Analytical interference in the corticotropin (ACTH) assay is an uncommon event (<1%).1 However, when it occurs, it can interfere with the diagnostic orientation and management of adrenal disease leading to inappropriate clinical decisions, especially when patients show alterations in imaging tests.
Case #1A 79-year-old woman was referred for a right adrenal mass (23×16mm) incidentally discovered in a thoracic-abdominal CT scan performed after trauma. The patient had always had cats. She did not report tachycardia, palpitations, headaches or hyperhidrosis, and did not show any clinical sign of hypercortisolism. Hyperpigmentation was absent.
Hormone analysis showed marked hypercorticotropinemia (ACTH 242pg/ml; normal range, N: 5–46) with serum cortisol (16.1mcg/dl; N: 3.7–19.4), nighttime (23:00) salivary cortisol (0.1mcg/dl; N<0.28)], and 24-h urinary free cortisol (UFC, 40mcg/24h; N: <140) within the normal range. Mineralocorticoid function [aldosterone 4.4ng/dl (N: 3–35.5), plasma renin activity, PRA 1.78ng/ml/h (N: 0.3–7.0)] and medullary adrenal function [24-h urinary metanephrines 168mcg/24h (N: 50–825)] were also normal. A second plasma ACTH determination confirmed hypercorticotropinemia (ACTH 311pg/ml).
The presence of hypercorticotropinemia with normal values of serum, urinary and night salivary cortisol in the absence of clinical adrenal dysfunction forced us to rule out ACTH dependent Cushing syndrome (ACTH-dependent CS) and Addison's disease. We performed a 1-mg dexametasone (23:00h) suppression test (serum cortisol 1.4mcg/dl; N<1.8) and a short ACTH (250mcg iv) stimulation test (serum cortisol at 0, 30, and 60min: 12.8, 21.3, and 23.3mcg/dl). Antiadrenal antibodies were also negative. A normal pituitary MRI and a negative 99mTc-EDDA/HYNIC-TOC scintigraphy ruled out the presence of a silent pituitary corticotropinoma and a neuroendocrine tumor, respectively.
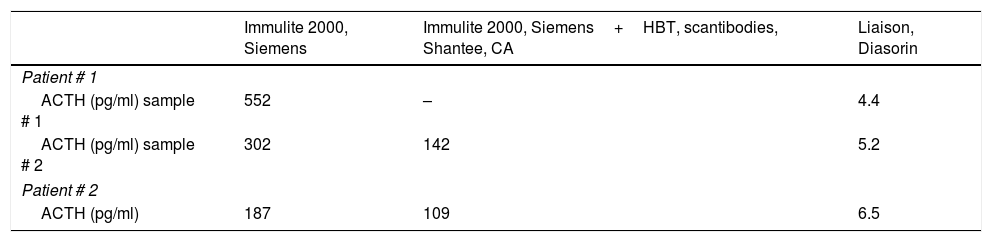
The diagnosis of a nonfunctioning adrenal incidentaloma with hypercorticotropinemia not associated with endogenous hypercortisolism or adrenal insufficiency was established. In suspicion of possible antibody interference we pretreated plasma sample of the patient with a heterophilic antibody blocking tube (HBT, Scantibodies, Shantee, CA) that captures potential heterophilic antibodies before hormone quantification. Basal ACTH after HBT remained still high (142pg/ml). Then ACTH was determined in the same plasma sample with other chemiluminiscent immunometric assay (Liaison, Diasorin) different from the first analyzer used (Immulite 2000, Siemens Healthcare Diagnostics), resulting in the low-normal range (4.4pg/ml) with the first analyzer while it was disproportionately high with the latter (552pg/ml). These results were confirmed using another separate plasma sample (5.2pg/ml and 302pg/ml with Liaison and Immulite 2000, respectively) (Table 1). According with these results, low-normal plasma ACTH concentrations might be associated with a slight autonomous hypersecretion of cortisol by the adenoma.
Plasma ACTH (pg/ml) concentrations in plasma samples of the patients after using different analyzers.
| Immulite 2000, Siemens | Immulite 2000, Siemens+HBT, scantibodies, Shantee, CA | Liaison, Diasorin | |
|---|---|---|---|
| Patient # 1 | |||
| ACTH (pg/ml) sample # 1 | 552 | – | 4.4 |
| ACTH (pg/ml) sample # 2 | 302 | 142 | 5.2 |
| Patient # 2 | |||
| ACTH (pg/ml) | 187 | 109 | 6.5 |
These findings were compatible with an analytical interference in ACTH measurement associated with Immulite 2000 analyzer. With the aim of determining other possible antibody interference we performed a serum protein electrophoresis for quantifying the three major classes of immunoglobulins (IgG, IgA, and IgM), which were normal. Lastly, the quantification of rheumatoid factor (RF) in the patient's serum was clearly high (RF 129IU/ml; N<40), suggesting a possible antibody interference with this immunoglobulin.
