en la eficacia de las estrategias preventivas de Salud Pública. Se pretende conocer la prevalencia de papilomavirus de alto riesgo (VPH-AR) en CIN2+ en mujeres no vacunadas en Galicia (España), el impacto esperado de la vacunación bivalente y la distribución del VPH 16 en lesiones escamosas.
Material y métodosSe estudiaron retrospectivamente 94 casos confirmados histológicamente de CIN2+ (2009-2010): 23 CIN2, 58 CIN3− carcinoma escamoso in situ (CIN3-CIS), 5 adenocarcinoma in situ (AIS) y 8 carcinoma escamoso invasivo (CES). Se utilizó Linear Array VPH Genotyping Test (Roche Diagnostics, Mannheim, Alemania) en muestras cervicales. El impacto de la vacunación se calculó según la cobertura vacunal autonómica, la prevalencia local de VPH-AR y datos publicados de eficacia (protección directa y cruzada).
ResultadosLa prevalencia de VPH-AR fue del 96,8%. Los genotipos más frecuentes fueron HPV 16 (48,8-58,2%) y HPV 31 (9,3-12,1%) considerando infecciones simples o infecciones simples-múltiples, respectivamente (atribución jerárquica). En lesiones escamosas, la prevalencia de VPH 16 en mujeres de hasta 45 años aumentó con la severidad de las lesiones (CIN3-CIS/CES; OR 4,2) y fue mayor que en las mujeres mayores (OR 5,5). La vacuna podría reducir la incidencia acumulada de CIN2+ un 50,6% (protección directa) o un 62,7% (protección directa y cruzada).
ConclusiónLa vacunación frente al VPH podría tener un gran impacto en mujeres menores de 45 años debido a la alta prevalencia de VPH 16 en las lesiones que presentan.
The knowledge of a 99.7% worldwide HPV prevalence in cervical carcinomas1 and the fact that persistence of a high risk human papillomavirus (HR-HPV) infection is essential for the development, maintenance and progression of high-grade cervical intraepithelial neoplasia (CIN)2,3 have changed cervical cancer prevention. Several longitudinal, randomized-controlled trials4–6 have demonstrated improved prevention of high-grade CIN and cervical cancer with the introduction of HPV testing in cervical screening. In the particular case of Galicia (Spain), an organized cervical cancer screening protocol was introduced by Galician Public Health Service in 2008 according to European and National recommendations (www.sergas.es). In brief, it was a Pap-based screening program. In case of detection of low-grade or high-grade cytological alterations women were referred to colposcopy. The HPV DNA testing was used as a triage test for colposcopy referral in case of atypical squamous cells of undetermined significance (ASC-US) in women older than 25 years of age.
Two HPV vaccines, namely Merck’ Gardasil® and GSK’ Cervarix®, approved by Federal Drug Administration (FDA) in 2006–2007 protect against acquisition of infections with oncogenic HPV types 16 and 18 and their lesions,7,8 which are responsible for about 70% of all cervical cancers.9 In September of 2008, free vaccination of girls aged 14 years against HPV 16 and 18 with the bivalent vaccine was introduced in Galicia (Spain). The impact of the vaccination on cervical cancer incidence is expected to take several decades to manifest itself.
The implementation of any new intervention requires surveillance in the general population to measure its efficacy. To provide a baseline HPV-type specific prevalence in precancerous and cancerous cervical lesions is essential for future comparisons in order to evaluate the impact of screening protocols and vaccination in cervical cancer prevention.
In this way, a retrospective study was performed in order to know the HPV prevalence in histologically confirmed cases of moderate-grade CIN or worse (CIN2+) which were detected in Galicia, Spain, prior to widespread HPV vaccination, the expected impact of bivalent vaccination in these cervical lesions and the distribution of HPV 16 in squamous lesions.
MethodsPatients, ethical statement and epidemiological dataWe conducted a retrospective study including consecutive cases of CIN2+ diagnosed during 2009–2010 in women attending the Gynecological Unit of Meixoeiro Hospital (University Hospital of Vigo, Galicia, Spain) for cervical cancer screening. Women had not been vaccinated with any HPV vaccine at time of diagnosis. Ninety-four women agreed to be enrolled.
This study received approval from the ethics committee of clinical investigation of Galicia (Santiago de Compostela, Spain). All study participants provided written informed consent before inclusion.
Data for age at first worst histological diagnosis, previous cytological screening and following treatment were reported. Nationality, race, oral contraceptive use, smoking, age at first sexual intercourse and lifetime number of sexual partners were collected by voluntary questionnaire filled in by the patients themselves and received by mail. When possible, they were compared with sexual risk behavior data obtained in the year 2009 from Galician general population by 7988 telephone interviews to women (aged 16–49 years) selected from a health database by simple random sampling (Galician risk behaviors information system, SICRI, unpublished data).
