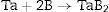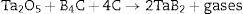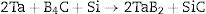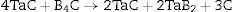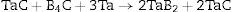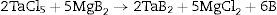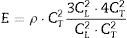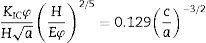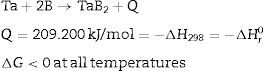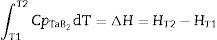TaB2 is a material from the Ultra High Temperature Ceramics group and is rather unexplored because it is difficult to procure the raw materials and to densify TaB2. Using SPS technique to realize reactive sintering processes of powders mixture according to the reaction Ta+2B→TaB2 makes it possible to achieve TaB2 in one technological step. The aim of the study was to determine the influence of heating rates on the synthesis reaction and on the multistage densification mechanisms during SPS processes. The mixture was sintered at constant parameters of 2200°C, 48MPa for 5min with the usage of heating rates from 50°C/min up to 400°C/min. The densification processes were studied through analyzing the shrinkage of powder compacts during SPS (Spark Plasma Sintering) processes. The comparison of the densification curves indicates that the reactions do not proceed completely at slow heating rates. Namely, too low heating rates contribute to the sintering of tantalum before the synthesis reaction and demonstrate the presence of boron in liquid state. The best material obtained in this study has Young's modulus 571GPa, Vickers hardness 20.7GPa (HV1) and indentation fracture toughness KIC 4.7MPam1/2.
El TaB2 es un material perteneciente al grupo de cerámicas de ultra alta temperatura muy poco investigado tanto por la complejidad de encontrar materias primas adecuadas, como por su difícil densificación. Mediante la técnica SPS se consiguen procesos de sinterización reactiva de mezcla de polvos de acuerdo a la reacción Ta+2B→TaB2 que permiten preparar TaB2 en un única etapa. El objetivo de este trabajo fue estudiar la influencia de las velocidades de calentamiento en la reacción de síntesis, así como determinar los mecanismos de densificación durante el proceso. La mezcla fue sinterizada a parámetros constantes de 2200°C, 48MPa durante 5min y a velocidades de calentamiento de 50°C/min a 400°C/min. Los procesos de densificación fueron estudiados mediante análisis dilatométrico de los compactos durante los procesos de SPS (sinterización por corriente eléctrica pulsada). La comparación de las curvas de densificación indica que las reacciónes son incompletas a velocidades de calentamiento bajas. Estas contribuyen a la sinterización del Tántalo antes de la reacción de síntesis y esto demuestra la presencia de boro en estado líquido. El mejor de los materiales preparados posee un valor del modulo de Young de 571GPa, una dureza Vicker de 20.7GPa (HV1) y resistencia a la fractura de 4.7MPam1/2.
The progress in advanced industries requires new, perfect materials. There is a demand for materials able to work at very high temperatures or in particularly aggressive environments. This demand opens many new opportunities and it provides a challenge for scientists to obtain new durable materials. The materials of the future should have a high hardness, resistance to oxidation and to thermal shock, chemical stability, good strength and, what is most important for these applications, it should have a high melting point and Young's modulus. Only several elements and compounds in the world have a melting point of above 3000°C [1]. These are mainly borides, carbides and nitrides of the transition metals from IV, V and VI group of the periodic table (like Ti, V, Cr, Zr, Nb, Mo, Hf, Ta and W). These ceramic materials have the hardness of above 20GPa, the Young's modulus of about 500GPa and additionally metal-like properties (electrical and thermal conductivity). All these characteristics make these materials attractive among the conventional ceramics. Tantalum diboride (TaB2) is a material which is rather unexplored due to the low availability of raw materials and due to problems with its densification [2]. The main properties of this material are: the density of 12.60g/cm3, the melting point at 3200°C and Vickers hardness of above 20GPa [3]. Most ceramic materials, before sintering, must be prepared by the various forming methods such as pressing, powder injection molding, direct coagulation casting and others [4]. A single-phase TaB2 is usually obtained in a few technological stages: pressureless synthesis, milling of the reaction products and finally sintering processes. The reactions can occur directly through the synthesis of the metal with boron (1) or through the reduction of its oxide (2) [3,5].
Due to its properties, tantalum diboride often occurs in other ceramic materials, for example according to the reactions [6–9]:
Zhang et al. [3] obtained a single-phase tantalum diboride material from TaB2 powder using Hot Pressing method. The powder was synthesized by the reduction of Ta2O5 using B4C and graphite (2) in an alumina furnace. The material obtained had the following properties: Young's modulus – 551GPa, Vickers’ hardness – 25.6GPa (HV0.5), flexure strength – 555MPa, and fracture toughness – 4.5MPam1/2.
Musa et al. [10] reported that the consolidation of TaB2 during RSPS (Reactive Spark Plasma Sintering) processes is significantly improved (up to ∼96% of apparent density) when the pressure applied is increased from 20MPa to 60MPa immediately after the synthesis reaction. The mechanical properties of the materials obtained in this study were consistent with the values reported by Zhang.
Licheri et al. [11] described two processes of obtaining monolithic tantalum diboride: (i) from precursors through Self-propagating High-temperature Synthesis followed by SPS (SHS-SPS) and (ii) directly by means of Reactive Spark Plasma Sintering (RSPS). The study showed a dual distribution of mean grain size, along with almost no residual porosity for the materials from RSPS processes and with diffused trapped porosity for TaB2/SHS-SPS which had corrupted the mechanical and oxidation performances.
In the study presented in this paper, the SPS/FAST (Spark Plasma Sintering/Field Assisted Sintering Technique) method was chosen for densification processes. This method was chosen because it enables a precise setting and control of heating rate, pressure and temperature, including high sintering temperatures. SPS/FAST also provides other technological and economical advantages in comparison to conventional sintering methods [12]. These advantages include: lowering the sintering temperature necessary to obtain fully dense materials, shorter holding time, a wide range of heating rates, the elimination of the forming step. Additionally, the application of pressure and pulse electric current which is flowing through the particles in SPS/FAST processes, makes it possible to obtain materials from powders which are difficult to sinter [13]. Moreover, when used for reactive sintering, the SPS/FAST method allows for the synthesis and the sintering to occur during one process. The consolidation of refractory ceramic powders in relatively lower conditions, combined with the elimination/reduction of some technological steps simplifies and shortens the sintering process while lowering the processing costs.
