Cystic pancreatic neoplasms are a heterogeneous group of pathology, and intraductal papillary mucinous neoplasia is becoming more common. The aim of this study is to review our series of cystic pancreatic neoplasms that underwent surgery and to evaluate the similarities with Fukuoka recommendations.
MethodsRetrospective review of our experience analyzing clinical and radiological data, indication for surgery and pathology study of 11 patients operated on in our centre from July 2011 to July 2015, aiming to evaluate the degree of agreement with the current consensus.
ResultsIn our series the majority of cases (7/11) had symptoms at diagnosis. Preoperative diagnosis was achieved in 10 patients using radiology and/or endoscopy. Indications for surgery were the presence of symptoms, radiological data suspicious of malignancy, and secondary branch neoplasia over 30mm. Pathological findings were malignancy in 6/11 cases (2 invasive neoplasia, 4 high grade dysplasia), moderate dysplasia in 2/11, low-grade dysplasia in 2/11 and no dysplasia in one patient.
ConclusionsSurgical indication of intraductal mucinous pancreatic neoplasms depends on the associated symptoms, size, location, risk and suspicion of malignancy.
Las neoplasias quísticas pancreáticas representan un grupo heterogéneo de enfermedades, donde la neoplasia mucinosa papilar intraductal está alcanzando protagonismo. El objetivo del estudio es revisar nuestra serie de neoplasias quísticas pancreáticas intervenidas y valorar la concordancia con las recomendaciones de Fukuoka.
MétodosRevisamos de forma retrospectiva nuestra experiencia analizando los datos clínicos y radiológicos, la indicación quirúrgica y el estudio histológico de los 11 pacientes intervenidos en nuestro centro desde julio de 2011 a julio de 2015 por esta enfermedad, con el objetivo de valorar la concordancia con los consensos actuales.
ResultadosEn nuestra serie la mayoría de los casos (7/11) presentaban síntomas al diagnóstico. El diagnóstico preoperatorio se alcanzó en 10 pacientes mediante radiología y/o ecoendoscopia. Las indicaciones quirúrgicas fueron presencia de síntomas, datos radiológicos de sospecha de malignidad y neoplasia de rama secundaria asintomática mayor a 30mm. Los hallazgos en estudio histológico fueron de malignidad en 6/11 (2 neoplasia invasiva, 4 displasia de alto grado), displasia moderada en 2/11, displasia de bajo grado en 2/11 y ausencia de displasia en un paciente.
ConclusionesLa indicación quirúrgica de las neoplasias mucinosas papilares intraductales de páncreas depende de los síntomas asociados, dimensiones, localización, riesgo y sospecha de malignidad.
Intraductal papillary mucinous neoplasms (IPMN) are included within the group of cystic neoplasms of the pancreas. Described for the first time by Ohashi in 1982,1 their differential diagnosis should include chronic pancreatitis, mucinous cystic tumours and pancreatic duct adenocarcinoma.
Preoperative diagnosis is based on determining the presence, lesion type and risk of malignancy, which is important given its impact on treatment and prognosis. One of the most striking characteristics of these lesions is the sequential progression towards malignancy, so they are considered precursor lesions of pancreatic cancer.
The criteria by Sendai,2 which have been updated with the publication of the criteria by Fukuoka in 2012,3 have raised interest in this disease and established an agreement for its treatment.
We have retrospectively reviewed the IPMN treated at our hospital in recent years with the aim to assess the accordance of treatment with the Fukuoka recommendations.
MethodsThis study included patients with histological diagnosis of IPMN treated at the Hospital Universitario de Getafe (Madrid, Spain) between July 2011 and July 2015.
For 11 patients, we collected data referring to age, sex, and presence of symptoms, preoperative diagnosis, surgical indication and histology of the resected specimen.
The preoperative study was conducted according to the presence or absence of symptoms and study findings, including tumour markers (CEA, CA 19.9), abdominal ultrasound (US), computed tomography, magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), endoscopic ultrasound (EUS) and fine-needle aspiration (FNA) for cytology studies. Radiological findings that were considered suspicious for malignancy included the presence of mural nodules and dilatation of the main pancreatic duct (MPD) of more than 10mm.
Indication for surgery was based on the presence of associated symptoms, lesion size, and the finding of data indicating surgery.
