Menopausal transition is critical for the development of early, subclinical vascular damage. Multiple factors, such as atherosclerosis, increased epicardial fat, and endothelial dysfunction can play a role. Hence, the objective of this study was the comparison of epicardial adipose tissue and carotid intima media thickness in order to establish the best predictor of carotid stiffness in middle-aged women with endothelial dysfunction.
MethodsA total of 43 healthy women aged 40–59 years old with endothelial dysfunction previously demonstrated by flow mediated dilation were recruited to have anthropometric, biochemical, hormonal and ultrasound determinations of carotid intima media thickness and epicardial fat thickness.
ResultsCarotid arterial stiffness parameters (local pulse wave velocity [4.7±0.7 vs 4.8±0.5 vs 5.6±0.5m/s, respectively, p<0.001], pressure strain elastic modulus [55.2±13.4 vs 59.2±11.8 vs 81.9±15.6kPa, respectively, p<0.001], arterial stiffness index β [4.4±1.4 vs 5.0±1.1 vs 6.4±1.3, respectively, p<0.001]) and epicardial fat thickness (2.98±1.4 vs 3.28±1.9 vs 4.70±1.0mm, respectively, p=0.007) showed a significant and proportional increase in the group of late post-menopausal women when compared to early post-menopausal and pre-menopausal groups, respectively. Among body fat markers, epicardial fat was the strongest predictor of local pulse wave velocity, independent of age.
ConclusionsIn menopausal women with endothelial dysfunction, menopausal transition is associated with increased carotid arterial stiffness and epicardial fat thickness, independent of age. Ultrasound measured epicardial fat was a better independent predictor of arterial stiffness than carotid intima media thickness in these women.
La transición menopáusica es crítica para el desarrollo de daño vascular subclínico precoz. Múltiples factores como la aterosclerosis, el aumento del tejido adiposo epicárdico (TAE) y la disfunción endotelial pueden desempeñar un papel en este proceso. El objetivo de este estudio fue comparar la medición del TAE y el espesor íntima media carotídeo (IMC) para establecer el mejor predictor de rigidez carotídea en mujeres de mediana edad con disfunción endotelial.
MétodosSe incluyeron 43 mujeres entre 40-50 años con disfunción endotelial demostrada por dilatación mediada por flujo. Se evaluaron variables antropométricas, bioquímicas, hormonales y se determinó por ultrasonografía el espesor de IMC y TAE.
ResultadosLos parámetros de rigidez arterial carotídea (velocidad de onda del pulso local [4,7±0,7 vs 4,8±0,5 vs 5,6±0,5m/sec, p<0,001], módulo de elasticidad de deformación de presión [55,2±13,4 vs 59,2±11,8 vs 81,9±15,6 Kpa, p<0,001], índice β de rigidez arterial [4,4±1,4 vs 5,0±1,1 vs 6,4±1,3 p<0,001]) y el espesor del TAE (2,98±1,4 vs 3,28±1,9 vs 4,70±1,0mm, p=0,007) mostraron un incremento significativo y proporcional en el grupo de mujeres en posmenopausia tardía comparado con los grupos de posmenopausia temprana y premenopausia respectivamente. Entre los marcadores de adiposidad el TAE fue el mejor predictor de la velocidad de onda del pulso independientemente de la edad.
ConclusionesEn mujeres menopáusicas con disfunción endotelial la transición menopáusica se asoció con un incremento en la rigidez arterial y espesor del TAE, independiente de la edad. El espesor del TAE fue mejor predictor independiente de rigidez arterial que el espesor IMC en estas mujeres.
Menopause is a physiological state in women and part of the natural aging process.1 While women are better protected against atherosclerosis during the fertile period, this effect can change after menopause.2 The sharp increase in coronary artery disease risk during the postmenopausal stage may reflect the cumulative impact of early, often asymptomatic, cardiovascular changes occurring during the menopausal transition, defined as the period between the end of the reproductive stage and the start of postmenopause.3 Recent observations pointed out the relation between the menopausal transition and the speed of progression of atherosclerosis.4
In addition to aging,5 endothelial dysfunction and excessive visceral fat accumulation have been suggested to contribute to subclinical cardiovascular diseases during a rapid menopausal transition. Previous studies showed that ovarian atresia in the early stages of menopause is associated with endothelial dysfunction.6 Healthy middle-aged early postmenopausal women have more endothelial dysfunction than those in perimenopause or in the late postmenopausal stages.7 The need of measureable biomarkers to detect early, asymptomatic vascular damages is therefore compelling. Carotid intima-media thickness (CIMT) has been used to predict an early endothelial dysfunction in menopausal women, although results are quite controversial.8,9 The impact of excessive visceral adiposity on the cardiovascular risk profile in the post-menopause has been evaluated before, whereas its role in the menopausal transition is not well established, yet. Epicardial fat is an emerging marker of visceral organ-specific adiposity that can be easily measured with standard cardiac ultrasound.10 Echocardiographic epicardial fat thickness has been related to subclinical atherosclerosis and metabolic syndrome.11,12 Recently epicardial fat has been associated with higher cardiovascular risk in pre- or post-menopausal women.13–15 Nevertheless, whether epicardial fat may predict an early and asymptomatic endothelial dysfunction during the critical menopausal transition has not been explored before.