In the Ta-B system there are five boride phases: TaB2, Ta3B4, Ta2B, TaB and Ta3B. Among them TaB2 is stable from room temperature to the melting point. Furthermore, the Ta-B phase diagram shows that tantalum diboride has a wide compositional range of stability-approximately 10at.% of boron [3].
Materials and methodsTantalum and boron powders were used as precursors to Reactive Spark Plasma Sintering processes (Table 1, Figs. 1 and 2). The quantitative and qualitative composition was determined according to the reaction (1) (Ta+2B→TaB2). A high-energy planetary mill with a silicon nitride milling bowl and balls was used in order to homogenize the mixture.
In order to verify the particle size and purity of the precursor powders, DLS (Dynamic Light Scattering) and BET (Brunauer Emmet Teller Adsorption Isotherm) methods were used.
FCT HP D5 furnace was used in order to carry out SPS sintering processes. The materials were sintered in vacuum (50Pa) using a graphite die with the diameter of 20mm. The amount of mixture for each sintering process was fixed at 15g. The minimum pressure to run an SPS process is 10MPa. During the first stage of the performed SPS processes, the value of the pressure applied was gradually increased from 10MPa to 50MPa. This value was reached at temperatures below the measuring range of the pyrometer, and thus below 400°C. The mechanical pressure of 50MPa was then maintained throughout the heating and annealing processes. During the cooling process, the pressure was gradually lowered from 50MPa to 16MPa. Each of the samples was sintered using the same parameters of pressure, temperature of 2200°C and duration of 5min. Variable heating rates of 50°C/min, 100°C/min, 200°C/min, 300°C/min and 400°C/min were used in order to determine the influence of this parameter on the synthesis reaction and on the properties of the obtained materials.
Several characterization methods were used in order to determine the properties of the obtained materials. Phase compositions were analyzed using X-ray diffraction (Panalytical Empyrean diffractometer; PDF4 database). Microstructures were examined using scanning electron microscopy (Jeol 6460LV microscope). Average grain size was measured with a mean linear intercept length technique. Apparent densities of the sintered samples were measured with the Archimedes’ method. The Young's modulus of the samples was measured based on the transmission velocity of ultrasonic waves through the sample, using a Panametrics Epoch III ultrasonic flaw detector, according to the following equation:
where E is the Young's modulus, CL is the velocity of the longitudinal wave, CT is the velocity of the transversal wave, ρ is the density of the material.The velocities of transverse and longitudinal waves were determined as the ratio of sample thickness and the relevant transmission time. Hardness of samples was determined by the Vickers method (at the load of 9.81N) using Future-Tech FLC-50VX hardness tester. Indentation fracture toughness was calculated from the length of cracks which appeared during Vickers indentation test (at the load of 98.1N), using Niihara's equation:
where KIC is the critical stress intensity factor, φ is the constrain factor, H is the Vickers hardness, E is the Young's modulus, a is the half of indent diagonal, c is the length of crack.The Young's modulus (E) and the average Vickers hardness values of both loads (HV1, HV10 for KIC calculations) were each calculated at least five different measurements.
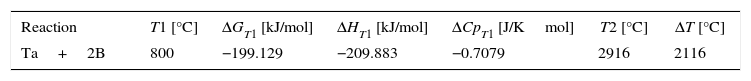
Results and discussionThermodynamic calculationsFor the synthesis reaction of tantalum and boron powders, demonstrated in Eq. (1), thermodynamic calculations were performed using the Thermocalc program version S. For these calculations, the SGTE Substances database version 4 and Barin [14] were used to provide appropriate information. The reaction Ta+2B→TaB2 (1) is highly exothermic and therefore the heat aspect should be taken into account in its Eq. (9). In the case of tantalum diboride, the values of the reaction heat Q, the reaction enthalpy −ΔHr0, and the formation enthalpy −ΔH298 all equal 209.200kJ/mol. Furthermore, free enthalpy (Gibbs energy, G) for this compound is always below zero, which means that the reaction is thermodynamically possible and spontaneous, independent of temperature.
Using the commonly known relationships among free enthalpy, enthalpy, entropy, specific heat of materials, and formula (10), it is possible to calculate the temperature increase which occurs during the combustion reaction of tantalum and boron (9).
where T1 is the reaction temperature; T2 is the system temperature after the reaction involving a change of internal energy (enthalpy); Cp is the specific heat.When the synthesis reaction is initiated at the temperature T1 of 800°C, the calculated reaction temperature increase should be about 2100°C (Table 2). This calculation is correct for a complete synthesis reaction and formation of stoichiometric TaB2 in adiabatic conditions (without heat exchange with the environment).
