The aim of this study was to determine the differences in percentage resistance in H. pylori clinical isolates using EUCAST breakpoints compared with previously used breakpoints. MIC value distribution in H. pylori clinical isolates was also studied.
MethodsSusceptibility to amoxicillin, tetracycline, metronidazole, clarithromycin, rifampicin and levofloxacin was performed by E-test in 824 H. pylori clinical isolates. EUCAST and previous breakpoints defined resistance as follows: MIC >0.12mg/L and ≥2mg/L for amoxicillin, >8mg/L and ≥8mg/L for metronidazole, >0.5mg/L and ≥1mg/L for clarithromycin, >1mg/L and ≥32mg/L for rifampicin, and >1mg/L and ≥4mg/L for tetracycline and >1mg/L levofloxacin.
ResultsOverall resistance rate by EUCAST and by previous breakpoints was 8.5% and 3.2% for amoxicillin, 0.6% and 0.1% for tetracycline, 39.2% and 39.7% for metronidazole, 51.2% and 51.2% for clarithromycin, 32% and 3.1% for rifampicin, and 6.7% and 6.7% for levofloxacin.
ConclusionsWhen using the different breakpoints for antimicrobial susceptibility testing, similar results were found with most antibiotics tested (tetracycline, metronidazole, clarithromycin, and levofloxacin), except for amoxicillin and rifampicin.
El objetivo de este estudio era determinar las diferencias en el porcentaje de resistencia de aislamientos clínicos de H. pylori usando los puntos de corte de EUCAST comparado con los puntos de corte usados anteriormente. También se estudió la distribución de los valores de CMI en los aislamientos de H. pylori.
MétodosLa sensibilidad de amoxicilina, tetraciclina, metronidazol, claritromicina, rifampicina y levo-floxacina se determinó mediante E-test en 824 aislamientos clínicos de H. pylori. Los puntos de corte utilizados fueron EUCAST: CMI >0,12mg/L para amoxicilina, >8mg/L para metronidazol, >0,5mg/L para claritromicina y >1mg/L para rifampicina, tetraciclina y levofloxacina. Los puntos de corte que se habían utilizado antes de EUCAST fueron: CMI ≥2mg/L para amoxicilina, ≥8mg/L para metronidazol, ≥1mg/L para claritromicina, ≥32mg/L para rifampicina, ≥4mg/L para tetraciclina y >1mg/L para levofloxacina.
ResultadosLa resistencia global con los puntos de corte EUCAST y con los puntos de corte anteriores fue: 8,5% y 3,2% para amoxicilina, 0,6% y 0,1% para tetraciclina, 39,2% y 39,7% para metronidazol, 51,2% y 51,2% para claritromicina, 32% y 3,1% para rifampicina y 6,7% y 6,7% para levofloxacina.
ConclusiónA pesar de la utilización de diferentes puntos de corte, se obtuvieron resultados de resistencia similares para la mayoría de los antibióticos probados (tetraciclina, metronidazol, claritrnnñomicina, y levofloxacino), con la única excepción de amoxicilina y rifampicina.
Helicobacter pylori is a Gram-negative spiral rod colonizing the gastric mucosa mainly at the antrum, producing gastric inflammation. Patient could remain without symptomatology for his o her whole life or develop several pathologies such as duodenal or gastric ulcer; mucosa-associated lymphoid tissue (MALT) or gastric cancer.1 Association with non-digestive diseases has also been described.2 When treatment is needed, several triple or quadruple therapies could be used. Amoxicillin, tetracycline, metronidazole and clarithromycin are the antimicrobials most frequently used combined with proton pump inhibitors or bismuth salts.3 Several papers have stressed the importance of doing H. pylori susceptibility testing before administering the treatment.4 However, different methodology could be performed for in vitro susceptibility testing. Several Societies and Committees have defined reference methods and breakpoints for categorized organisms as susceptible or resistant to antimicrobial agents.