As for the surgical technique, pancreaticoduodenectomy, central pancreatectomy, distal pancreatectomy or total pancreaticoduodenectomy were performed according to the location of the lesion.
After the surgical intervention, the IPMN were classified by anatomical criteria (main branch, secondary branch, or mixed) and histological criteria (intestinal, gastric, pancreatobiliary or oncocytic).
Statistical AnalysisWe conducted a descriptive study of the data, representing the quantitative variables by means and range (minimum–maximum). We did not use percentages because the series had less than 25 cases.
ResultsOut of the 11 patients treated, 8 were males and 3 females, with a mean age of 64.3±8.5 years (range: 52–79).
As for the presence of symptoms, 7 patients presented some type of associated symptom (recurrent pancreatitis in 5, biliary sepsis due to obstructive jaundice in one, abdominal pain in another), while the other 4 were incidental findings.
Elevated CEA levels (>5ng/mL) were found in 2, and elevated CA 19.9 (>37U/mL) in one, which reached levels >700U/mL.
The preoperative study included abdominal ultrasound in one patient, abdominal computed tomography in 7, MRCP in 11 and EUS in 5. The diagnosis was IPMN in 10 cases: 3 in main branches and 7 in secondary branches. In only one case was the FNA cytology study definitive after uncertain radiology results.
The mean radiological size of the lesions studied was 33.7mm (range: 20–55) for the IPMN of the main branch and 26.4mm (range: 18–35mm) for those in secondary branches.
In 3 cases, radiological findings showed evidence that was suspicious for malignancy: presence of a mural node on ultrasound in one case, and dilatation of the MPD>10mm in 2 cases.
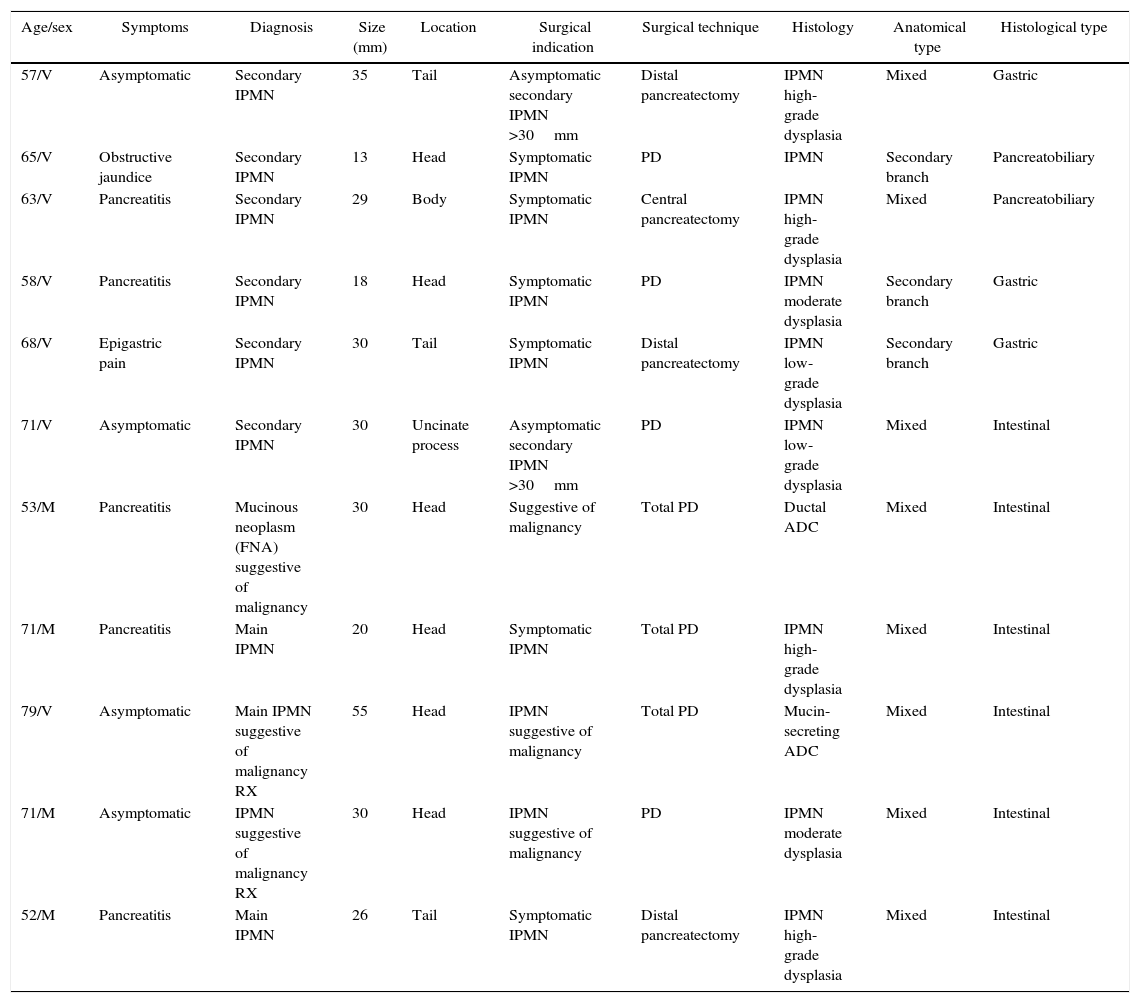
The surgical indications and techniques performed are represented in Table 1, showing that total pancreatectomy was necessary in 3 cases: in one patient due to multifocal disease detected on intraoperative ultrasound, and mucinous discharge during pancreatic resection in 2 cases.
Case Series.
| Age/sex | Symptoms | Diagnosis | Size (mm) | Location | Surgical indication | Surgical technique | Histology | Anatomical type | Histological type |
|---|---|---|---|---|---|---|---|---|---|
| 57/V | Asymptomatic | Secondary IPMN | 35 | Tail | Asymptomatic secondary IPMN >30mm | Distal pancreatectomy | IPMN high-grade dysplasia | Mixed | Gastric |
