Although the prevalence of MNG is widespread throughout the world, its pathogenesis is poorly understood, and the complex interactions of both genetic predisposition and the individuals’ environment are likely. However, to the best of our knowledge, it remains unknown whether there is a relationship between vitamin D status and prevalence or pathogenesis of euthyroid MNG. Therefore, the goal of the present study was determination of vitamin D status in euthyroid MNG as well as exploration of the correlation between vitamin D status & TSH levels.
MethodsA total of 77 patients diagnosed with euthyroid MNG and 50 subjects without goiter were matched according to age, weight and BMI as control group in this case control study.
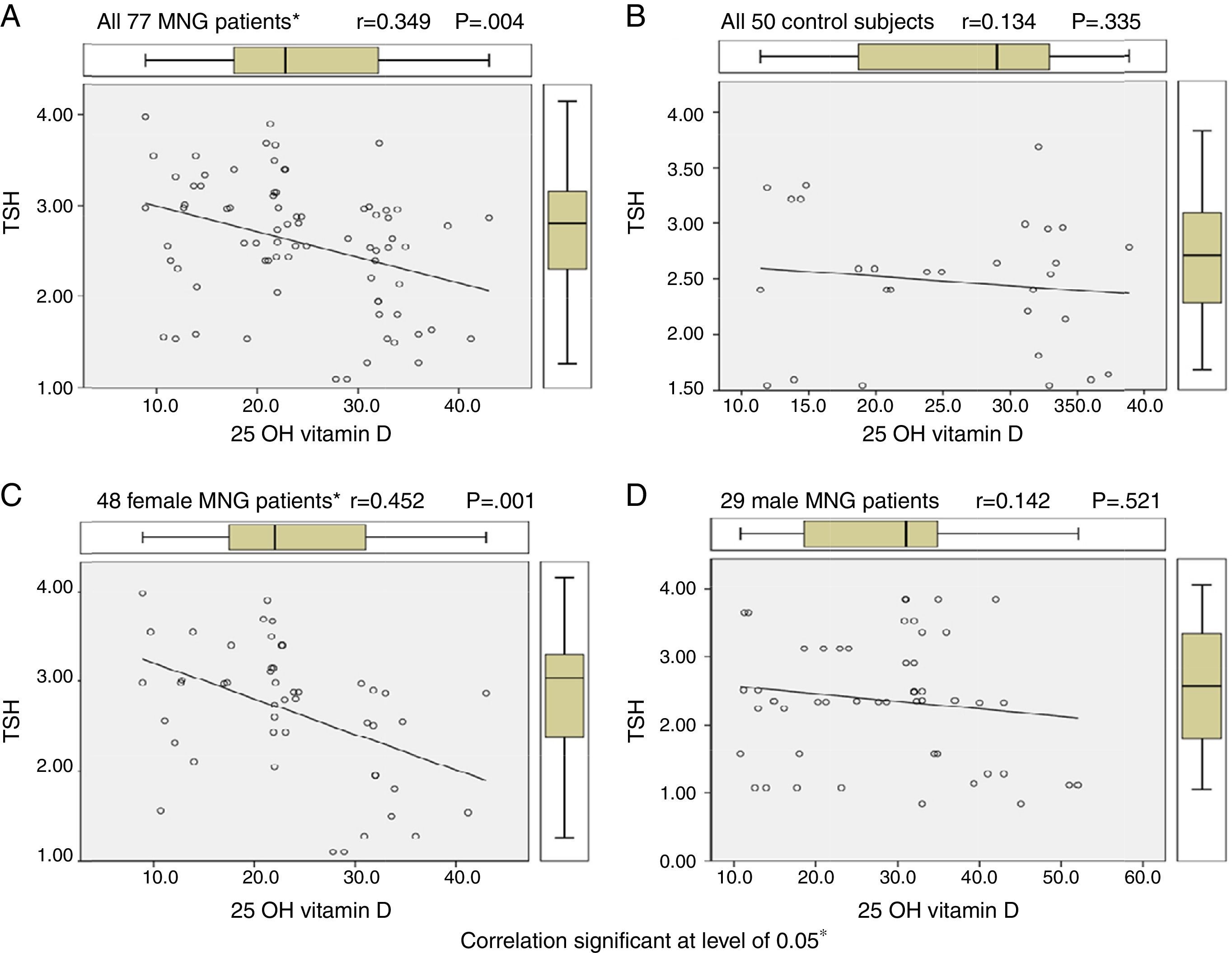
ResultsWe found that patients with euthyroid MNG had statistically significant lower mean of [25(OH)D] (24.21±8.68ng/mL) in comparison with its mean in control subjects (28.37±10.91ng/mL, P value=0.019). The 28 sufficient vitamin D MNG patients had statistically significant lower level of TSH than 49 insufficient vitamin D MNG patients. Vitamin D and TSH levels correlate with vitamin D levels in MNG patients in Pearson correlation. Also 25 OH vitamin D was a significant independent predictor for TSH levels among euthyroid MNG patients in regression analysis.