Hence, this study was aimed to evaluate whether there is a relation between epicardial fat, subclinical atherosclerosis, and climacteric stages in women with established endothelial dysfunction.
Patients and methodsStudy designWe performed a cross-sectional observational study including 43 healthy women, aged between 40 and 59 years old, with endothelial dysfunction detected by flow-mediated dilation of the brachial artery. Endothelial function was assessed using the protocol described by Celermajer et al.16 by cardiologist blinded of the clinical features of the patients. Endothelial dysfunction was defined as a brachial artery flow-mediated dilation response <5.0%.16
A total of 133 patients were consecutively screened in the climacteric section of the National Institute of Endocrinology. Out of these 133 only 52 women were found to have endothelial dysfunction, and 43 women agreed to participate in the study. These women were then subdivided as peri-menopausal, early postmenopausal (referring to the first five years) and late postmenopausal stage (from five years after menopause up to 64 years old). They were then referred to the Echocardiography Service in “Manuel Fajardo” Hospital to undergo ultrasound assessment of CIMT, arterial stiffness and epicardial fat thickness.
Clinical (age, heart rate, systolic (SBP) and diastolic blood pressure (DBP), climacteric syndrome stages, anthropometrics (waist circumference [WC], body mass index [BMI]), and biochemical variables (fasting glucose, total cholesterol and triglycerides, total estradiol and follicle-stimulating hormone [FSH]), were also collected from these 43 women.
Anthropometric parametersWith the patient previously undressed, waist circumference was measured as the abdominal perimeter at the middle point between the end of the costal arch and the anterior superior iliac crest in the standing position with minimal respiration. Body mass index was calculated as: weight in kg/(size in m)2. Body fat percentage was determined using a Body composition Analyzer (OMROM). The patient's age, sex, weight in kg and size in cm were inputs in the device. Then the patient held it with the hands extended. Body mass percentage was later calculated by means of electric impedance.
Biochemical parametersFasting glucose, total cholesterol and the triglycerides were analyzed with the reagents RapiGluco-Test, Colestest and Monotriglitest (EPB Carlos J. Finlay, Havana, Cuba). Blood glucose was processed in an Eppendorf Glucose Analyzer using the enzymatic and colorimetric method. Triglycerides were processed in a Hitachi 7170A analyzer, made in Tokyo, Japan.
Hormonal determinationsFSH (follicle-stimulating hormone) was measured in a follicular stage in menstruating women with immunoradiometric assay (IRMA). Its normal serum concentration is 0.6–9.5UI/ml in the follicular phase in menstruating women while in postmenopausal women it is 30–135mUI/ml. Estradiol was determined in follicular stage in menstruating women by means of ECLI (Electrochemiluminiscence immunoassay) using a Cobas E411 immunoanalyzer and applying as normal values in follicular phase (46.0–607pmol/l) and in postmenopausal women (18.4–201pmol/l).
Ultrasound parametersCIMTCarotid arteries were imaged using a 7.5-MHz linear-array transducer (Aloka Alfa 10 Mitakashi, Tokio, Japan). A depth of 4cm was used. Imaging was performed with the patient in the supine position, and the head was rotated 30°–45° away from the side and examined and retroflexed by approximately 20°. CIMT of the distal 1cm of the far wall was measured from both the right and left common carotid arteries as the distance from the leading edge of the intima-media to the trailing edge of the adventitia using a semiautomated border detection program (QLAB software).
Arterial stiffnessSubjects were studied after resting supine for 15min in a temperature-controlled environment. Both the left and right common carotid arteries were imaged as previously described. Local pulse wave velocity (LPWV) was determined using a high-definition echo-tracking system, as previously described.17,18
Epicardial fat thicknessEpicardial fat thickness was measured according to the method proposed and validated by Iacobellis et al.19 Epicardial fat was identified as the echo-free space between the outer wall of the myocardium and the visceral layer of pericardium. Epicardial fat thickness was measured in the parasternal long-axis view, perpendicularly on the free wall of the right ventricle at end-systole in three cardiac cycles. Maximum epicardial fat thickness was measured at the point on the free wall of the right ventricle along the midline of the ultrasound beam, perpendicular to the aortic annulus, used as anatomical landmark for this view. The average value of three cardiac cycles was considered.