Histological diagnosesCervical biopsy specimens sampled under a colposcopic guide were considered for diagnosis. Additional biopsies were studied in case of conization or hysterectomy. The worst histological diagnosis was considered for the patient classification. Diagnoses of moderate dysplasia-cervical intraepithelial neoplasia grade 2 (CIN2), severe dysplasia-cervical intraepithelial neoplasia grade 3-carcinoma in situ (CIN3-CIS), invasive squamous cell carcinoma (SCC), adenocarcinoma in situ (AIS), and adenocarcinoma (AC) were referred to here as CIN2+. CIN3+ excluded CIN2.
HPV detection and genotypingA total of 46 deparaffined biopsies and 48 endocervical exudates samples collected at CIN2+ diagnosis time or within the previous 4 months were studied. DNA was isolated from biopsies using QIAamp DNA MiniKit (Qiagen, Hilden, Germany) and from exudates using a QIAamp MinElute Media Kit (Qiagen, Hilden, Germany). Amplification and detection were carried out using the Linear Array HPV Genotyping Test (Linear Array. Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions.
Additional specific PCR was performed in case of HPV 33, 35 and/or 58 infection10 in order to detect properly coinfection of any of these three genotypes with HPV 52.
For this work, HPV genotypes were classified as high-risk (HR-HPV, IARC Group 1 carcinogens: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) and probable/possible-high-risk (pHR-HPV, IARC Groups 2A/B carcinogens: 26, 34, 53, 66, 67, 68, 69, 70, 73, 82 – including IS39 subtype) following the classification proposed by the International Agency for Research on Cancer Monograph Working Group.11 According the different grades of dysplasia, HPV genotypes were also classified in two groups: (a) the eight most common HPV genotypes detected in cervical cancer worldwide9 divided into 3 subgroups (HPV 16 and 18; HPV 45; HPV 31, 33, 35, 52 and 58) and (b) others.
Attribution of HPV genotypesAttribution of HPV genotypes was performed as it was described previously.12 The minimum estimate (ME) was calculated as the frequency of each HPV genotype in single infections. The highest estimate (HE) was given by the crude prevalence of HPV genotypes in single and multiple infections, where the total number of genotype-specific infections observed is divided by the total number of HPV positive studied lesions. In addition, a proportional attribution (HE, proportional) of multiple infections was estimated: a case was attributed proportionally according to the frequency of each HPV genotype as a single infection. The hierarchical attribution (HE, hierarchical) of multiple infections was also estimated: a case was attributed to the HPV genotype present in a multiple infection found with a higher frequency in single infections.
For the comparison of HPV genotype-specific prevalence between the different grades of dysplasia, only the ME and the HE with the hierarchical attribution of multiple infections were used.
For the study of HPV 16 prevalence in squamous lesions by age and severity of lesions, only the HE with the hierarchical attribution of multiple infections was used.
Impact of the vaccinationThe potential impact of the vaccination on the cumulative incidence of CIN2, CIN2+ and CIN3+ was calculated. The expected relative reduction was estimated for each histological group as the addition of the products of the multiplication of prevaccine HPV prevalence, vaccine coverage and vaccine efficacy for each HPV type.
For the prevaccine HPV prevalence, data of type-specific prevalence (HE, hierarchical attribution, excluded negative cases, expressed as percentage) in CIN2, CIN2+ and CIN3+ obtained in this study were used. For the direct protection, data of HPV 16 and 18 prevalence were used. For the cross-protection, data of HPV 31, 33 and 45 prevalence were used.
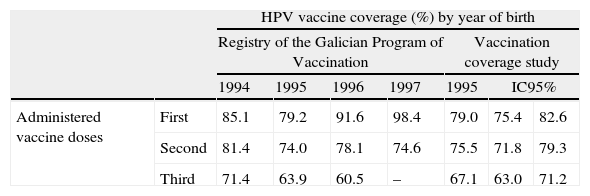
Regional vaccine coverage was available from registration data of vaccination points – both public and private – from the Registry of the Galician Program of Vaccination for women born between 1994 and 1997 (last accession: June of 2012) (Table 1). The data from women born in 1995 were also confirmed by a vaccine coverage study performed during the year 2011. Vaccine coverage of 0.80 was considered based on data from women born in 1994. Mean of coverage with one, two and three doses was calculated for this group of women.
HPV bivalent vaccine coverage. Coverage (%) by year of birth and number of administered doses.
| HPV vaccine coverage (%) by year of birth | ||||||||
| Registry of the Galician Program of Vaccination | Vaccination coverage study | |||||||
| 1994 | 1995 | 1996 | 1997 | 1995 | IC95% | |||
| Administered vaccine doses | First | 85.1 | 79.2 | 91.6 | 98.4 | 79.0 | 75.4 | 82.6 |
| Second | 81.4 | 74.0 | 78.1 | 74.6 | 75.5 | 71.8 | 79.3 | |
| Third | 71.4 | 63.9 | 60.5 | – | 67.1 | 63.0 | 71.2 | |
A bivalent vaccine's efficacy of 1 and 0.923 against CIN2+ associated with HPV 16 and HPV 18, respectively, was considered.8 A bivalent vaccine's cross-protective efficacy of 0.834, 0.763 and 1 against CIN2+ associated with HPV 31, HPV 33 and HPV 45, respectively, was considered.13
Data analysisFor all calculations of means, 95% confidence interval (95% CI) was calculated. Means were compared using t-Student test (SPSS version 15.0 for Windows, SPSS Inc., Chicago, IL).