ConclusionsPatients with euthyroid MNG have lower levels of vitamin D and TSH levels correlate with vitamin D levels in euthyroid MNG patients. In addition, 25 OH vitamin D was a significant independent predictor for TSH levels among euthyroid MNG patients. We recommend hypovitaminosis D evaluation and correction in patients with MNG.
A pesar de su amplia prevalencia en todo el mundo, se sabe poco de la patogénesis del BMN. Es probable que existan interacciones complejas de la predisposición genética y el entorno de los sujetos. No obstante, sigue sin saberse si existe una relación entre el estado de vitamina D y la prevalencia o la patogénesis del BMN eutiroideo. Por ello, el objetivo de este estudio era determinar el estado de vitamina D en el BMN eutiroideo, y explorar la correlación entre las concentraciones de vitamina D y TSH.
MétodosEn este estudio de casos y controles se emparejó por edad, peso e IMC a 77 pacientes diagnosticados BMN eutiroideo y a 50 sujetos sin bocio como grupo de control.
ResultadosLos pacientes con BMN eutiroideo tenían una concentración media de (25[OH]D) (24,21±8,68ng/ml) significativamente inferior a la hallada en los sujetos de control (28,37±10,91ng/ml, valor de p=0,019). Los 28 pacientes con BMN y vitamina D suficiente tenían valores de TSH estadísticamente inferiores a los 49 pacientes con BMN y vitamina D insuficiente. Las concentraciones de vitamina D y de TSH se correlacionan con los valores de vitamina D en los pacientes con BMN en la correlación de Pearson, y la 25 OH vitamina D era un factor predictivo independiente de los valores de TSH en los pacientes con BMN eutiroideo en un análisis de regresión.
ConclusionesLos pacientes con BMN eutiroideo tienen concentraciones más bajas de vitamina D y los valores de TSH se correlacionan con los de vitamina D en esos pacientes. Además, la 25 OH vitamina D era un factor predictivo independiente importante de la concentración de TSH en los pacientes con BMN eutiroideo. Se recomienda la evaluación de la hipovitaminosis D y su corrección en los pacientes con BMN.
Nontoxic goiter is a diffuse or nodular enlargement of the thyroid gland that does not result from an inflammatory or neoplastic process and is not associated with abnormal thyroid function.1
Multinodular non-toxic goiter (MNG) is the most common endocrine condition.2 MNG prevalence varies according many factors, including iodine intake, age, smoking, radiation, body weight, body mass index, etc., while the prevalence of nodular thyroid disease (as well as goiter) inversely correlates with the population's iodine intake. Based on ultrasound investigation, a frequency of thyroid nodular disease as high as 30–40% (in women) and 20–30% (in men) of the adult population has been reported in iodine-deficient areas.3
Goiter, an indicator of iodine deficiency, is a major public health problem for populations living in iodine deficient environment.4
The universal salt ionizations (USI) program was launched in 1996 to prevent iodine deficiency disorders (IDDs). In China, the USI program decrease The overall goiter prevalence was 22.8% prior to 1996 and this was halved (declined to 12.6%).5 Also, similar result by Indian studies prevalence of goiter was 65.9% in an iodine-deficient area, which decreased to 27.7% two decades after the USI program.6
In spite of this success, high prevalence of goiter in India persisted despite iodine sufficiency by the USI program. The cause and effect relationship between iodine deficiency and goiter requires further elucidation.7
It is clear now that although iodine deficiency has a pivotal role in pathogenesis of goiter and nodule formation, it does not fully explain the pathogenesis of MNG. Although the prevalence of MNG is widespread throughout the world, its pathogenesis is poorly understood. A multifactorial etiology based on complex interactions of both genetic predisposition and the individuals’ environment is likely.8
On the other hand, vitamin D is an essential element for bone metabolism and skeletal health, and yet its deficiency can cause rickets in children as well as an increased propensity for osteoporosis. It may also affect extra-skeletal health and risk factor for diabetes mellitus, cancers, multiple sclerosis and other autoimmune diseases, atherosclerosis, and infectious diseases.9
Recently, a lot of studies explore the correlation of thyroid disorder with vitamin D. Tamer et al. 201110 related vitamin D insufficiency in hypothyroidism. Xu et al. 201511 confirmed that low vitamin D status may increase the risk of Graves’ disease. Also Komorowski 201312 revealed that impaired vitamin Metabolism may play an important role in thyroid follicular cell oncogenesis.
