Insulin overdose with more than 1000 units of glargine is uncommon and prolonged support, beyond the serum half life for glargine is usually required. Glargine is a long-acting insulin with low aqueous solubility at neutral pH.1 Following subcutaneous (s.c.) injection, the solution forms micro-precipitates that slowly release monomers and dimers. Decline in serum glucose begins by 1h. Full activity is apparent by 5h and generally persists 20±4h.2 Reduction in serum glucose may persist longer than serum glargine levels suggest.3 Reports of glargine overdose, including this one, approximate the effect of glargine based on the amount of dextrose needed to maintain euglycemia.5
We report a 12-year-old non-diabetic, 42.5-kg female who attempted suicide with glargine that belonged to a family member. She was treated for depression and self-mutilation but had no prior suicide attempts. On the day of admission, she injected multiple doses of glargine (100units/ml, total=20ml, =2000units) sub-cutaneously in the back of the left arm. One to two hours later, she was found unresponsive and hypoglycemic [blood glucose was 1.4mmol/l] alongside two empty vials of glargine and a used syringe. Intravenous glucose (IV) (7.5g) was given by emergency medical services. Her serum glucose increased [6.3mmol/l], but she remained unresponsive. In the ambulance and emergency room her serum glucose dropped as low as 0.5mmol/l. Physical examination revealed a thin, Caucasian female with old superficial self-inflicted lacerations on her forearm, but no other significant findings. Initial laboratory investigation revealed negative drug and alcohol screens, but hypokalemia (potassium=3mmol/l, [normal: 3.5–5.5mmol/l]) that normalized after addition of potassium (30mequiv./l) to her IV plus 5 bolus injections of potassium (10mEq. each).
Continuous IV glucose (7.64mgglucose/kg/min) was initiated, but multiple boluses of dextrose (10g) were required to maintain blood glucose >3.8mmol/l (total received=15.5mgglucose/kg/min). The glucose infusion was then increased (8.4mgglucose/kg/min) and a general diet (3 meals and 3 snacks) was added. During the next 4 days, IV glucose averaged 11.6mgglucose/kg/min and additional boluses were also required. Due to recurrent nocturnal hypoglycemia, uncooked cornstarch (1.5g/kg/dose as an oral slurry in cold water) was given every 3h beginning on the fourth night. That night she no longer required bolus dextrose, and her IV dextrose was weaned from 25% to 10% and entirely off by 130h.
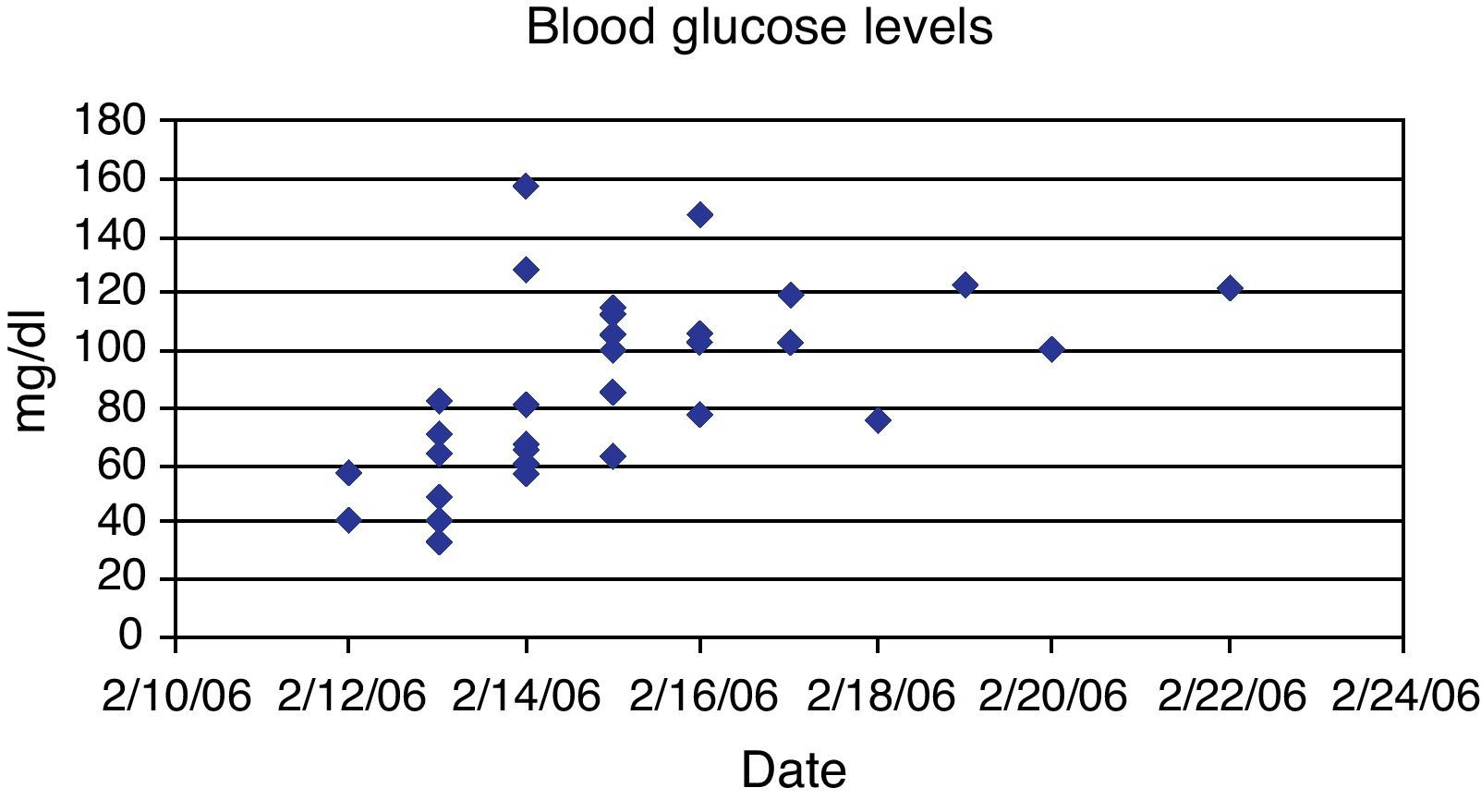
This case provides several insights. First, when injected in large s.c. depot, the biological half-life of glargine appears to be different than predicted from published data based on conventional doses of glargine in patients with diabetes mellitus.3 With conventional dosing, glargine has a relatively flat biological response curve and a time to onset that is slower than NPH insulin.1 There is however, a slightly higher incidence of hypoglycemia in the first 15h after glargine administration. In one case report, the patient was found comatose 4h after the subcutaneous injection of 300 units of glargine and 300 units of aspart. The patient required 20% glucose infusion to normalize blood sugar and had the last episode of hypoglycemia 30h after insulin injection.11 In our case, no other form of insulin was injected, but severe hypoglycemia developed by 1–2h suggesting that large doses of glargine have a rapid onset of action. We also found the duration of action to be longer than predicted. In this case, hypoglycemia recurred over 63h and required IV glucose support for 130h (Fig. 1). Endogenous insulin production, as reflected by serum C-peptide, was suppressed for 3 days and did not fully recover until 10 days. Few cases of insulin glargine overdose have