The National Committee for Clinical Laboratory Standard (NCCLS) (now the Clinical and Laboratory Standards Institute, CLSI) proposed in 1999 and continued recommending H. pylori breakpoints only for clarithromycin and using an agar dilution.5 The British Society for Antimicrobial Chemotherapy proposed the Epsilometer test (E-test)6 and recommended breakpoints for four antimicrobials. Several studies conducted by the European Helicobacter pylori Study Group also used the E-test and proposed breakpoints for six antimicrobials.7
The European Committee for Antimicrobial Susceptibility Testing (EUCAST) was initiated by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) to harmonize minimum inhibitory concentration (MIC) breakpoints across Europe.8 In March–April 2011 the EUCAST group proposed breakpoints for six antimicrobials used to treat H. pylori.9 The breakpoints are based on the epidemiological cut-off value (ECOFF), on clinical validation or on the study of resistance mechanisms.
The adoption of new guidelines or changes in breakpoints can have an important effect on antimicrobial-resistance surveillance.10
The aim of this study was to determine the resistance percentage in H. pylori clinical isolates using EUCAST breakpoints comparing the results obtained with others previously used. MIC value distribution in H. pylori clinical isolates was also studied.
MethodsPatients and H. pylori strains824 H. pylori strains were isolated from antral gastric mucosal biopsy specimens obtained from symptomatic patients from January 1, 2007 to December 2014. 641 (77.8%) were children (median age 8.99±3.3) and 183 (22.2%) were adults (median age 43.1±15.9). 59.1% were females and 46.8% were males. No data of previous eradication treatment were available.
Biopsies were cultured in selective and non-selective media obtained commercially (Blood-supplemented Columbia Agar plates and Pylori agar, BioMerieux). H. pylori strains were cultured under microaerobic conditions obtained in an anaerobiosis jar with a microaerobic gas-generating envelopment. Strains were identified by colony and Gram stain morphology, and urease, oxidase and catalase positive test. From November 2012, any strain with a rare resistance was confirmed to be H. pylori by MALDI-TOF.
Susceptibility methodsSusceptibility to amoxicillin (AMX), tetracycline (TET), metronidazole (MET), clarithromycin (CLA), rifampicin (RIF) and levofloxacin (LEV) was performed by the E-test.7 The bacteria were subcultured for 48h in Blood-supplemented Columbia agar and a bacterial suspension adjusted to 107CFU/mL was inoculated directly onto Mueller-Hinton agar supplemented with 5% sheep blood obtained commercially (bioMerieux).
E-test was applied over the culture media within 30min of inoculation. Plates containing the E-test were incubated under microaerobic atmosphere. After 72h of incubation, the Minimal Inhibitory Concentration (MIC) was determined by considering the point were elipse growth cut with the scale number in the E-test strip.
To analyze the data each MIC value was adjusted to the next higher twofold dilutions (as studied by broth microdilution or agar dilution).
Interpretative criteriaThe MICs obtained in the entire period were analyzed according to the breakpoints previously used and to EUCAST breakpoints (Table 1).5,6,9,11,12
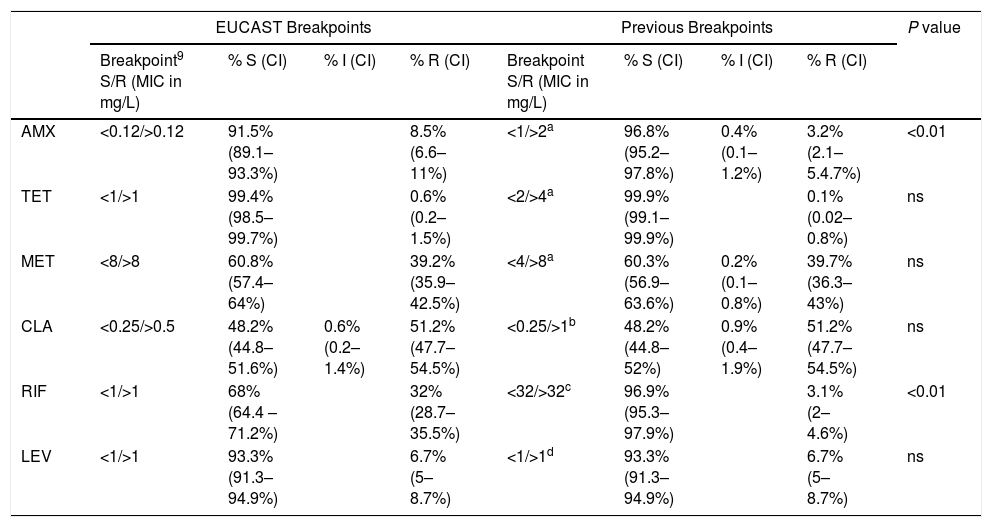
Assignment of H. pylori clinical isolates to interpretative categories according to the EUCAST breakpoint and the previous breakpoint (percentage and confidence interval, CI). The antibiotic tested were amoxicillin, AMX, tetracycline, TET, metronidazole, MET, clarithromycin, CLA, rifampicin, RIF and levofloxacin, LEV.