Qualitative variables were compared with the chi-square test. Odds ratios (OR) and their 95% confidence intervals (CI) were estimated (EPIDAT version 3.114).
A two-sided p-value <0.05 was considered statistically significant.
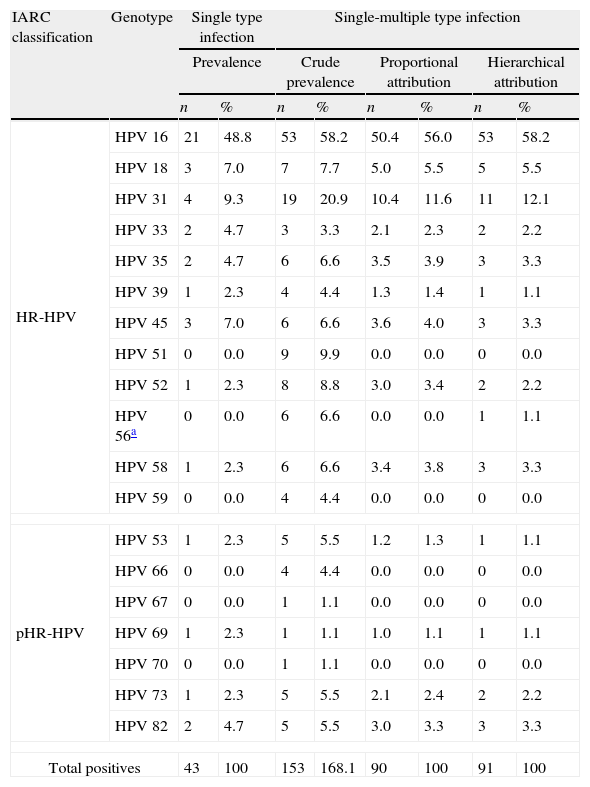
ResultsA total of 94 women between 18 and 82 years of age at CIN2+ diagnosis time (mean age 37.5 years. 95% CI, 35.1–39.8) were included in this study. Histological diagnoses were: CIN2 (n=23), CIN3-CIS (n=58), AIS (n=5) and SCC (n=8). Presence of HR-HPV was detected in 91/94 (96.8%) CIN2+ cases: 43 (47%) single infections and 48 (53%) multiple infections. Type-specific prevalence is shown in Table 2. HPV 16 and 31 were the most frequent genotypes in single and multiple infections. Genotypes other than HPV 16 and 18 were present between 44.2% (ME) and 36.3% (HE, hierarchical).
Attribution of HPV types to CIN2+ cases.
| IARC classification | Genotype | Single type infection | Single-multiple type infection | ||||||
| Prevalence | Crude prevalence | Proportional attribution | Hierarchical attribution | ||||||
| n | % | n | % | n | % | n | % | ||
| HR-HPV | HPV 16 | 21 | 48.8 | 53 | 58.2 | 50.4 | 56.0 | 53 | 58.2 |
| HPV 18 | 3 | 7.0 | 7 | 7.7 | 5.0 | 5.5 | 5 | 5.5 | |
| HPV 31 | 4 | 9.3 | 19 | 20.9 | 10.4 | 11.6 | 11 | 12.1 | |
| HPV 33 | 2 | 4.7 | 3 | 3.3 | 2.1 | 2.3 | 2 | 2.2 | |
| HPV 35 | 2 | 4.7 | 6 | 6.6 | 3.5 | 3.9 | 3 | 3.3 | |
| HPV 39 | 1 | 2.3 | 4 | 4.4 | 1.3 | 1.4 | 1 | 1.1 | |
| HPV 45 | 3 | 7.0 | 6 | 6.6 | 3.6 | 4.0 | 3 | 3.3 | |
| HPV 51 | 0 | 0.0 | 9 | 9.9 | 0.0 | 0.0 | 0 | 0.0 | |
| HPV 52 | 1 | 2.3 | 8 | 8.8 | 3.0 | 3.4 | 2 | 2.2 | |
| HPV 56a | 0 | 0.0 | 6 | 6.6 | 0.0 | 0.0 | 1 | 1.1 | |
| HPV 58 | 1 | 2.3 | 6 | 6.6 | 3.4 | 3.8 | 3 | 3.3 | |
| HPV 59 | 0 | 0.0 | 4 | 4.4 | 0.0 | 0.0 | 0 | 0.0 | |
| pHR-HPV | HPV 53 | 1 | 2.3 | 5 | 5.5 | 1.2 | 1.3 | 1 | 1.1 |
| HPV 66 | 0 | 0.0 | 4 | 4.4 | 0.0 | 0.0 | 0 | 0.0 | |
| HPV 67 | 0 | 0.0 | 1 | 1.1 | 0.0 | 0.0 | 0 | 0.0 | |
| HPV 69 | 1 | 2.3 | 1 | 1.1 | 1.0 | 1.1 | 1 | 1.1 | |
| HPV 70 | 0 | 0.0 | 1 | 1.1 | 0.0 | 0.0 | 0 | 0.0 | |
| HPV 73 | 1 | 2.3 | 5 | 5.5 | 2.1 | 2.4 | 2 | 2.2 | |
| HPV 82 | 2 | 4.7 | 5 | 5.5 | 3.0 | 3.3 | 3 | 3.3 | |
| Total positives | 43 | 100 | 153 | 168.1 | 90 | 100 | 91 | 100 | |
HR-HPV: high-risk HPV genotypes; pHR-HPV: probable/possible high-risk HPV genotypes.11
One HPV 56 and 66 multiple infection was only included in the crude prevalence and in the hierarchical attribution as HPV 56 infection based on the IARC classification of carcinogenic risk. It was considered that the absence of HPV 56 monoinfection was only related with the low number of samples studied.