In the same manner, recent studies show direct effect of vitamin D on TSH levels. Chailurkit et al. 201313 have reported direct effect of vitamin D status on TSH levels in younger individuals. Also similar result in middle-aged and elderly only.9
However, to the best of our knowledge, it remains unknown whether there is a relationship between vitamin D status and prevalence or pathogenesis of euthyroid.
Therefore, the determination of hypovitaminosis D in patients with & euthyroid MNG deserves more attention. The goal of the present study was determination of vitamin D status in euthyroid MNG as well as exploration of the correlation between vitamin D status and TSH levels. This research will be an initial step for full evaluation role of vitamin D in pathogenesis of euthyroid MNG.
Patients and methodsIn this case control study from June 2013 to May 2016 that was conducted at Endocrinology unit, Mansoura University in Egypt, a total of 77 patients (29 male & 48 female) diagnosed with multiple nodular goiter were studied. We selected a Control group of 50 subjects (18 male & 32 female) who were matched according to age, weight, and body mass index (BMI). All patients and control subjects were from Dakahlia and Damietta governorates coast Mediterranean Sea in north of Nile delta.
All procedures performed in this study were in accordance with ethical standard of Declaration of Helsinki 1964. Informed consent was obtained from all individual participants included in this study.
Inclusion criteria were as follows: age between 18 and 65, both sex, MNG diagnosed by thyroid ultrasound, adequate hepatic and renal function, normal thyroid function. Euthyroidism was defined as the absence of hypothyroidism or hyperthyroidism.
Exclusion criteria were as follows: abnormal thyroid function, positive thyroid autoimmunity (anti TPO or anti thyroglubin AB above normal value), hypocalcaemia or hypercalcemia, patient with autoimmune disease, presence of any endocrine disorders (including diabetes), hepatic (including HCV IgG or HBV s Ag positive) or renal diseases, taking vitamin D supplements or medications that influence vitamin D metabolism or levels. Drugs such as oral contraceptives pills, estrogen, glucocorticoids and iodine therapy were excluded.
Laboratory evaluations were performed including Fasting FT4, FT3, TSH, and serum 25-hydroxyvitamin D [25(OH)D] levels; Thyroid ultrasonography; Anti TPO assay in 33 patients and 28 subject in control group, HCV IgG and HBV s Ag; Routine laboratory assessment (complete blood picture, liver function tests, blood sugar and serum creatinine); and also assessment of serum calcium, phosphorus and albumin. In addition Anti TPO assay were done in 27 patients Antithyroglobulin antibody was done in 13 patients outside our hospital before this study.
25(OH) D serum level was detected using (Calbiotch Inc., Austin Dr, Spring Valley, USA) enzyme-linked immunosorbent assay (ELISA) kit. Vit D status was indicated by the 25(OH) D serum levels. Vitamin D insufficiency and deficiency at levels below was set as (30ng/mL) and (20ng/mL), respectively.
Statistical analysisAll data were analyzed using the SPSS statistical version 22.0. An independent t test was used for comparison of continuous variables. Categorical data were analyzed by the Pearson Chi-square. Linear regression analysis was used to examine the relationship between TSH values with others parameters (which significantly correlated with TSH levels in Pearson correlation) in order to determine the predictors of TSH levels in MNG patients. A probability value of (P) <0.05 was considered statistically significant for all tests.