Statistical analysisContinuous variables are expressed as mean±SD and were compared with the ANOVA test. Pearson's correlation coefficient was used to determine the correlation between ultrasonographic and echocardiographic parameters with estradiol levels. Also, a multiple linear regression model was performed to assess the independent correlation of study parameters with local pulse wave velocity. Statistical analysis was performed using SPSS 21.0 Statistical Software (SPSS, Chicago, IL). A bilateral value of p<0.05 was established to define the statistical significance.
ResultsA total of 43 women, mean age 51.6±41.3 years, were included in the study. They were divided according to their menopausal stage as follows: peri-menopausal (n=11), early postmenopausal (n=16) and late postmenopausal (n=16).
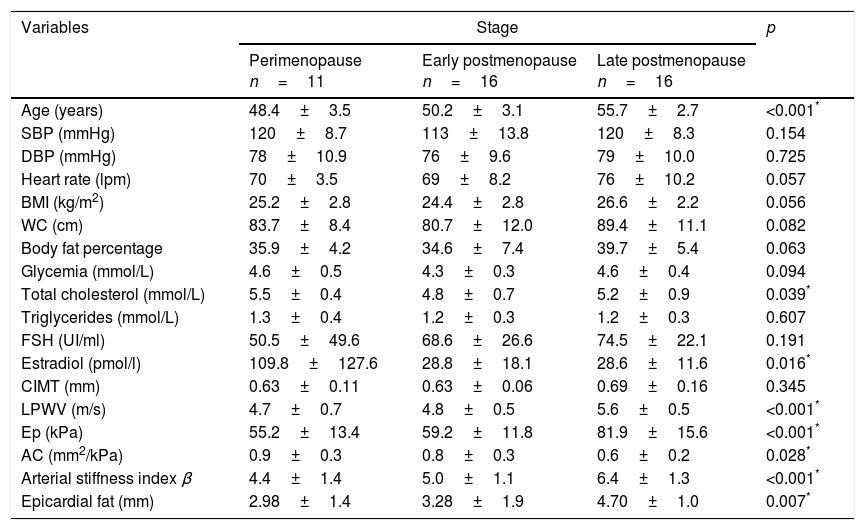
Comparative analysisAge was significantly higher in peri-menopausal women when compared to those in transition to late postmenopause (48.4±3.5 vs 50.2±3.1 vs 55.7±2.7 years, respectively, p<0.001). No significant difference within the other clinical and anthropometric variables was observed. Among the biochemical parameters, total cholesterol was greater in peri-menopausal women, lower during early postmenopause and again slightly greater in late postmenopausal women. The estradiol levels were significantly lower in women with both early and late postmenopause compared to those in the peri-menopausal stage (Table 1).
Distribution of clinical, anthropometric and biochemical variables according to menopausal stage.
| Variables | Stage | p | ||
|---|---|---|---|---|
| Perimenopause n=11 | Early postmenopause n=16 | Late postmenopause n=16 | ||
| Age (years) | 48.4±3.5 | 50.2±3.1 | 55.7±2.7 | <0.001* |
| SBP (mmHg) | 120±8.7 | 113±13.8 | 120±8.3 | 0.154 |
| DBP (mmHg) | 78±10.9 | 76±9.6 | 79±10.0 | 0.725 |
| Heart rate (lpm) | 70±3.5 | 69±8.2 | 76±10.2 | 0.057 |
| BMI (kg/m2) | 25.2±2.8 | 24.4±2.8 | 26.6±2.2 | 0.056 |
| WC (cm) | 83.7±8.4 | 80.7±12.0 | 89.4±11.1 | 0.082 |
| Body fat percentage | 35.9±4.2 | 34.6±7.4 | 39.7±5.4 | 0.063 |
| Glycemia (mmol/L) | 4.6±0.5 | 4.3±0.3 | 4.6±0.4 | 0.094 |
| Total cholesterol (mmol/L) | 5.5±0.4 | 4.8±0.7 | 5.2±0.9 | 0.039* |
| Triglycerides (mmol/L) | 1.3±0.4 | 1.2±0.3 | 1.2±0.3 | 0.607 |
| FSH (UI/ml) | 50.5±49.6 | 68.6±26.6 | 74.5±22.1 | 0.191 |
| Estradiol (pmol/l) | 109.8±127.6 | 28.8±18.1 | 28.6±11.6 | 0.016* |
| CIMT (mm) | 0.63±0.11 | 0.63±0.06 | 0.69±0.16 | 0.345 |
| LPWV (m/s) | 4.7±0.7 | 4.8±0.5 | 5.6±0.5 | <0.001* |
| Ep (kPa) | 55.2±13.4 | 59.2±11.8 | 81.9±15.6 | <0.001* |
| AC (mm2/kPa) | 0.9±0.3 | 0.8±0.3 | 0.6±0.2 | 0.028* |
| Arterial stiffness index β | 4.4±1.4 | 5.0±1.1 | 6.4±1.3 | <0.001* |
| Epicardial fat (mm) | 2.98±1.4 | 3.28±1.9 | 4.70±1.0 | 0.007* |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; WC: waist circumference; FSH: follicle stimulating hormone; CIMT: carotid intima media thickness; LPWV: local pulse wave velocity; Ep: elastance; AC: arterial compliance.