Case #2A 71-year-old woman was referred for follow-up of a papillary thyroid carcinoma. She referred no contact with animals. An abdominal CT scan performed for follow-up of her neoplasia detected bilateral adrenal nodules (15mm right and 11mm left).
Initial hormone investigations showed normal urine metanephrines (120mcg/24h), serum aldosterone (13ng/dl), and PRA (2.46ng/ml/h). Her baseline cortisol (15.7mcg/dl) and dehydroepiandrosterone sulfate (199ng/ml) levels were also normal. However, her plasma ACTH level was strikingly high (251pg/ml). A second plasma ACTH determination in the Immulite instrument (Siemens) confirmed hypercorticotropinemia (ACTH 187pg/ml). To complete the hormonal study we carry out the following investigations: urinary free cortisol, 32.5mcg/24h; salivary cortisol at 23:00, 0.13mcg/dl; and serum cortisol after 1mg dexamethasone, 2.10mcg/dl. Plasma ACTH concentration measured on Immulite was 109pg/ml after HBT. In this sample plasma ACTH quantified in the Liaison instrument (Diasorin) was 6.49pg/ml. The patient had normal serum immunoglobulin concentrations, and the RF test was negative (<20U/ml). Results of this patient suggest a slight hypersecretion of cortisol (subclinical hypercortisolism) with low serum ACTH and overnight 1mg dexamethasone suppression test slightly above the normal value (1.8mcg/dl).
ACTH measurement after serial dilutions of plasma samples at 1/5 and 1/10 in both patients revealed a concentration that exceeded the coefficient of variation (10%) of this method, suggesting a possible interference. Moreover, the post-polyethylene glycol recovery resulted in undetectable plasma ACTH levels not only in both patients but also in a control plasma sample.
ACTH analytical interference has been occasionally reported mainly associated to Siemens Immulite analyzers.1–4 Interfering antibodies can be heterophilic antibodies, human anti-animal antibodies (HAAA), and RF.5,6 Analytical interference in the first patient could be attributable to the presence of RF in plasma. However, in the second one the cause remains undetermined.
RF is present in about 5–10% of the general population and in up to 70% of patients with rheumatoid arthritis, although it may precede the disease for many years. In addition, it can appear in a large number of autoimmune systemic diseases, in various infectious diseases, and in many inflammatory conditions and malignancies.7 RF can produce falsely elevated levels when immunometric methods such as enzyme-linked immunosorbent assay (ELISA) or multiplex immunoasays (MIA) are used.5 RF has been associated with several hormonal interferences leading to erroneous high levels of thyrotropin,8,9 free thyroxine,10 gonadotropins (FSH, LH, HCG), and prolactin.9 Recently, a single center study performed in 437 consecutive patients with incidentally discovered adrenal adenomas, a non-suppressed ACTH concentration (>20pg/ml), and a non-suppressed cortisol concentration after 1mg overnight dexamethasone suppression test (>1.8mcg/dl) identified 4 patients with antibody interference in ACTH immunoassay. In one of them RF was responsible for the interference.1
Analytical interference associated with Immulite 2000 ACTH assay might be likely due to the use of two different antibodies for ACTH quantification; one of them, a murine monoclonal anti-ACTH antibody and, the other one, a rabbit polyclonal anti-ACTH antibody that could react with serum RF or other unknown antibodies; however, Liaison ACTH immunoassay uses two monoclonal anti-ACTH antibodies which improve their specificity.
In conclusion, the interference in ACTH immunoassay due to the presence of RF or other autoantibodies in plasma can be unmasked with the use of other analyzers avoiding inappropriate clinical decisions. In the case of an unexpected analytical result a good collaboration between clinical and laboratory professionals is essential to rule out the possibility of any interference in the analytical technique.
Ethical approvalI confirm that we have obtained written informed consent of the patients.
ContributorshipBiochemical analysis: AGC and LJ. Interpretation of data: all authors. Manuscript writing: PI & JJD. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of competing interestsNone.