Results77 MNG patients and 50 age, weight and BMI matched subjects as control group were enrolled in case–control study that was conducted at Department of endocrinology, Mansoura University in Egypt from June 2013 to May 2016. This study included 29 (37.7%) males and 48 (62.3%) females in patient group and 18 (36%) males and 32 (64%) females in control group and they were matched according to age, weight and BMI.
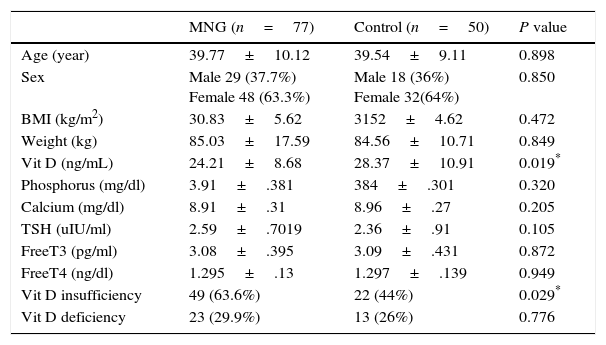
The 77 patients with euthyroid MNG had statistically significant lower level of 25(OH)D levels (24.21±8.68ng/mL) in comparison with its level in 50 control subjects (28.37±10.91ng/mL) (P value=0.019). Non-statistically significant difference had been found in the mean of age, BMI, TSH, Free T3, Free T4, Calcium and Phosphorus. Prevalence of vitamin D insufficiency & deficiency was 63.6% & 29.9% in MNG patients versus 44% & 26% in control subjects as described in Table 1.
Comparison of clinical and laboratory characteristics of patients with euthyroid MNG versus control subjects.
| MNG (n=77) | Control (n=50) | P value | |
|---|---|---|---|
| Age (year) | 39.77±10.12 | 39.54±9.11 | 0.898 |
| Sex | Male 29 (37.7%) Female 48 (63.3%) | Male 18 (36%) Female 32(64%) | 0.850 |
| BMI (kg/m2) | 30.83±5.62 | 3152±4.62 | 0.472 |
| Weight (kg) | 85.03±17.59 | 84.56±10.71 | 0.849 |
| Vit D (ng/mL) | 24.21±8.68 | 28.37±10.91 | 0.019* |
| Phosphorus (mg/dl) | 3.91±.381 | 384±.301 | 0.320 |
| Calcium (mg/dl) | 8.91±.31 | 8.96±.27 | 0.205 |
| TSH (uIU/ml) | 2.59±.7019 | 2.36±.91 | 0.105 |
| FreeT3 (pg/ml) | 3.08±.395 | 3.09±.431 | 0.872 |
| FreeT4 (ng/dl) | 1.295±.13 | 1.297±.139 | 0.949 |
| Vit D insufficiency | 49 (63.6%) | 22 (44%) | 0.029* |
| Vit D deficiency | 23 (29.9%) | 13 (26%) | 0.776 |
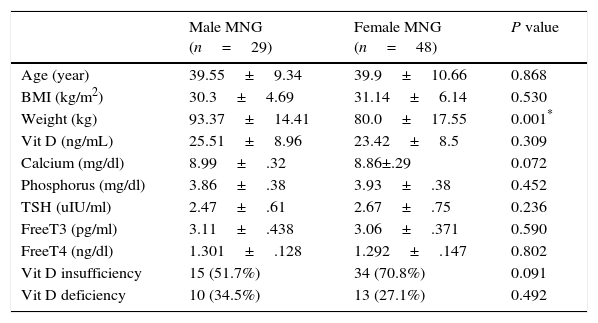
Gender did not affect 25 OH vitamin D levels in MNG patients. 29 euthyroid MNG males had statistically significant higher means of weight only in comparison with its means in 48 female euthyroid MNG patients. But non-statistically significant difference had been found in the mean of age, BMI, TSH, Free, Free T4, calcium, phosphorus and 25 OH vitamin D as described in Table 2.