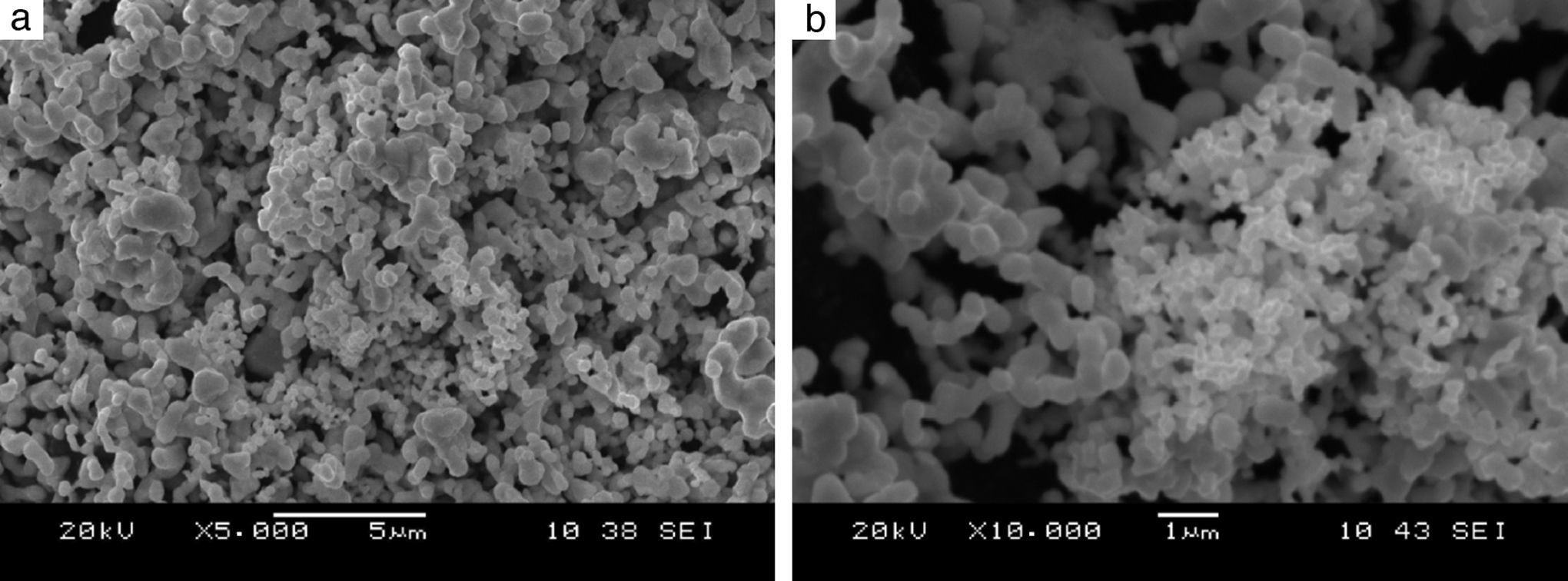
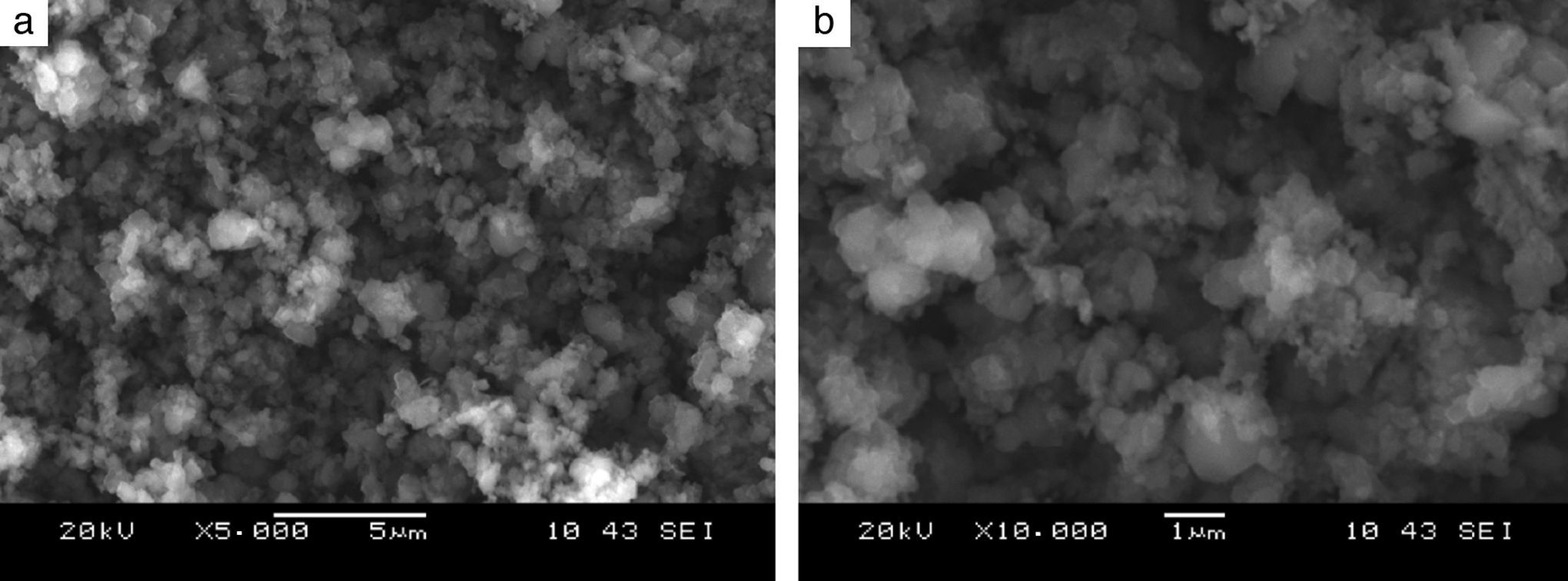
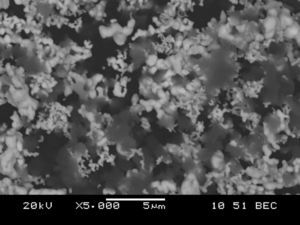
Precursors characteristicsThe morphology of the tantalum powder used as one of the precursors for Reactive Spark Plasma Sintering processes indicates that the powder was produced by the electrolytic method (see Fig. 1). The particles of this powder form agglomerates with the relatively high specific surface area of 1.48m2/g. The particle size of 1–5μm, declared by the powder provider, is, in fact, the size of the agglomerates and not of the particles. In the case of the boron powder, microscopic observations of the powder show that the particles are of a nanometer size (Fig. 2), whereas the powder provider declares the particle size value of 1–2μm. The specific surface area of the boron powder is 10.5m2/g, which is ten times bigger than in the case of fine tantalum powder. Reliable information about the morphology of both powders used can be obtained from examining the distribution of particle size fractions, the value of specific surface area, and the electron images of the precursors. Fig. 3 presents a backscatter electron image of the tantalum and boron mixture, after the milling process. The image shows that the milling process results in a homogeneous distribution of particles from both powders in the mixture. The milling process does not have a very significant influence on the shape, grain size and reduction of the agglomerates (Table 3).