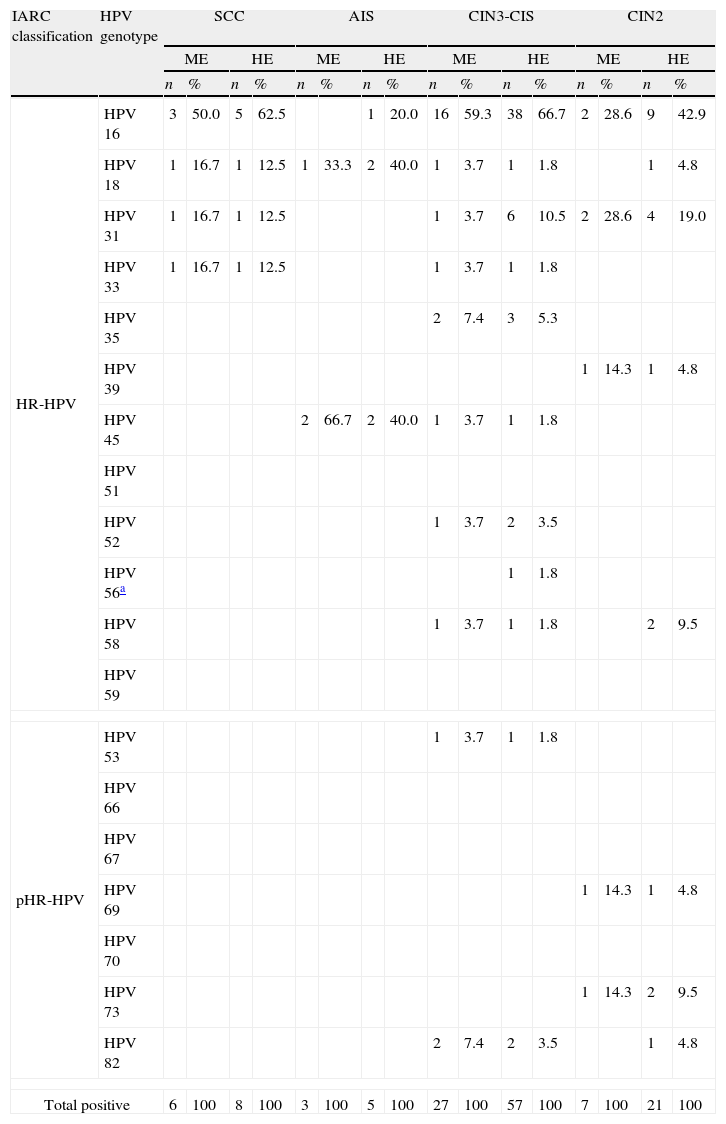
HPV prevalence for each grade of histological dysplasia is shown in Table 3. The most frequent types (HE, hierarchical) in CIN2 and CIN3-CIS were also HPV 16 and 31. HPV 16, 18 and 45 were the detected genotypes in AIS whereas HPV 16, 18, 31 and 33 were the detected genotypes in SCC.
Attribution of HPV types for the different grades of histological diagnoses.