Sex difference in euthyroid MNG patients.
| Male MNG (n=29) | Female MNG (n=48) | P value | |
|---|---|---|---|
| Age (year) | 39.55±9.34 | 39.9±10.66 | 0.868 |
| BMI (kg/m2) | 30.3±4.69 | 31.14±6.14 | 0.530 |
| Weight (kg) | 93.37±14.41 | 80.0±17.55 | 0.001* |
| Vit D (ng/mL) | 25.51±8.96 | 23.42±8.5 | 0.309 |
| Calcium (mg/dl) | 8.99±.32 | 8.86±.29 | 0.072 |
| Phosphorus (mg/dl) | 3.86±.38 | 3.93±.38 | 0.452 |
| TSH (uIU/ml) | 2.47±.61 | 2.67±.75 | 0.236 |
| FreeT3 (pg/ml) | 3.11±.438 | 3.06±.371 | 0.590 |
| FreeT4 (ng/dl) | 1.301±.128 | 1.292±.147 | 0.802 |
| Vit D insufficiency | 15 (51.7%) | 34 (70.8%) | 0.091 |
| Vit D deficiency | 10 (34.5%) | 13 (27.1%) | 0.492 |
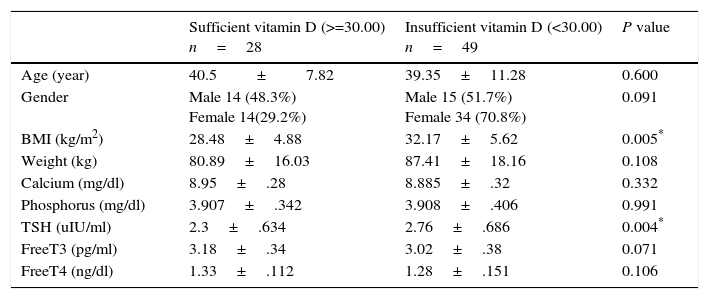
Vitamin D insufficiency associated with higher levels of TSH. The 28 sufficient vitamin D euthyroid MNG patients with (25 OH vitamin D>=30.00) had statistically significant lower level of TSH and BMI (2.38±.69IU/mL & 28.19±4.77kg/m2 respectively) in comparison with its level in 49 insufficient vitamin D euthyroid MNG patients with 25 OH vitamin D<30 (2.79±.67IU/mL & 32.66±5.67kg/m2 respectively) and P value (=0.04 & 0.04 respectively) as described in Table 3.
Comparison of clinical and laboratory characteristics according vitamin D sufficiency in euthyroid MNG patients.
| Sufficient vitamin D (>=30.00) n=28 | Insufficient vitamin D (<30.00) n=49 | P value | |
|---|---|---|---|
| Age (year) | 40.5±7.82 | 39.35±11.28 | 0.600 |
| Gender | Male 14 (48.3%) Female 14(29.2%) | Male 15 (51.7%) Female 34 (70.8%) | 0.091 |
| BMI (kg/m2) | 28.48±4.88 | 32.17±5.62 | 0.005* |
| Weight (kg) | 80.89±16.03 | 87.41±18.16 | 0.108 |
| Calcium (mg/dl) | 8.95±.28 | 8.885±.32 | 0.332 |
| Phosphorus (mg/dl) | 3.907±.342 | 3.908±.406 | 0.991 |
| TSH (uIU/ml) | 2.3±.634 | 2.76±.686 | 0.004* |
| FreeT3 (pg/ml) | 3.18±.34 | 3.02±.38 | 0.071 |
| FreeT4 (ng/dl) | 1.33±.112 | 1.28±.151 | 0.106 |
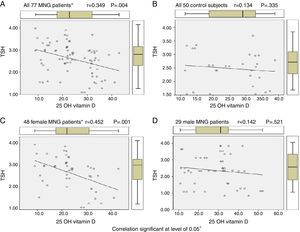
By using Pearson correlation to evaluate correlation between vitamin D levels and TSH levels in euthyroid MNG patients, all 77 MNG patients and 48 female patients show statistically significant correlation (r=0.349 & 0.452, P value 0.004 & 0.001 respectively) while 29 male MNG patients & 50 control subject had no statistically significant correlation as shown in Table 4 and Figure 1.