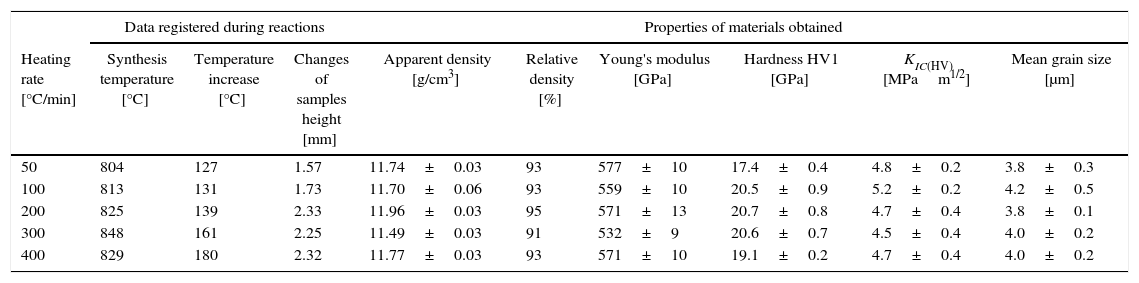
The synthesis reactions of the tantalum and boron powder mixture (9), were initiated in the Reactive Spark Plasma Sintering processes, at the temperature of about 800°C. The energy changes in the sintering system resulted in exothermic combustion reactions; a temperature increase of about 150°C was observed and registered. The values of the temperature increase registered were directly correlated with the heating rate, as presented in Table 4.
Data registered during reactions of Ta+2B mixture; properties of materials obtained at 2200°C during 5min.
| Data registered during reactions | Properties of materials obtained | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Heating rate [°C/min] | Synthesis temperature [°C] | Temperature increase [°C] | Changes of samples height [mm] | Apparent density [g/cm3] | Relative density [%] | Young's modulus [GPa] | Hardness HV1 [GPa] | KIC(HV) [MPam1/2] | Mean grain size [μm] |
| 50 | 804 | 127 | 1.57 | 11.74±0.03 | 93 | 577±10 | 17.4±0.4 | 4.8±0.2 | 3.8±0.3 |
| 100 | 813 | 131 | 1.73 | 11.70±0.06 | 93 | 559±10 | 20.5±0.9 | 5.2±0.2 | 4.2±0.5 |
| 200 | 825 | 139 | 2.33 | 11.96±0.03 | 95 | 571±13 | 20.7±0.8 | 4.7±0.4 | 3.8±0.1 |
| 300 | 848 | 161 | 2.25 | 11.49±0.03 | 91 | 532±9 | 20.6±0.7 | 4.5±0.4 | 4.0±0.2 |
| 400 | 829 | 180 | 2.32 | 11.77±0.03 | 93 | 571±10 | 19.1±0.2 | 4.7±0.4 | 4.0±0.2 |
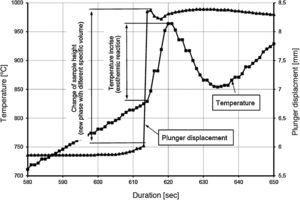
In SPS apparatus, the mixture for sintering is placed in a graphite die, with the sample pressed between two graphite plungers (Fig. 4). The temperatures reached in the sintering processes carried out in this study were too high to enable the use of a thermocouple inside the material. The temperature is measured at the bottom of the top plunger and not inside the sample. Due to