| IARC classification | HPV genotype | SCC | AIS | CIN3-CIS | CIN2 | ||||||||||||
| ME | HE | ME | HE | ME | HE | ME | HE | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| HR-HPV | HPV 16 | 3 | 50.0 | 5 | 62.5 | 1 | 20.0 | 16 | 59.3 | 38 | 66.7 | 2 | 28.6 | 9 | 42.9 | ||
| HPV 18 | 1 | 16.7 | 1 | 12.5 | 1 | 33.3 | 2 | 40.0 | 1 | 3.7 | 1 | 1.8 | 1 | 4.8 | |||
| HPV 31 | 1 | 16.7 | 1 | 12.5 | 1 | 3.7 | 6 | 10.5 | 2 | 28.6 | 4 | 19.0 | |||||
| HPV 33 | 1 | 16.7 | 1 | 12.5 | 1 | 3.7 | 1 | 1.8 | |||||||||
| HPV 35 | 2 | 7.4 | 3 | 5.3 | |||||||||||||
| HPV 39 | 1 | 14.3 | 1 | 4.8 | |||||||||||||
| HPV 45 | 2 | 66.7 | 2 | 40.0 | 1 | 3.7 | 1 | 1.8 | |||||||||
| HPV 51 | |||||||||||||||||
| HPV 52 | 1 | 3.7 | 2 | 3.5 | |||||||||||||
| HPV 56a | 1 | 1.8 | |||||||||||||||
| HPV 58 | 1 | 3.7 | 1 | 1.8 | 2 | 9.5 | |||||||||||
| HPV 59 | |||||||||||||||||
| pHR-HPV | HPV 53 | 1 | 3.7 | 1 | 1.8 | ||||||||||||
| HPV 66 | |||||||||||||||||
| HPV 67 | |||||||||||||||||
| HPV 69 | 1 | 14.3 | 1 | 4.8 | |||||||||||||
| HPV 70 | |||||||||||||||||
| HPV 73 | 1 | 14.3 | 2 | 9.5 | |||||||||||||
| HPV 82 | 2 | 7.4 | 2 | 3.5 | 1 | 4.8 | |||||||||||
| Total positive | 6 | 100 | 8 | 100 | 3 | 100 | 5 | 100 | 27 | 100 | 57 | 100 | 7 | 100 | 21 | 100 | |
ME: minimum estimate, single type infection; HE: highest estimate, single type and multiple type infection (hierarchical attribution). The most frequent type in single type infection is attributed to the case with multiple type infection.
HR-HPV: high-risk HPV genotypes; pHR-HPV: probable/possible high-risk HPV genotypes.11
CIN2: cervical intraepithelial neoplasia grade 2; CIN3-CIS: cervical intraepithelial neoplasia grade 3; SCC: invasive squamous cell carcinoma; AIS: adenocarcinoma in situ.
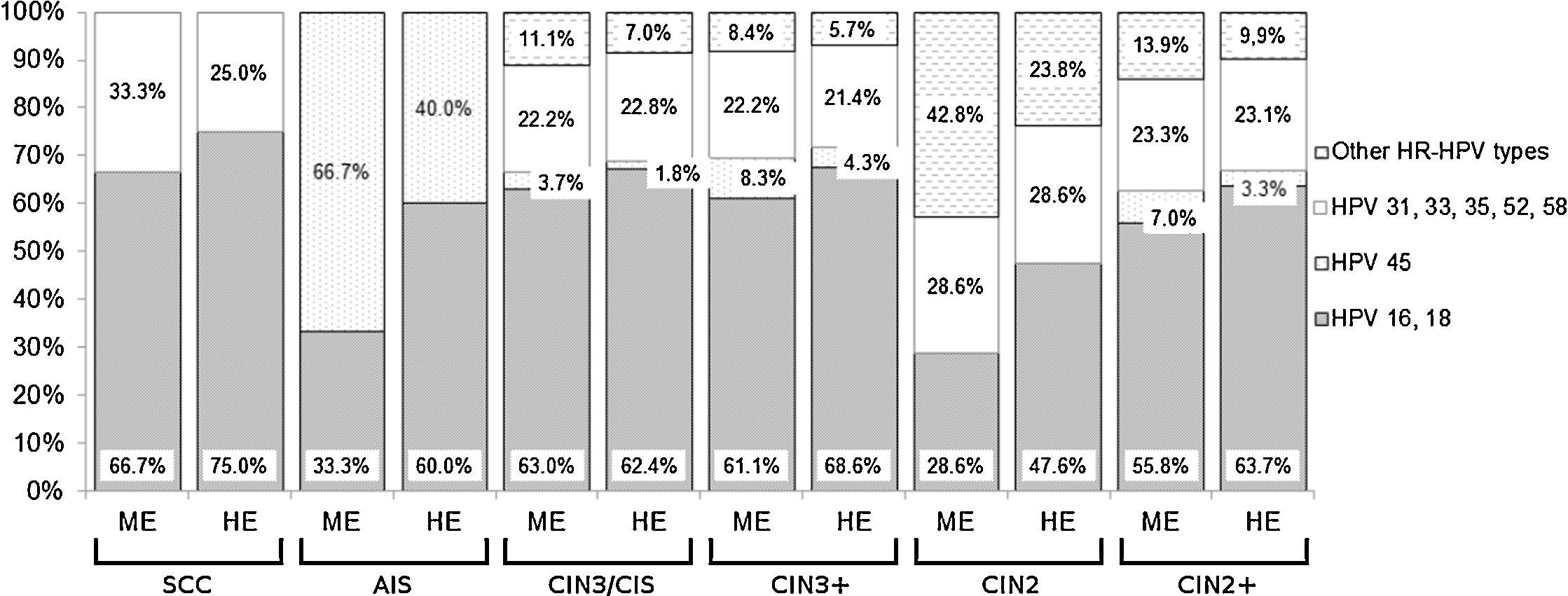
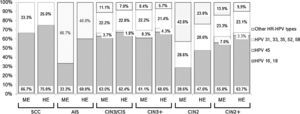
The cumulative detection of the eight most common HPV genotypes detected in cervical cancer worldwide (HPV 16, 18, 31, 33, 35, 45, 52, 58) is shown in Fig. 1. Some cases have been associated with HPV genotypes other than these eight types: 42.8% (ME) to 23.8% (HE, hierarchical) of CIN2 but less than 9.0% of CIN3+ (Fig. 1). The combined detection of HPV 16 and/or 18 ranged from 28.6% (ME) to 47.6% (HE, hierarchical) in CIN2 and from 61.1% to 68.6% respectively in CIN3+ (Fig. 1).