| EUCAST Breakpoints | Previous Breakpoints | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breakpoint9 S/R (MIC in mg/L) | % S (CI) | % I (CI) | % R (CI) | Breakpoint S/R (MIC in mg/L) | % S (CI) | % I (CI) | % R (CI) | ||
| AMX | <0.12/>0.12 | 91.5% (89.1–93.3%) | 8.5% (6.6–11%) | <1/>2a | 96.8% (95.2–97.8%) | 0.4% (0.1–1.2%) | 3.2% (2.1–5.4.7%) | <0.01 | |
| TET | <1/>1 | 99.4% (98.5–99.7%) | 0.6% (0.2–1.5%) | <2/>4a | 99.9% (99.1–99.9%) | 0.1% (0.02–0.8%) | ns | ||
| MET | <8/>8 | 60.8% (57.4–64%) | 39.2% (35.9–42.5%) | <4/>8a | 60.3% (56.9–63.6%) | 0.2% (0.1–0.8%) | 39.7% (36.3–43%) | ns | |
| CLA | <0.25/>0.5 | 48.2% (44.8–51.6%) | 0.6% (0.2–1.4%) | 51.2% (47.7–54.5%) | <0.25/>1b | 48.2% (44.8–52%) | 0.9% (0.4–1.9%) | 51.2% (47.7–54.5%) | ns |
| RIF | <1/>1 | 68% (64.4 –71.2%) | 32% (28.7–35.5%) | <32/>32c | 96.9% (95.3–97.9%) | 3.1% (2–4.6%) | <0.01 | ||
| LEV | <1/>1 | 93.3% (91.3–94.9%) | 6.7% (5–8.7%) | <1/>1d | 93.3% (91.3–94.9%) | 6.7% (5–8.7%) | ns | ||
S=susceptible, I=Intermediate, R=resistant, ns=no significant.
95% Confidence intervals (95%CI) of prevalence rates were calculated. Data were analyzed using EpiInfo 6.04 (CDC, USA) computer software.
ResultsThe percentage of strains that are susceptible, intermediate or resistant by using the EUCAST breakpoints and the previously used breakpoints is in Table 1. The overall percentage of resistance to the 6 antibiotics by the new EUCAST breakpoints and by the previous breakpoints was 8.5% and 3.2% for amoxicillin, 0.6% and 0.1% for tetracycline, 39.2% and 39.7% for metronidazole, 51.2% and 51.2% for clarithromycin, 32% and 3.1% for rifampicin and 6.7% and 6.7% for levofloxacin.