LPWV showed a significant increase from peri-menopausal and early postmenopausal stage to late postmenopause (4.7±0.7 vs 4.8±0.5 vs 5.6±0.5m/s respectively, p<0.001). Similarly, Ep (55.2±13.4 vs 59.2±11.8 vs 81.9±15.6kPa, respectively, p<0.001) and arterial stiffness index β (4.4±1.4 vs 5.0±1.1 vs 6.4±1.3 respectively, p<0.001) increased proportional and significantly through menopausal stages, while AC was significantly reduced (0.9±0.3 vs 0.8±0.3 vs 0.6±0.2mm2/kPa respectively, p=0.028).
Epicardial fat thickness also showed a significant increase through menopausal stages (2.98±1.4 vs 3.28±1.9 vs 4.70±1.0mm respectively, p=0.007) (Table 1).
Correlative analysisSimple regression analysis was performed to assess individual correlation between study parameters. Arterial stiffness index β, Ep, LPWV and CIMT showed significant and negative correlation with estradiol levels.
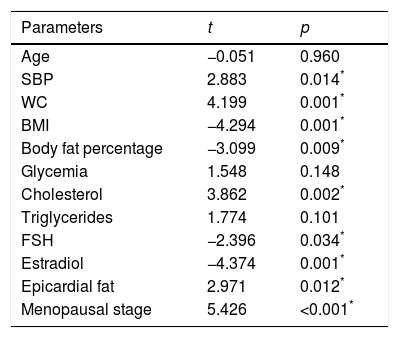
Multiple regression models were performed to assess the independent predictors of LPWV. When all the study parameters were considered, climacteric stage, SBP, BMI, WC, body fat percentage, cholesterol level, estradiol and FSH, as well as epicardial fat thickness, showed a significant association with LPWV (Table 2).
Multiple lineal regression of study parameters and their relation with local pulse wave velocity.
| Parameters | t | p |
|---|---|---|
| Age | −0.051 | 0.960 |
| SBP | 2.883 | 0.014* |
| WC | 4.199 | 0.001* |
| BMI | −4.294 | 0.001* |
| Body fat percentage | −3.099 | 0.009* |
| Glycemia | 1.548 | 0.148 |
| Cholesterol | 3.862 | 0.002* |
| Triglycerides | 1.774 | 0.101 |
| FSH | −2.396 | 0.034* |
| Estradiol | −4.374 | 0.001* |
| Epicardial fat | 2.971 | 0.012* |
| Menopausal stage | 5.426 | <0.001* |
SBP: systolic blood pressure; BMI: body mass index; WC: waist circumference; FSH: follicle stimulating hormone.
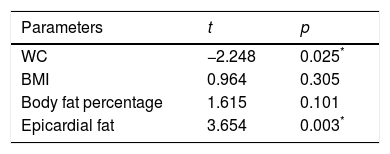
We then run a multiple regression model including only the body fatness markers such as WC, BMI, body fat percentage and epicardial fat thickness, as independent predictors of LPWV. After adjustment for age, epicardial fat thickness was the strongest predictor of LPWV (t=3.654, p=0.003) (Table 3).
DiscussionMenopausal transition seems to be critical for the development of early subclinical vascular damages. The estrogen deficit from peri-menopausal through the postmenopausal stage has been suggested to cause endothelial dysfunction. This predisposes to inflammation, vasoconstriction and an increase in the vascular permeability, which may lead to the development of atherosclerosis.7
In this study we wanted to evaluate the best predictors of that vascular damage in middle aged women with an established endothelial dysfunction. For the first time we found that (1) the transition from early to late postmenopause was associated with an increase in carotid arterial stiffness and epicardial fat thickness and (2) epicardial fat thickness was the best predictor of arterial stiffness during late postmenopause, independently of age.