Cumulative relative contribution of the eight most common HPV types in cervical cancer worldwide by histological category. ME: minimum estimate of type-specific contribution; HE: highest estimate of type-specific contribution (hierarchical attribution); SCC: invasive squamous cell carcinoma; AIS: adenocarcinoma in situ; CIN3-CIS: cervical intraepithelial neoplasia grade 3; CIN3+: SCC, AIS and CIN3-CIS considered together; CIN2: cervical intraepithelial neoplasia grade 2; CIN2+: SCC, AIS, CIN3-CIS and CIN2 considered together.
Considering only direct protection of the vaccine, with the introduction of the bivalent vaccine in Galicia, it is expected to reduce the cumulative incidence of CIN2+ by 50.6%, of CIN2 by 37.9%, of CIN3+ by 54.5%.
If cross-protection of bivalent vaccine is also considered, it is expected to reduce the cumulative incidence of CIN2+ by 62.7%, of CIN2 by 50.6% and of CIN3+ by 66.4%.
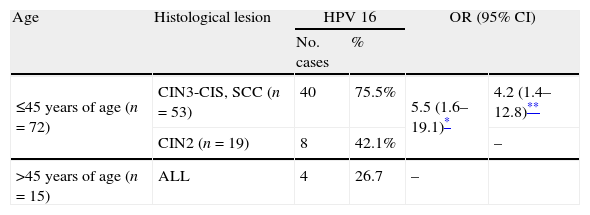
HPV 16 was predominant and its prevalence ranged between 28.6% (ME) and 42.9% (HE, hierarchical) in CIN2 and between 52.8% and 62.9% in CIN3+. HPV 16 prevalence (HE, hierarchical) by age and diagnosis in squamous lesions is shown in Table 4: this genotype was significantly more prevalent in women aged 45 years or younger. And, between them, its prevalence was significantly higher in CIN3-CIS/SCC than in CIN2. Considering direct protection of the vaccine, it is expected to reduce by 62.6% the cumulative incidence of CIN3-CIS/SCC in women younger than 45 years of age.
HPV 16 prevalence excluding AIS. HPV 16 prevalence among HPV positive women by age and histological lesion, excluding AIS.
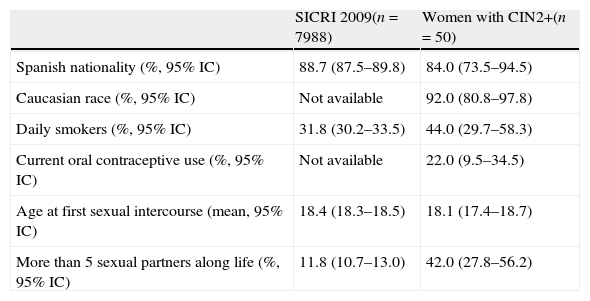
Epidemiological data were available from 50 women between 18 and 52 years of age (mean age 36.2 years. 95% CI, 34.1–38.2) who agreed to answer a voluntary questionnaire: CIN2 (n=14), CIN3-CIS (n=30), AIS (n=2) and SCC (n=4). The characteristics of these patients and the comparison with SICRI data are shown in Table 5.
Epidemiological characteristics. Data from the risk behaviors information system (SICRI) were obtained from women aged 16–49 years in the year 2009. Data of this study were from women aged 18–52 years in the years 2009–2010.