| 65/V | Obstructive jaundice | Secondary IPMN | 13 | Head | Symptomatic IPMN | PD | IPMN | Secondary branch | Pancreatobiliary |
| 63/V | Pancreatitis | Secondary IPMN | 29 | Body | Symptomatic IPMN | Central pancreatectomy | IPMN high-grade dysplasia | Mixed | Pancreatobiliary |
| 58/V | Pancreatitis | Secondary IPMN | 18 | Head | Symptomatic IPMN | PD | IPMN moderate dysplasia | Secondary branch | Gastric |
| 68/V | Epigastric pain | Secondary IPMN | 30 | Tail | Symptomatic IPMN | Distal pancreatectomy | IPMN low-grade dysplasia | Secondary branch | Gastric |
| 71/V | Asymptomatic | Secondary IPMN | 30 | Uncinate process | Asymptomatic secondary IPMN >30mm | PD | IPMN low-grade dysplasia | Mixed | Intestinal |
| 53/M | Pancreatitis | Mucinous neoplasm (FNA) suggestive of malignancy | 30 | Head | Suggestive of malignancy | Total PD | Ductal ADC | Mixed | Intestinal |
| 71/M | Pancreatitis | Main IPMN | 20 | Head | Symptomatic IPMN | Total PD | IPMN high-grade dysplasia | Mixed | Intestinal |
| 79/V | Asymptomatic | Main IPMN suggestive of malignancy RX | 55 | Head | IPMN suggestive of malignancy | Total PD | Mucin-secreting ADC | Mixed | Intestinal |
| 71/M | Asymptomatic | IPMN suggestive of malignancy RX | 30 | Head | IPMN suggestive of malignancy | PD | IPMN moderate dysplasia | Mixed | Intestinal |
| 52/M | Pancreatitis | Main IPMN | 26 | Tail | Symptomatic IPMN | Distal pancreatectomy | IPMN high-grade dysplasia | Mixed | Intestinal |
After the histological study of the resection specimen, evidence of non-invasive IPMN was seen in 9 patients and invasive cancer in 2. Among the non-invasive neoplasms, we observed high-grade dysplasia/carcinoma in situ (CIS) in 4 out of 9 patients, moderate grade/borderline in 2, low-grade/adenoma in 2 and no evidence of dysplasia in only one patient. Among the invasive neoplasms, evidence of invasive ductal adenocarcinoma (pT1N1) was seen in one patient, and mucin-producing adenocarcinoma (pT3N0) was found in another; both of these cases had previous suspicion of malignancy. Therefore, the presence of malignancy (CIS and invasive neoplasm) was found in 6 out of the 11 patients studied. Multifocal disease was found in 5 patients, 3 of which underwent total pancreatectomy due to intraoperative findings; in the other 2, intraoperative resection margins were negative and there has been no radiological recurrence to date after one and 4 years of follow-up.