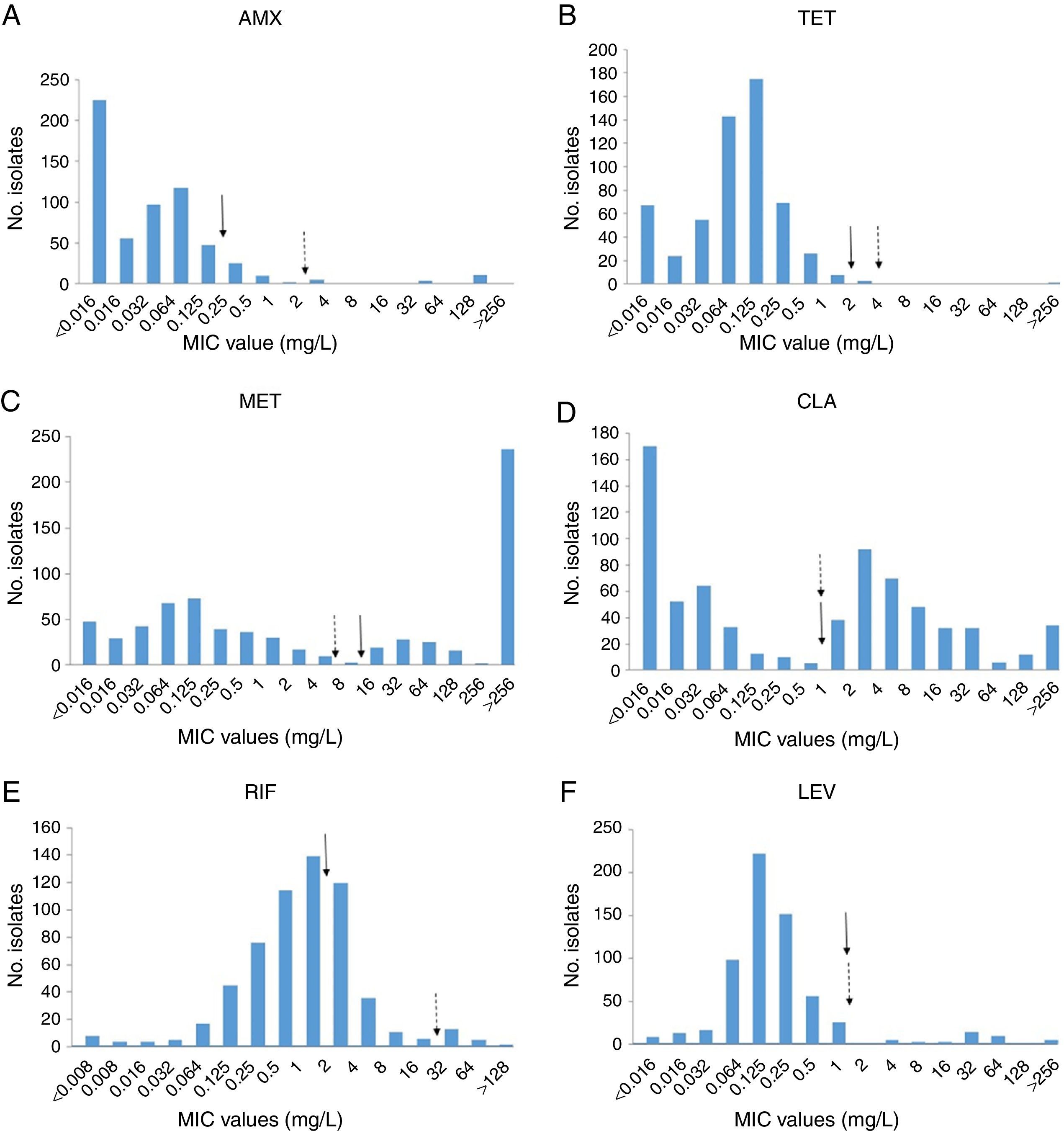
The distributions of MICs for amoxicillin, tetracycline, metronidazole, clarithromycin, rifampicin and levofloxacin in H. pylori clinical isolates are shown in Fig. 1.
Distribution of antibiotic MICs for H. pylori clinical isolates. MICs were determined by E-Test and adjusted to the highest two-fold dilution values. MICs of amoxicillin (A) tetracycline (B), metronidazole (C), clarithromycin (D), rifampicin (E) and levofloxacin (F) are shown. Filled arrows indicate the EUCAST resistance breakpoints and dashed arrows the previously used breakpoints. Exe Y shows the number of H. pylori isolates for each MIC value and Exe X shows each MIC value (mg/L). Arrows indicates the EUCAST (
) or previous () resistance breakpoint.Several triple or quadruple therapies are recommended for treatment of H. pylori infection, those being omeprazole, clarithromycin and amoxicillin which are the most frequently used. However, infection by a clarithromycin-resistant strain is a risk factor for treatment failure13 and other antimicrobial agents used are tetracycline, metronidazole, levofloxacin or rifabutin.14 Treatment for H. pylori infection is usually started on an empirical basis, and when an infecting strain is resistant to the antimicrobial agents used, its successful eradication is hampered.