| SICRI 2009(n=7988) | Women with CIN2+(n=50) | |
| Spanish nationality (%, 95% IC) | 88.7 (87.5–89.8) | 84.0 (73.5–94.5) |
| Caucasian race (%, 95% IC) | Not available | 92.0 (80.8–97.8) |
| Daily smokers (%, 95% IC) | 31.8 (30.2–33.5) | 44.0 (29.7–58.3) |
| Current oral contraceptive use (%, 95% IC) | Not available | 22.0 (9.5–34.5) |
| Age at first sexual intercourse (mean, 95% IC) | 18.4 (18.3–18.5) | 18.1 (17.4–18.7) |
| More than 5 sexual partners along life (%, 95% IC) | 11.8 (10.7–13.0) | 42.0 (27.8–56.2) |
Regarding clinical presentation and treatment, women (n=86) with CIN2, CIN3-CIS or AIS were younger at the worse histological diagnosis time than those (n=8) with SCC: 35.8 years (95% CI, 33.8–37.8) vs 55.0 years (95% CI, 40.1–69.9) (p=0.019). Among women with SCC, the two patients infected by HPV 16 and 18 were 49.5 years of age (95% CI, 22.1–76.9). Absence of previous cytological control was observed in 5 cases: 3/59 (5.1%) CIN3-CIS cases and 2/8 (25.0%) SCC cases. All women received treatment except one patient who refused and another who moved outside the city. Eighty-seven women were referred to conization, 4 to hysterectomy and 2 to lymphadenectomy. The mean time between first histological diagnosis of CIN2+ and treatment was 13.8 weeks (95% CI, 11.8–15.8). Four CIN2 and 2 CIN3-CIS cases were detected in very young women (less than 25 years of age) and they received treatment.
Regarding the three patients without HR-HPV detection, a low risk genotype (HPV 11) was detected in one case and HPV 31 was detected one year before diagnosis in other case.
DiscussionThis study provides data for the local distribution of HPV genotypes among unvaccinated women with CIN2+ in Galicia, Spain. This knowledge could be useful for future evaluations of the impact of the bivalent vaccination that was introduced recently, allow identification of local problems of primary or secondary prophylaxis, of HPV transmission and special needs of Public Health Service interventions.
The data confirmed that HPV 16 is found in the majority of cervical dysplasia, especially in CIN3-CIS and SCC, where more than 60% of the cases were related to this genotype, in agreement with previous data for European patients with invasive disease.9,15 HPV 31 was the second most frequent genotype in single or multiple infections, as it was published for CIN2 and CIN3-CIS cases from Spain15 but higher than it was described previously by others.16 Differences may be attributable to the relative proportion of CIN2 cases included in the study or to slight differences in the calculation methods. HPV 18 and HPV 45 prevalences were very similar and higher in SCC and AIS. An early detection of invasive cases related to HPV 16 and 18 was found, as it was described previously.9,15
The eight most common HPV genotypes (HPV 16, 18, 31, 33, 35, 45, 52 and 58) in invasive cervical cancer worldwide have been studied separately: only genotypes belonging to this group have been found in AIS, SCC and around 90% of CIN3-CIS cases. Given the prevalence of HPV 31 in CIN2+ and of HPV 33 and 45 in CIN3+ it seems interesting to study their persistence after infection in order to evaluate the cost-effectiveness of referring women to colposcopy in case of detection of these genotypes regardless of cytology result as it was suggested previously.17
Excluding AIS, HPV 16 prevalence (hHE) in women younger than 45 years of age was higher than that in older women. A decline in HPV 16 positivity in women with age and a corresponding increase in positivity for other genotypes has been observed in previous studies.18,19 Referring only to this youngest group of women, HPV 16 prevalence (hHE) also increased in severe lesions (CIN3-CIS/SCC).
The local vaccine coverage was satisfactory (80%, 71.4% three doses) for the first vaccinated cohort (women born in 1994). The local expected vaccine impact was a reduction in the cumulative incidence of CIN2+ by 50.6% and of CIN3+ by 54.5% but higher (62.7% and 66.4%, respectively) considering also its cross-protective efficacy. This is consistent with the expected impact of the vaccine that was published recently by other authors in invasive cervical cancer20 and in the HSIL in Spain.21 It is important to note that duration of direct and cross-protective efficacy is unknown. Some authors have suggested a potential waning of effect of the cross-protection of bivalent vaccine.13,22 The duration of protection against vaccine and non-vaccine type HPVs will have an important effect on the consequences of vaccination.
Data of vaccine efficacy observed in clinical trials have been used in this study for the expected vaccine impact calculations. A high vaccine effectiveness against vaccine type infection has been published recently.23 Several factors as herd protection or vaccine effectiveness of incomplete series of vaccination could modify the vaccine impact.
Although the role of the most frequent carcinogenic types in cervical cancer worldwide has been well established,9 the carcinogenicity of less frequent HPV genotypes or their role in multiple infections is still being studied. Referring to crude data, the most frequent non-vaccine (direct of cross-protection) high risk genotypes were HPV 51, 52, 35, 56 and 58. Their crude prevalence (HE) is not expected to be modified by vaccine introduction, but their relative contribution to CIN2+ might be influenced and therefore the impact of the vaccination might be different than estimated.
Based on the current evidence, the recommended screening practices should not change for women with history of HPV vaccination: at least 34% of CIN2+ will continue to happen and data that could support changes in the starting age or the screening interval are missing. At this time, little is known about the real effect of vaccination on the HPV genotype distribution and vaccine effectiveness.23 The performance of cytology and HPV testing (the two recommended methods for screening24), the duration of protection and the screening adherence after vaccination are still unknown.