these restrictions and due to the fact that in the sintering process heat exchange with the environment takes place, it is not possible to directly compare the values of temperature increase registered during real-life sintering processes with the values obtained from thermodynamic calculations. However, the values obtained by both these means prove the high exothermic character of this synthesis reaction.
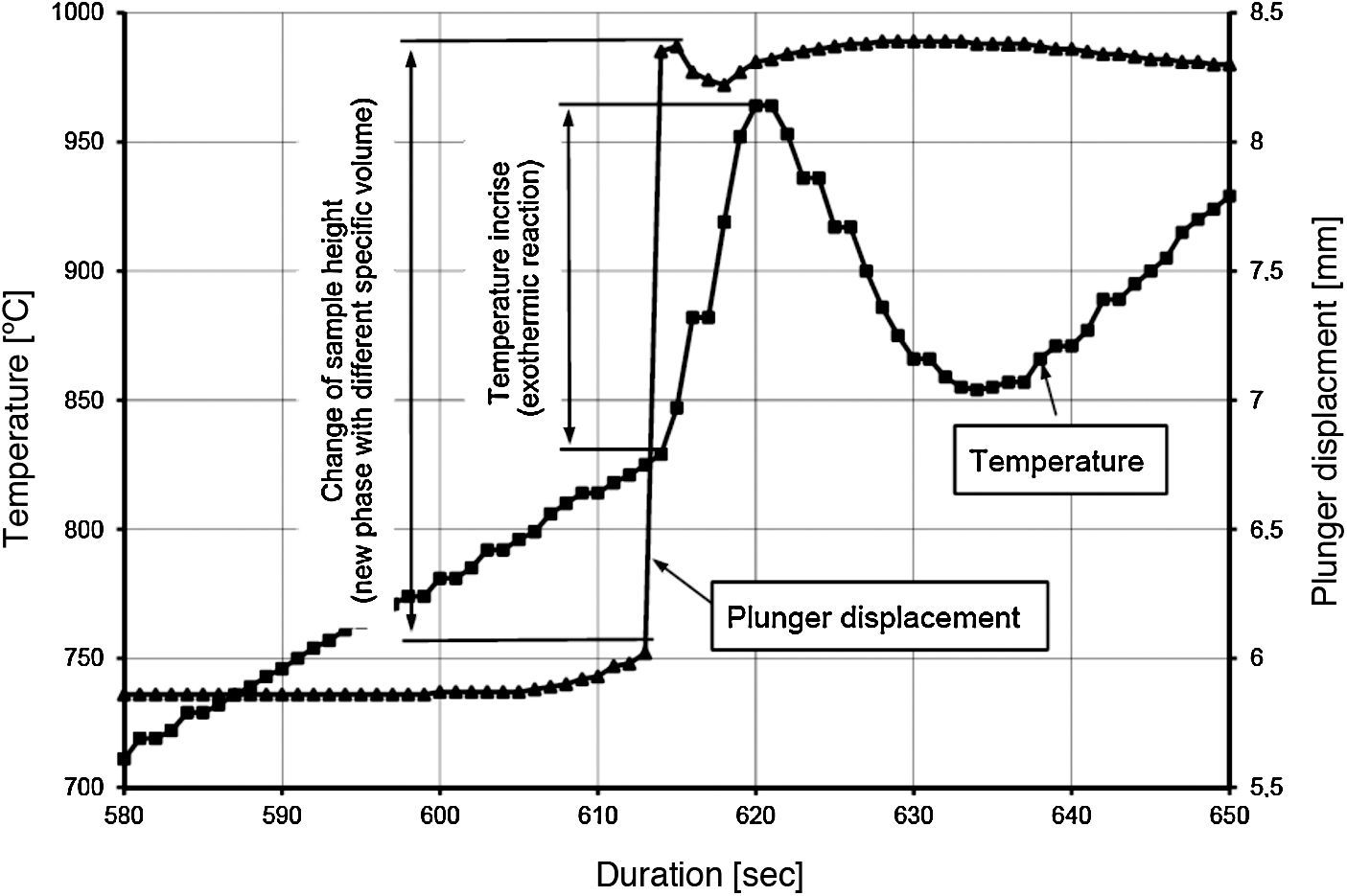
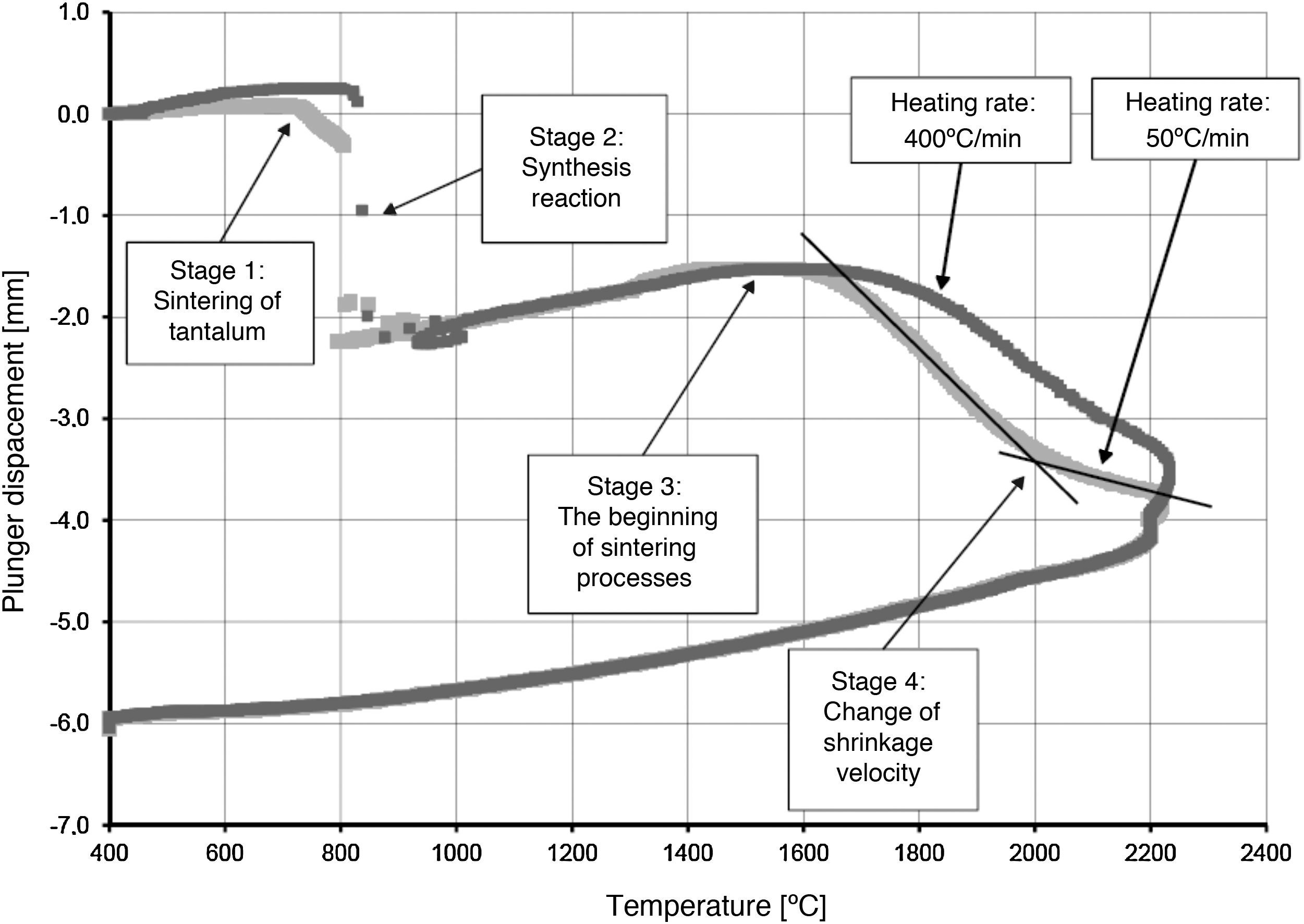
Temperature changes and plunger displacement were measured throughout the sintering processes in order to observe the synthesis reactions and the densification mechanisms. Rapid plunger displacement at the time of the reaction was well visible and determined (Table 4). Changes in the height of the samples were different for each heating rate applied; the range of the changes was from 1.57 to 2.33mm. These changes demonstrate that each synthesis reaction resulted in the formation of a new phase, whose volume differed from the volume of the initial mixture. The thermal expansion of the graphite components (i.e. the plungers and the die) has an influence on the registered values of sample height change. For all the samples, the sintering temperature was 2200°C; the duration of the annealing was 5min. Therefore, the participation of thermal expansion of the graphite components is the same in all cases, allowing to compare the changes in the height within a given sample as well as between the samples. The detailed values registered during the reactions (such as synthesis temperature, temperature increase and changes in the height of the samples) are presented in Table 4. The temperature increase and the plunger displacement that occurred for a sample heated at the rate of 200°C/min is presented in Figs. 5 and 6.