DiscussionIPMN represent 1%–3% of exocrine pancreatic neoplasms and 20%–50% of cystic neoplasms of the pancreas, although their actual incidence is not clear because in most cases they are small asymptomatic lesions.4
They received a variety of names until the World Health Organization, in their classification published in 1998,5 defined the term as we currently know it, mainly to differentiate it from mucinous cystic tumours. They are defined as intraductal epithelial neoplasms, characterized by segmental or diffuse dilatation of the pancreatic ducts, comprised by mucin-producing cells, with papillary epithelial proliferation, and associated variable degrees of atypia.4
Fundamentally, associated symptoms include repeated episodes of pancreatitis as the mucin produced by the lesion plugs the MPD or secondary branches. This also leads to dilatation of the duct, as in our series, where 6 out of 11 patients presented pancreatobiliary symptoms.
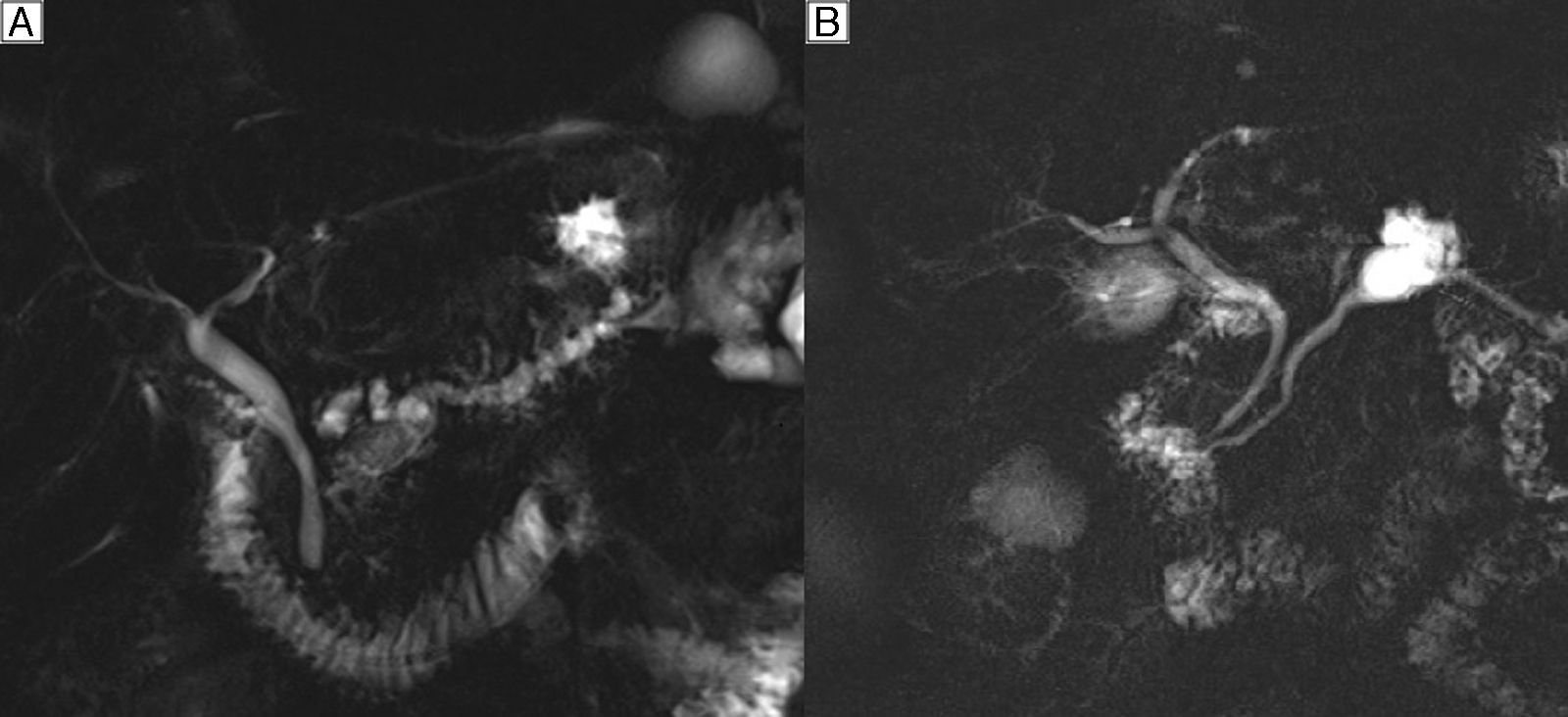
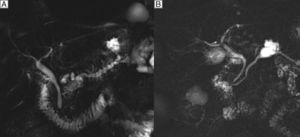
The preoperative diagnosis is based on determining the presence, anatomical type and risk of malignancy. In the IPMN of the main branch (20% in frequency), the diagnostic suspicion is established by the dilatation of the MPD greater than 5mm with no other cause of obstruction (Fig. 1A). In the secondary branches (40% in frequency), diagnostic suspicion is based on a communication with a main duct smaller than or equal to 5mm (Fig. 1B). In the mixed type (40% in frequency), there is involvement of both the MPD as well as the secondary branches.3
Additionally, the risk of malignancy associated with the lesion should be determined. In general, the predictive factors for malignancy and invasive carcinoma in IPMN found in the literature include: the presence of jaundice, lesions in the main branch or mixed lesions, mural nodules and dilatation of the MPD.6 In those of the secondary branches, an additional factor is tumour size greater than or equal to 30mm; it is rare to find malignancy in cases with lesions smaller than 20mm.6