It will be necessary to know the fully vaccinated status of the population at an age likely to be prior to HPV exposure. Surveillance systems including CIN3 and Pap registries and monitoring HPV type specific CIN2+ cases will be crucial. The detection of non-vaccine genotypes might be important in HPV type specific screening, particularly in women older than 45 years of age in Galicia.
As the most evident and serious abnormal results determined by cytology, HPV tests, and colposcopy are caused by HPV 16, the positive predictive value for CIN2+ for these three methods will decrease in vaccinated women. In Galicia, the risk of CIN2+ in women younger than 45 years of age may be linked preferentially to HPV 16, so if data demonstrate reductions in cancer risk for vaccinated women, it may be reasonable to delay screening until the age of 25 years. In addition it may be possible to expand the screening intervals if the long-term effectiveness of the vaccine is proven and if HPV testing is added to cervical cytology.
Women included in this study belonged to the general population that was attending a Gynecological Unit for cervical cancer screening. The analysis of their epidemiological data would allow us to detect any bias and to know if the conclusions are representative of the local population. The mean age at first sexual intercourse was lower than it was described previously by other authors.25 This could be attributable to the range of birth year of women that answered the questionnaire in this study (1957–1991) but also to geographical characteristics. It was remarkable that 42.0% of women with CIN2+ included in this study had had more than 5 lifetime sexual partners in contrast with 11.8% in SICRI survey or 5.9% in AFRODITA survey.25 This could be explained by the fact that a relative risk has been found by other authors for SCC and adenocarcinoma, in case of more than 6 lifetime sexual partners.26
The largest number of cases was detected in women aged between 30 and 40 years. This finding was in concordance with age trends of high-grade cervical lesions that were published previously27 where a peak was observed at less than 30 years of age in North America but at relative older age in Europe (25–40 years). This is also related with the age-specific prevalence of HR genotypes described recently in Spain.28 These authors studied general non-vaccinated population and found that HPV prevalence was highest in women aged 18–25 years and decreased with increasing age.
Invasive cases were less common and corresponded to the oldest patients as it would be expectable in a population with a cervical cancer screening program. Four CIN2 and 2 CIN3-CIS cases were detected in very young women (less than 25 years of age), so it would be interesting to investigate the impact of the introduction of the current program of cervical cancer screening in the overtreatment of very young women as half of them would have regressed in the short term.29
This retrospective study allowed us to know the characteristics of the CIN2+ cases detected just after the implementation of an organized screening protocol of the Public Health Service and to know the baseline local prevalence of HPV genotypes in cervical cancer prior to mass HPV vaccination. Few data have been published of the prevalence of HPV genotypes in CIN2+ in Galicia, Spain. In this study the role of the 8 most frequent HPV genotypes in cervical cancer worldwide in CIN2+ in Galicia, Spain was shown, especially HPV 16, 18, 31, 33 and 45. The highest prevalence of CIN2+ in women aged between 30 and 40 years and the contribution of HPV 16 in CIN2/CIN3-CIS/SCC in women aged 45 years or younger were observed. The calculation of the vaccine impact was based on recent local data of vaccine coverage and HPV prevalence. This knowledge could be useful in Public Health Service evaluations about screening or vaccination policies.
One limitation of the present study is represented by the limited sample size; besides this, half of the patients did not participate with the voluntary questionnaire of epidemiological data. The absence of some HR-HPV monoinfections related with the reduced number of samples was a limitation to attribute properly the multiple infections. For example, in the case of HPV 51, 56, 59, 66, 67 or 70 multiple infections with other HR or pHR-genotype detected in monoinfection in this work, the proportional or hierarchical attribution was added to the other genotype. Although it was considered to be important to include all consecutive cases of CIN2+ along the study period, since it was a retrospective study, in some cases we could not offer the patients the opportunity to be included because they were not alive at inclusion time. Another limitation is that it is difficult to calculate the real impact of the vaccine nowadays until the duration of its protection is well established and the contribution of genotypes in coinfections can be assessed.
ConclusionsAlthough HPV 16 is the most frequent genotype in CIN2+, other genotypes not included in the vaccine like HPV 31, 33, 35, 45, 51, 52, 56 and 58 are also common, suggesting that the development of new vaccines against a higher number of genotypes is important. HPV vaccination could have a great impact in women younger than 45 years of age because of the high prevalence of HPV 16 in their lesions. Presence of multiple high-risk genotypes in CIN2+ might influence the vaccination impact.
FundingThis work was supported by Conselleria de Sanidade, Galicia, Spain.
Conflict of interestThe authors declare no conflict of interest.
We thank Malvar A (Department of Epidemiology, Galician Public Health Service, Spain) for critical review of the manuscript. We thank Biomedical Foundation of the University Hospital of Vigo, Spain for the statistical analysis and the English revision of the manuscript. We are also grateful to Taboada P (Peixe Software, SLNE, Spain) for the online software donation for data collection.