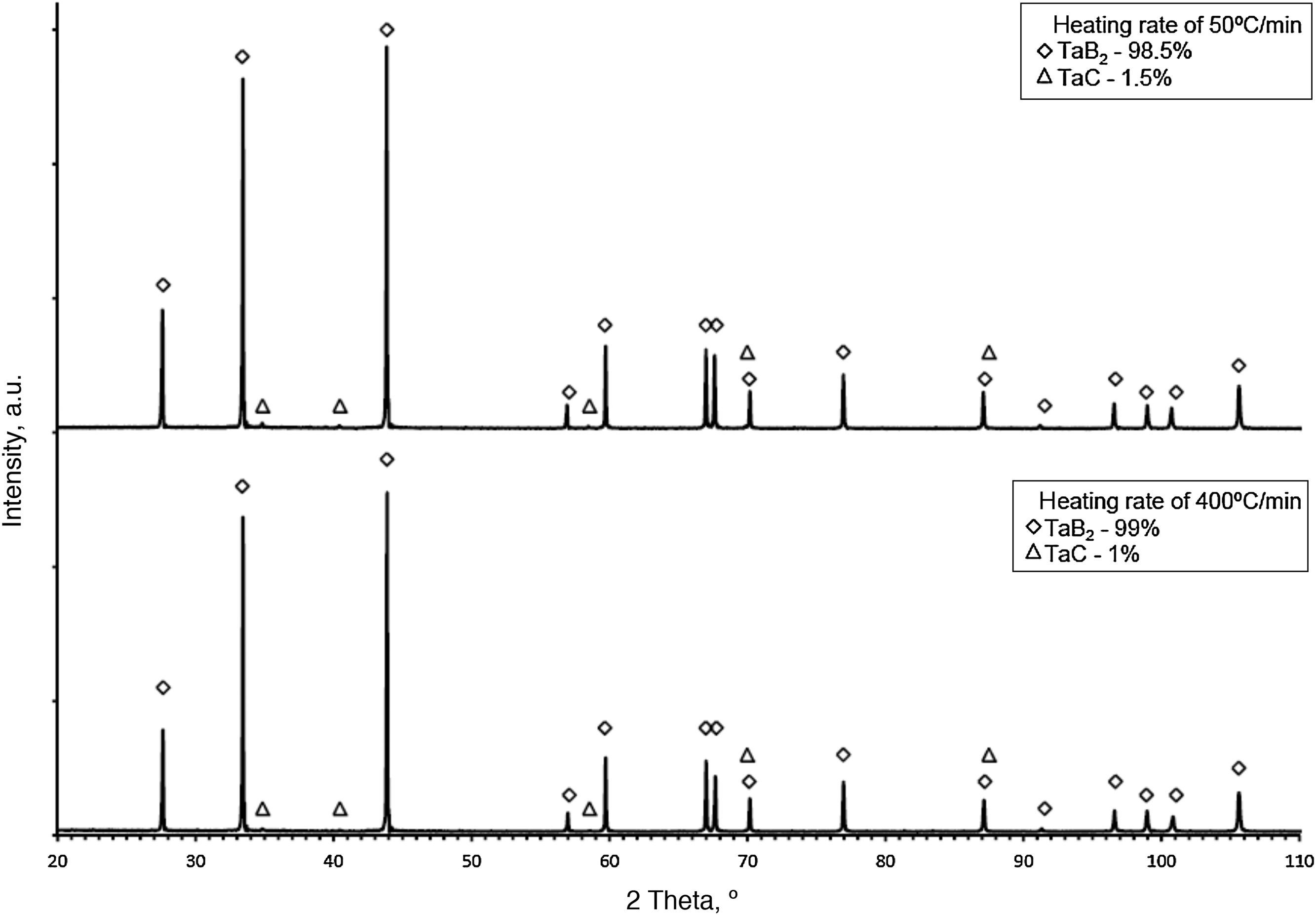
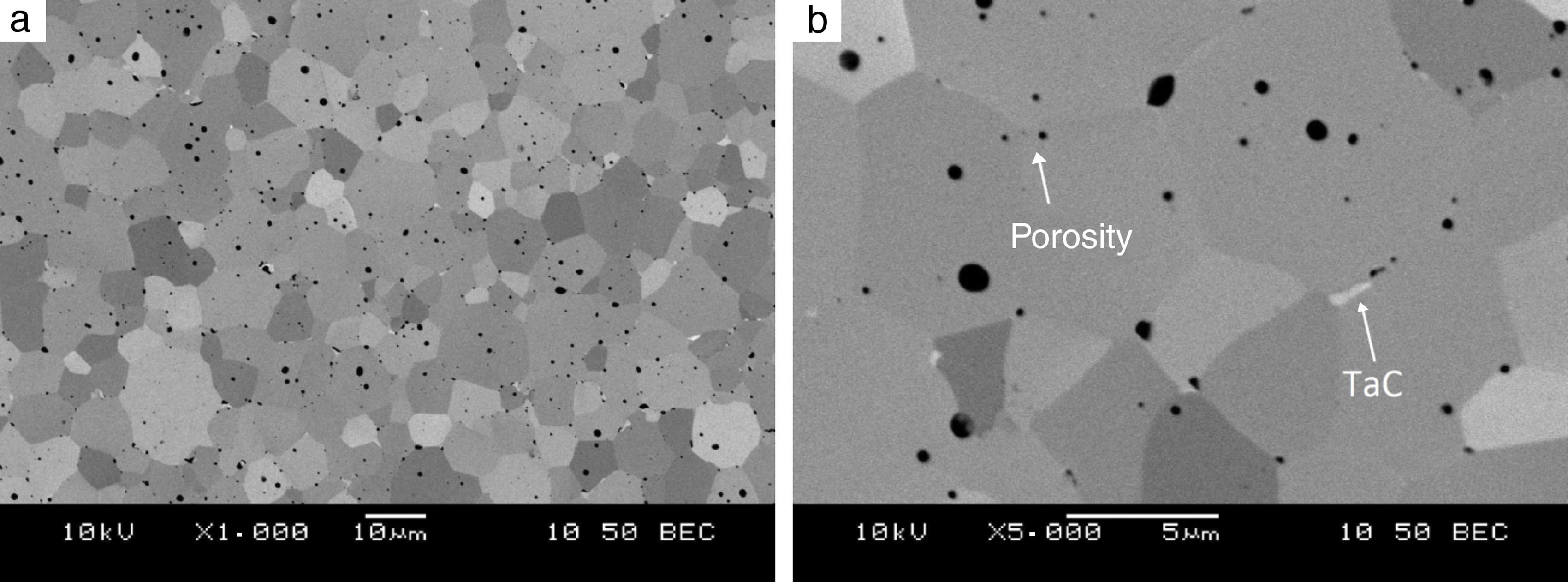
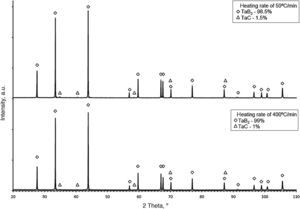
The synthesis reactions were confirmed by phase composition analyses of materials sintered at various heating rates. In each case, the formation of tantalum diboride took place. Apart from TaB2, tantalum carbide (TaC) in small amounts was observed on the XRD patterns (up to 2%). TaC was formed due to the proximity of the sintered material to the graphite die and plungers. Residual porosity and traces of TaC (small bright areas in Fig. 7b) and are visible on the microstructure. The differences in color of tantalum diboride grains (Fig. 7a) result from the crystallographic orientation of the grains. In the study, the grain size of the materials obtained using reactive SPS processes did not depend on the heating rate applied. The grain size of the materials was determined by the temperature and the duration of the sintering process. For sintering processes in the temperature of 2200°C during 5min, the grain size of the material obtained was in the range of 3.8–4.2μm.