Based on these data, the Fukuoka criteria, published in 2012, defined “high risk signs” as the presence of a solid component with uptake inside the cyst or MPD dilatation greater or equal to 10mm. “Findings for concern” include cyst size greater than or equal to 30mm, thickened cyst walls, mural nodules with no uptake, MPD diameter between 5 and 9mm, and abrupt change in size with distal atrophy or presence of lymphadenopathies.3 They also recommend radiological tests for all pancreatic cysts larger than 10mm in order to assess the existence of these characteristics.
In contrast, the American guidelines published in 20157 do not recommend additional evaluation in the case of cystic lesions smaller than 30mm without dilatation of the MPD or presence of a solid component. MRCP is recommended over other imaging tests because it provides better resolution to identify septa or nodules and the communication with pancreatic ducts.8
CEA and CA 19.9 determinations are recommended, even though CA 19.9 is elevated in less than 20% of non-invasive IPMN.
According to these findings, the management algorithm for IPMN according to the Fukuoka criteria3 include:
- •
Surgical treatment in cases with symptoms, IPMN of the main branch larger than 10mm or IPMN of a secondary branch with “high-risk findings” (obstructive jaundice, high-risk signs on radiology or positive/suspicious cytology).
- •
EUS in cases of secondary branch IPMN with “findings for concern” observed on imaging tests. FNA is conclusive in 40%–50% of cases, without forgetting that the absence of atypia does not exclude the presence of malignancy. In cases of suspicious or positive cytology, MPD involvement or mural nodules on EUS, surgical intervention is recommended.
Thus, an initial MRCP is recommended and, depending on the findings, either surgery or extended studies with EUS are recommended.
In cases of asymptomatic secondary branch IPMN >30mm with no high-risk findings, treatment is controversial. The decision should be individualized, depending on patient age and EUS/FNA findings. Resection should be considered especially in young patients due to the accumulated risk for malignancy (annual malignancy rate 2%–3%).
In our series, the surgical indications followed the recommendations of the 2012 guidelines based on the presence of symptoms and suspected malignancy (MPD dilatation or presence of mural nodes). In the cases of asymptomatic secondary IPMN >30mm, the indication was based on the patient's young age in one case, and, in the other, on the uncertain cytology results.