The amoxicillin breakpoint, proposed by EUCAST, was based on the epidemiological cut-off value although no evidence exists which determines whether treatment is successful for infections caused by isolates with MICs >0.12mg/L. Metronidazole cut-off was the current and widely accepted breakpoint, but there is no clinical validation. Clarithromycin breakpoints have been clinically validated and isolates with MIC above 0.5mg/L have a resistance mechanism (23S RNA mutation). Tetracycline breakpoint correlates with mutations in 16S RNA, levofloxacin breakpoint with gyrA mutations, and rifampicin breakpoint with rpoB mutation, although there are no outcome data and there is no clinical validation.9
EUCAST guidelines for antimicrobial susceptibility testing (AST) are being implemented in European Laboratories.15 Adoption of new guidelines and breakpoints can have a significant effect on reports and prior to implementing new guidelines, the consequences should be considered to prevent misunderstandings in interpretations. In this study AST's interpretation of the EUCAST breakpoint compared with the previous one was investigated and EUCAST will lead to significantly more isolates of H. pylori being resistant to rifampicin and amoxicillin. However, there are no important changes with the rest of the antimicrobial agents used for treatment of this infection, A total of 8.5% of the strains were amoxicillin-resistant when using the EUCAST breakpoints compared with 3.2% for the previous breakpoints. Amoxicillin is included in most treatments, such as sequential, concomitant and hybrid treatment; resistance to this antibiotic is clinically important and requires attention. On the other hand, there are no clinical data to confirm if strains with MIC of 0.25–0.5mg/L, although they are amoxicillin-resistant according to EUCAST breakpoints, could be eliminated with oral amoxicillin of 1g/12h. In our study most of the amoxicillin-resistant strains had low MICs.
In the study done by Kim et al.16 no amoxicillin breakpoint was used to categorized the strains, although 5.6% of strains studied in 1994 and 18.5% of the strains studied in 2003 had MIC >0.5mg/L. Moreover, two of these strains in 1994 and 3 in 2003 had an MIC of 8mg/L. Wu et al.17 reported a surprisingly high prevalence of amoxicillin resistance using a breakpoint of >0.5mg/L, 71.9%, with 115 out of 153 being higher than 0.125 and 44 strains with MIC >16mg/l.
An increase of the resistance to tetracycline has been described by Kim et al.16 reporting a resistance rate of a 5.9% in 1987 and 12.3% in 2003 with MICs >4mg/L. Moreover, Wu et al.17 reported a surprisingly high prevalence of tetracycline resistance, 58.8% by using a breakpoint of >16, with 104 out of 153 being higher than 1 and 79 strains with MIC >32mg/l.
Metronidazole has been widely prescribed for infections such as parasitic or female genital infections and could contribute to the high resistance rate found everywhere.
In a European Study using the new EUCAST breakpoints, H. pylori resistance rates for adults were 17.5% for clarithromycin, 14.1% for levofloxacin and 34.9% for metronidazole, and were higher for clarithromycin and levofloxacin in Western/Central and Southern Europe (resistance was higher than 20%) than those in Northern European countries (resistance lower than 10%).18 Moreover, an association was found between outpatient quinolone use and the proportion of levofloxacin resistance and between the use of long-acting macrolides and clarithromycin resistance.
MIC distribution of the strains tested according to the MIC value was bimodal for metronidazole and clarithromycin, whilst a continuous distribution was observed for amoxicillin, tetracycline, rifampicin and levofloxacin. The wild type population is the subpopulation of isolates with no detectable acquired resistance mechanisms,19,20 but the mechanism of resistance was not studied herein.
There is a need for continuous surveillance of resistance to antimicrobial agents in H. pylori infections as well as in other infections. The knowledge of the breakpoints used in each study is mandatory when comparing data obtained from different studies.
Recently Boyanova et al.21 compared EUCAST with previously used breakpoint in 299 strains in Bulgaria and found similar results: although there were differences for 3 of the antibiotics tested, the differences in the resistant percentage was lower than 4%. Unfortunately, rifampicin resistance is determined only with EUCAST.
According to the results of this study, no matter which breakpoint was used, similar results were found for the antibiotics with high clinical relevance, whilst there were differences with amoxicillin and rifampicin.
Conflict of interestNone declared.
This study was supported, in part by Fondo de Investigaciones Sanitarias grant FIS 08/1775 (Instituto de Salud Carlos III, Ministerio de Economía y Competitividad)
This work has enjoyed the cooperation of the Research Fund Health FIS 08/1775 (Institute of Health Carlos III, Ministry of Economy and Competitiveness).