The study by Casiglia et al.20 which included 1031 women ranging in age from 18 to 95 years, found a significant increase of LPWV on postmenopausal women compared to pre-menopausal women, but that association disappeared after adjusting for age, as confirmed by others.21,22
On the contrary, our study found a significant relation between LPWV and the menopausal stage, independent of age. We also showed (except for AC) a significant correlation between carotid arterial stiffness parameters and estradiol levels. Zaydun et al.23 demonstrated that women who had experienced the menopause at least 6 years previously showed a significant risk of belonging to the highest PWV tertile, independent of age and other atherosclerotic risk factors, an association probably related, at least in part, to estrogen deficiency. The study performed by Nagai et al.24 examined the influence of age and estrogen replacement therapy on common carotid arterial stiffness. The arterial stiffness index was significantly lower on postmenopausal estrogen replacement therapy users compared to postmenopausal non-users, even after adjusting for age. These findings are important if we consider that increase of pulse wave velocity on postmenopausal women is related to a higher probability of stroke, coronary artery disease and death.25
Interestingly, in this study we examined the role of markers of general body fatness (WC, BMI and body fat percentage) and visceral adiposity such as epicardial fat thickness, in predicting LPWV.
We showed that ultrasound-measured epicardial fat increased through menopause stages, although was not significantly correlated with estradiol levels, and independently predicted LPWV, better than other body fat indices.
Epicardial fat thickness constitutes an emerging marker of cardiometabolic risk that has been linked to insulin resistance,26 subclinical atherosclerosis,27 metabolic syndrome and high ApoB/ApoA1 ratio,28 as well as coronary artery disease29 in the Cuban population and has shown a good correlation with abdominal visceral adipose tissue on menopausal women.30
Cakir et al.31 found that epicardial fat thickness was significantly greater in post-surgical menopause women than in those in a physiological menopause, independent of age. The SWAN study showed that late peri-/postmenopausal women have greater (9.88%) epicardial fat compared with pre-/early perimenopausal women independent of age, obesity, and other covariates.
Epicardial fat has been reported before to correlate with arterial stiffness.32,33 Arterial stiffness and endothelial dysfunction represent different aspects of vascular disease. However, there is certainly some crosstalk between these two pathophysiological processes. Albeit this study cannot establish mechanistic causes of the relation between epicardial fat and arterial stiffness, some speculations are tempting. Proinflammatory activity of EAT may affect inflammation in the vascular wall, promoting atherosclerotic changes and increased arterial stiffness. Thus, epicardial fat thickness increase throughout menopausal transition could play an important role in arterial stiffness augmentation. Other possible mechanisms for the effects of epicardial fat on the vasculature may include: (1) reduced catalase expression related to oxidative stress; (2) increased expression of secretory type II phospholipase A2 related to endothelial dysfunction; (3) induced cell-surface expression of adhesion molecules participating in the atherosclerotic process and (4) higher nuclear factor kappa B and c-Jun N-terminal kinase activity related to innate inflammatory response.10
Our findings suggest that echocardiographic determination of epicardial fat could serve as an easy, reliable, noninvasive, and readily available tool to detect early vascular damages during menopausal transition, in comparison to endothelial dysfunction and arterial stiffness studies which are technically challenging and far more time consuming.
In regards to CIMT, similar to Bechlioulis et al.9 and contrary to other reports,8 our study did not find any relation between this parameter and the different menopausal stages, although CIMT had a significant negative correlation with estradiol levels.
ConclusionsIn menopausal women with endothelial dysfunction, late postmenopausal stage is linked to arterial stiffness and epicardial fat thickness increase, independent of age. Among body fatness markers, ultrasound measured epicardial fat thickness resulted as the best predictor of arterial stiffness in these women.
Study limitationsSample size was relatively small, but we accurately selected patients with an established endothelial dysfunction. Statistical power was sufficient to detect significant differences in the study parameters. No cause-effect conclusion can be drawn from our study. This study was not design to examine the superiority of epicardial fat thickness over CIMT as cardiovascular risk marker. We did not include a control group. However, women with different menopausal phases represented internal comparators to each other group in this cross sectional study. Vitamin D levels, previously reported to correlate with epicardial fat in menopausal women,17 were not evaluated in this study.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors state that they have no conflicts of interest.