Radiological follow-up is indicated in cases of asymptomatic IPMN of secondary branches <30mm and main branches smaller than 10mm, including CT/MRI/MRCP every 2 years. In lesions measuring 10–20mm, follow-up would be annual for 2 years, and in 20–30mm lesions EUS should be done after 6 months.3 Meanwhile, other documents recommend MRI after one year, and every 2 years thereafter until a total of 5, if there are no changes.7 If changes in the cyst are detected, EUS is recommended.7
The recommendations made in the guidelines are based on the prognostic implication of the anatomical classification of the IPMN. Those of the main branch present a frequency for malignancy (CIS and invasive neoplasm) of 61.6% (range 36%–100%) and a frequency of invasive carcinoma of 43.1% (range 11%–81%), with 5-year survival rates from 31% to 54%. Meanwhile, those of secondary branches (40%) associate a frequency of malignancy of 25.5% (range 6.3%–46.5%), with invasive neoplasm in 17.7% (range 1.4%–36.7%).3,4 The relatively low frequency of malignancy in the secondary branch lesions would justify the possibility for conservative treatment in many of them, regardless of size.
In our series, the presence of invasive neoplasm was seen in 2 cases of the 11 patients studied, both with lesions suspicious of malignancy in the preoperative period, and in situ/high grade dysplasia in 4 patients, 3 of whom were symptomatic and one indicated due to a size greater than 30mm.
There is still much debate about the appropriate resection types. In main branch IPMN, pancreaticoduodenectomy is the most frequent procedure as most lesions are located in the head of the pancreas/uncinate process, the decision should be made depending on the findings in the resection margins. Meanwhile, in secondary branch lesions the extension of the resection is determined by the tumour location and size,2,3 although this is still a controversial topic. In some cases, non-anatomical partial resections could be performed as long as there are no doubts about the malignancy of the lesion, while in multifocal cases we must consider total pancreatectomy versus resection of the lesion with the highest oncologic risk and follow-up.
Histologically, IPMN are classified according to the type of epithelium that the lesion presents: gastric (70%) is the most frequent in secondary branches, related with the development of duct carcinoma; intestinal (20%) is the most frequent type in the main duct and is related with colloid carcinoma; pancreatobiliary (7%) is related with the development of ductal carcinoma; and, oncocytic (3%) is the least common and least understood.5
Pathologically, the prognostic implications are defined by the presence or absence of malignancy. It is currently thought that the development of invasive carcinoma in the context of IPMN follows an adenoma-carcinoma sequence in approximately 5 years, which would mean an evolution from low-grade dysplasia (adenoma) to moderate dysplasia (borderline) to high-grade dysplasia (carcinoma in situ) to invasive carcinoma.9,10 Invasive carcinoma can be tubular/ductal, present in >50% pancreatobiliary and 10%–30% of gastric types; or, it can be colloid, present in 30%–50% of intestinal types, which has the best prognosis.
Five-year survival rates in invasive IPMN are around 31%–62%, compared to 9%–20% in adenocarcinoma, while taking into account the fact that prognosis is related with tumour size, presence of metastasis, degree of differentiation and perineural or vascular invasion.4
Even though our series of cases is limited in number and it is a retrospective review, there are few cases published in our setting.
Our therapeutic approach coincides with the Fukuoka criteria in terms of the need for surgical intervention in patients with high-risk symptoms or signs, and most patients of our series presented with invasive carcinoma or dysplasia. Furthermore, we also concur with the indication for individualized treatment in secondary-branch IPMN larger than 30mm.
In conclusion, there is current consensus for the diagnostic management and surgical indications of IPMN. However, there is still controversy about what types of surgery should be used and the adequate management in multifocal cases.
Authors’ ContributionStudy design: Alba Manuel Vázquez, Alberto Carabias Hernández and Paloma Sanz Muñoz.
Data collection: Alberto Carabias Hernández, Teresa Carrascosa Mirón, Ainhoa Valle Rubio, Javier Mínguez García, Paloma Sanz Muñoz and Ana Serantes Gómez.
Analysis and interpretation of the results: Alba Manuel Vázquez, Teresa Carrascosa Mirón, Ainhoa Valle Rubio, Javier Mínguez García and Ana Serantes Gómez.
Article composition: Alba Manuel Vázquez.
Critical review and approval of the final version: Alberto Carabias Hernández and José María Jover Navalón.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Manuel Vázquez A, Carabias Hernández A, Carrascosa Mirón T, Valle Rubio A, Mínguez García J, Sanz Muñoz P, et al. ¿Qué hacer ante una neoplasia mucinosa papilar intraductal de páncreas? Nuestra experiencia. Cir Esp. 2016;94:467–472.