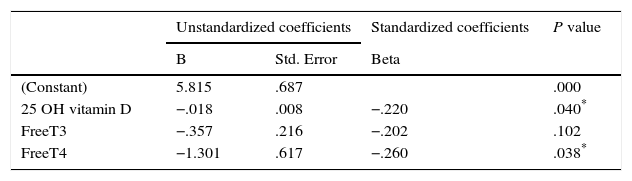
In regression analysis for determination of predictors of TSH levels in euthyroid MNG, we found that 25 OH vitamin D and free T4 were significant independent predictors for TSH levels among euthyroid MNG patients as shown in Table 5.
DiscussionThe role of 25 OH vitamin D in pathogenesis thyroid nodules is unexplored yet; also, relation of 25 OH vitamin D to MNG and TSH levels is still unclear. In the present study, we found that patients with euthyroid MNG had statistically significant lower level of 25 OH vitamin D in comparison with its level in age, weight and BMI matched individual without goiter as control group The previous result denoted that patient with euthyroid MNG had lower level of 25-hydroxyvitamin D [25(OH)D] levels.
Also in this study, we found that the sufficient vitamin D euthyroid MNG patients with 25 OH vitamin D>=30.00 had statistically significant lower level of TSH in comparison with their levels in insufficient vitamin D euthyroid MNG patients with 25 OH vitamin D<30. This result indicated the vitamin D insufficiency setting of TSH at higher levels in MNG. Although there was statistically significant difference in TSH levels but this difference was small; this small difference may be explained by selection of euthyroid patients only and patients with higher TSH than normal excluded from present study. In addition, the role of 25 OH vitamin D in pathogenesis of nodules is not explained only by setting TSH at higher levels but other mechanisms may also be involved.
Also, in this study by using Pearson correlation, we found TSH levels correlate with 25(OH) D levels in MNG patients and female patients while male patients & control subject had no statistically significant correlation. Also, in regression analysis, we found that 25 OH vitamin D and free T4 were significant independent predictors for TSH levels among euthyroid MNG patients. We considered our regression analysis results non-conclusive because of the limited number of participants.
Such results were partially similar to that reported by Zhang et al. 20149 study which reported an association between vitamin D and serum TSH levels in middle-aged and elderly males with higher 25(OH) D levels. They found that higher 25(OH) vitamin D levels were independently associated with lower TSH in males with euthyroidism aged over 40 years. Also Chailurkit et al. 201313 had reported that high vitamin D status in younger individuals is associated with low circulating thyroid-stimulating hormone (TSH), but only in younger (age, 15–44 years) individuals but not for all individuals.
There were differences between our result and other previous 2 population studies: firstly, the previous studies were conducted on large sample population; secondly, the previous studies were conducted on Asian race and ethnicity which was previously reported to be related with TSH levels14; thirdly our study was concerned with patients with multinodular goiter although the previous studies had patients with nodule, but not as this study; and fourthly, autoimmunity was included in previous studies while excluded in our study.
The preceding studies, indicate that TSH secretion is modulated by sex hormones, genetic susceptibility, or environmental factors,9 and our results may indicate relationship between vitamin D status and serum TSH levels.
The pathogenesis of MNG encompasses processes of diffuse follicular hyperplasia, focal nodular proliferation and eventual acquisition of functional automaticity. The development of MNG is a result of long-term exposure of the thyroid gland to proliferative stimuli, such as iodine deficiency, goitrogens and inborn error of thyroid hormone synthesis. All of the above results in insufficient thyroid hormone production and stimulates pituitary secretion of thyroid stimulating hormone (TSH).15
It is clear now that although iodine deficiency has a pivotal role in pathogenesis of goiter and nodule formation but it does not fully explain the pathogenesis of MNG.8 Relation of Vi D to pathogenesis of MNG is still unclear. The question is whether vitamin D is playing a role in pathogenesis of nodule formation? And who?
Our results indicated the vitamin D insufficiency setting of TSH at higher levels in MNG. The previous effect of vitamin D insufficiency may be responsible for promotion of growth of nodules in presence of other goitergens and thus more complications. Also, other previous studies evaluated the role of vitamin D on pituitary gland in mammals. Sar M et al. 198016 suggested for the first time providing evidence that vitamin D has a central effect on the modulation of thyrotropin secretion at the level of the rat pituitary. Also Gelbard, et al. 198017 found specific binding of high-affinity sites of 1 alpha,25-dihydroxyvitamin D(3) suggesting a physiologic role for 25-dihydroxyvitamin D(3) in the pituitary.