An important aspect of the synthesis reaction during the SPS process is the heating rate. Depending on the heating rates used (from 50°C/min to 400°C/min), different values of temperature increase and different changes in samples height during the combustion reaction were recorded (Table 4).
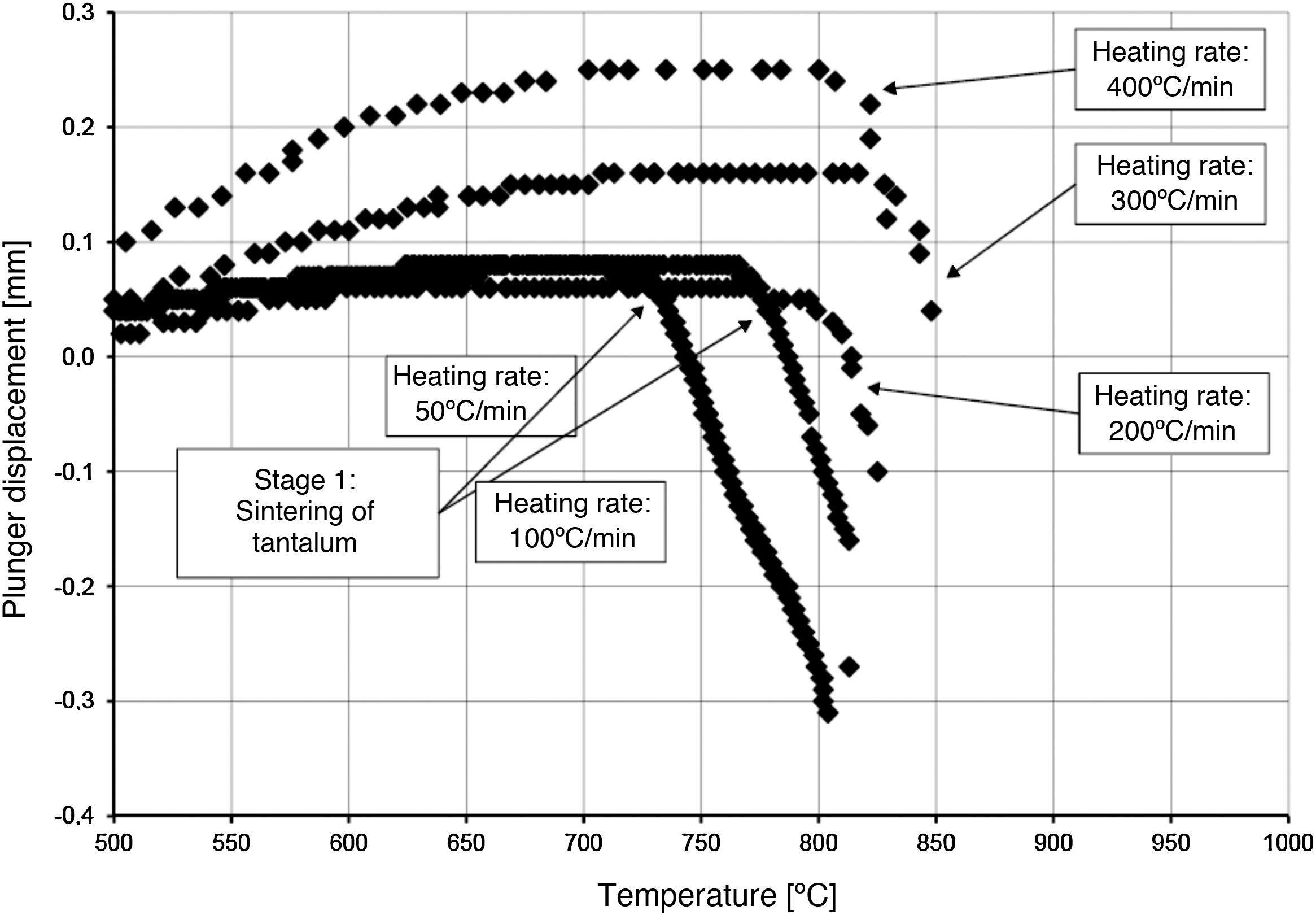
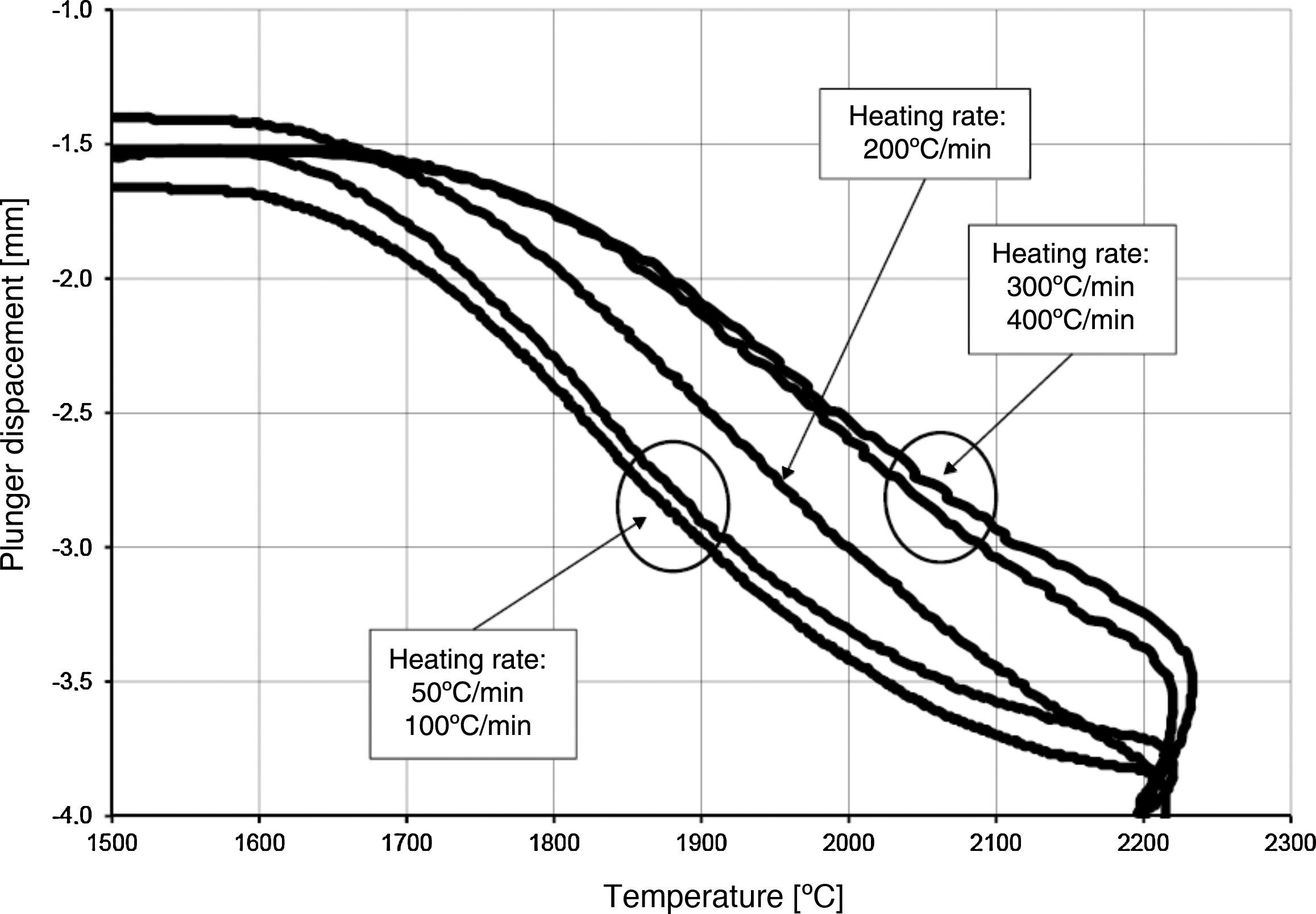
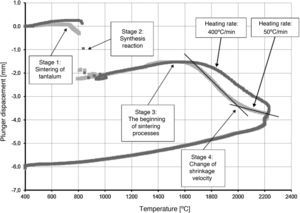
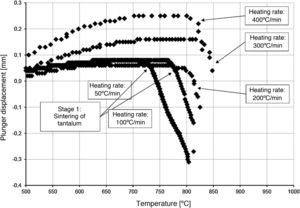
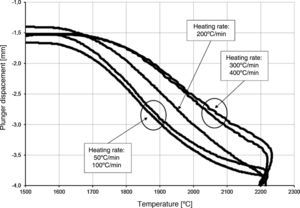
The densification process was studied by analysing the shrinkage of the powder compacts. A few distinct stages of densification were determined. The change of samples height during the entire SPS process, for marginal values of heating rates (50°C/min and 400°C/min) is presented in Fig. 8. The compaction graph indicates that the synthesis reaction of the material heated at the rate of 50°C/min has not proceeded completely. The comparison of the densification curves for each heating rate (Fig. 9) shows that the sintering of tantalum begins before synthesis reaction, when too low heating rates are applied. The sintering process of tantalum can be concluded based on the well visible change of the densification rate in temperature of about 700°C, that is immediately before synthesis reaction. This change of the densification rate was observed for the heating rates of 50°C/min and 100°C/min (Fig. 9). With further temperature increase during the heating process, in stage 2 (Fig. 8) combustion synthesis reaction with a rapid change of sample volume was observed. The initiating temperature of the combustion reaction in SPS processes was about 800°C for the precursors used: electrolytic tantalum powder with the specific surface area of 1.48m2/g and nanometer grain size boron powder with the specific surface area of 10.50m2/g. The sintering shrinkage for all the materials studied began at the temperature of about 1500°C (Fig. 8, stage 3).