been reported at doses ranging from 26–1000 units.4,6 A previously reported overdose of 1000units of glargine had hypoglycemia lasting for 106h and required IV dextrose for 130h.6 The fact that our patient, despite injecting twice as much glargine, had last documented hypoglycemia 63h after injection as compared to 106h in the previous case. This suggests that either use of higher dextrose concentrations (25% vs. 5–10%) was more effective or use of corn starch may have been beneficial. Cornstarch (1.5g/kg body weight as oral slurry in cold water) was given at bedtime and every 3h overnight (12AM, 3AM, 6AM) beginning 79h after injection and may have helped to prevent hypoglycemia.10
The second finding of interest was the magnitude of parenteral glucose required to sustain this patient. We initiated therapy based on published guidelines for insulin overdose (6–9mgglucose/kg/min)7 but additional hourly bolus infusions of glucose were still required. During the first few days, the patient received an average of 11.6mgglucose/kg/min which is approximately 6-fold greater than the estimated glucose production rate for adults (1.71±0.57mgglucose/kg/min) and near estimates of maximal insulin-mediated glucose disposal (14.9±1.0mgglucose/kg/min).8 This is also similar to reports by Mégarbane et al., in which glucose (15–100g/h) was required to treat very large insulin overdose.9 They found no relationship between the amount of glucose required to sustain euglycemia and the amount of insulin injected suggesting that maximal glucose disposal rates can be achieved with large insulin overdose.9
The final observation from this patient was the response to uncooked cornstarch. Late after the injection, the patient was able to maintain daytime euglycemia with a combination of intravenous glucose and oral intake but developed hypoglycemia overnight. Raw cornstarch has been used successfully to prevent or reduce nocturnal hypoglycemia in patients with glycogenolytic defects but is occasionally associated with an increase in intestinal gas formation.10 Due to the slow hydrolysis and sustained release of glucose from the polymers in uncooked cornstarch, we hypothesized this might help to prevent nocturnal hypoglycemia. Oral cornstarch (1.5g/kg body weight as slurry in cold water) at bedtime and every 3h thereafter was successful in preventing nocturnal hypoglycemia for the last 5 nights of her hospitalization without a need for supplemental intravenous glucose bolus or glucagon.
Other options used in treatment of hypoglycemia include glucagon and octreotide. Glucagon is the primary counter regulatory hormone to insulin. Glucagon administered IV, IM, or subcutaneously is safe, well tolerated and effective in restoring blood glucose levels and consciousness.12–14 The principal function of glucagon is to maintain glucose production through glycogenolysis and gluconeogenesis, mostly mediated via the liver.15 Octreotide is a long-acting analog of somatostatin that suppresses insulin release at a site distal to the sulfonylurea receptor is also used for treatment of hypoglycemia. Rapid infusion of high doses of glucose can result in acute hepatic injury. Earlier excision of an insulin depot could potentially prevent this problem and hasten recovery.16 The hepatic function on our patient were normal. These options were considered if patient were to be unresponsive to cornstarch treatment.
Managing this patient gave us valuable insights into insulin glargine overdose. We found that the onset of action was more rapid than predicted and the duration of action was prolonged. The suggested dextrose infusion rates of 6–9mgglucose/kg/min were inadequate. Oral intake of mixed meals was helpful in preventing hypoglycemia, but oral intake of uncooked cornstarch may have been of additional benefit in management.