Smith et al. 198918 concluded in vivo evidence for an interaction and a possible regulatory role of 1,25 vitamin D on pituitary TSH secretion. They found that exogenous vitamin D administration significantly suppressed pituitary TSH secretion in the basal secretion. All previous studies concluded a role for vitamin D in regulation and modulation of TSH secretion.
The active metabolite of vitamin D has antiproliferative, apoptosis and differentiation inducing as well as immunomodulatory effects.19 In the same manner, few studies evaluated effect of vitamin D on follicular cell and concluded that multiple mechanisms were hypothesized for this effect.
In mammal, Boonsong Ongphiphadhanakul et al. 199220 conclude that 1,25(OH)2D3 has a physiologic role for 1,25(OH)2D3 in the growth and function of thyroid follicular cells. Berg et al. 199121 showed that calcitriol at physiological concentrations inhibits both basal and TSH-stimulated cAMP production in rat thyroid cells which indicates that calcitriol may modulate the effect of TSH on thyroid function and growth Also Berg et al. 199422 had shown vitamin D analog to influence rat thyroid follicular cells by directly inhibiting thyrotropin-stimulated iodide uptake in a dose dependent manner, with inhibition of the TSH stimulated adenylyl cyclase activity and inhibition of cell growth.
Also in humans, Chailurkit et al. 201313 had shown that vitamin D influences thyrocytes directly by attenuating thyrotropin (TSH)-stimulated iodide uptake and cell growth. Clinckspoor et al. 201223 study inquired about the association between vitamin D status and the development of thyroid cancer.
Therefore, from these studies, it is clear that vitamin D affects TSH secretion and action on follicular cell, and directly affects follicular cell growth. Vitamin D modulates cell growth. Anti-apoptotic and prodifferentiating effects of 1,25(OH)2D3 have been described in several tumors types in preclinical models [24].
We hypothesized that vitamin D insufficiency may have a role in nodular formation and promotion of growth by multiple mechanisms firstly by setting TSH at higher level, secondly by absence of attenuating effect of vitamin D on TSH-follicular cell actin, and thirdly by lack of antiproliferative, anti-apoptotic and prodifferentiating effect of vitamin D promoting nodules growth. All above mechanisms promote nodular formation and growth in presence of other goitergens.
Limitation of this study were: firstly, relatively small number of control and patients as we focused on euthyroid MNG patients; secondly, absence of evaluation of thyroid autoimmunity assay in all patients; thirdly, absence of iodine status evaluation but generally WHO classified Egypt as adequate iodine intake country & Optimal iodine nutrition with 148μg/l median value of urinary iodine25; the upper Egypt and new valley region (desert oasis) have higher level of iodine deficiency state, while our patient and control subject were from coastal and north Nile delta governorates. No recent studies evaluated iodine status in our locality but national study estimated Total goiter prevalence (TGP) among children in coastal governorates 6.6% and Lower Egypt (Nile delta governess) ranging from 1.2 to 8.6%. In addition, a recent study in coastal governorate reported median value of urinary iodine to be 150μg/l with TGP 9.9–20%.26
To the best of our knowledge, this study is the first study that discusses relationship between vitamin D levels & euthyroid MNG as well as TSH levels..
In conclusion, patients with euthyroid MNG have lower levels of vitamin D and vitamin D insufficiency setting of TSH at higher levels in MNG. TSH levels correlate with vitamin D status; also, 25 OH vitamin D was a significant independent predictor for TSH levels among euthyroid MNG patients. We recommend vitamin D evaluation in patients with euthyroid MNG and correction of vitamin D insufficiency. Our results will direct attention of endocrinologists and surgeon towards the value of assessment of vitamin D status in thyroid nodules.
Ethical standardsAll procedures performed in this study were in accordance with ethical standards of our institutional in accordance with ethical standard of declaration of Helsinki 1964. Informed consent was obtained from all individual participants included in this study.
Conflict of interestThe authors declare that they have no conflict of interest.
We are grateful to all the patients who consented to be part of this study, and the control subjects who generously approved of getting involved in it. Special thanks go to the medical staff in Mansoura hospital, who spared no effort in providing me with moral and material support.