The differences in the slope degree of the densification curves, depending on the heating rates applied, can be observed also in the temperatures of above 2000°C. Fig. 10 presents densification curves for all the heating rates applied in this study, in the temperature range from 1500°C to 2200°C. As a result of further heating at heating rates of 50°C/min and 100°C/min, melting of boron occurred (melting point at 2076°C) and the sintering process continued with the liquid boron phase (Figs. 8 and 10, stage 4). The presence of liquid boron after the two synthesis reactions means that the reactions were not completely processed. This phenomenon is caused by the fact that during the densification process the sintering of tantalum occurs in these heating rates (Fig. 9). This is confirmed by a smaller change in the samples height during the synthesis reaction: 1.53mm for 50°C/min, 1.73mm for 100°C/min and above 2.2mm for the heating rates of 200°C/min, 300°C/min and 400°C/min. Further heating can cause the diffusion of boron to TaB2. As the result, the content of boron in TaB2 may vary in the range of 61–72at.% B [3], according to the Ta-B phase diagram.
Sintering at the temperature of 2200°C during 5min results in sinters with the relative density of 91–95% and Young's modulus of about 570GPa. The average Vickers hardness (HV1) of materials heated at the rates from 100°C/min to 400°C/min was above 20GPa. Only the material heated at the rate of 50°C/min had the hardness of 17.4GPa. This could have been caused by the presence of a small amount of precursors (below the detection level of the XRD method) in the material after the sintering processes. The indentation fracture toughness of all the materials studied was about 4.8MPam1/2, and it did not differ significantly when taking into account the measurement uncertainty.
The main properties of the materials obtained are similar to those found in the literature. Zhang et al. [3] reported that tantalum diboride material obtained using Hot Pressing method is characterized by a relative density of about 97–98%, but these values were received using image analysis software. The TaB2 materials have the Young's modulus of 551GPa, the Vickers hardness of 25.6GPa (HV0.5), and the fracture toughness of 4.5MPam1/2. The difference between Vickers hardness values obtained in this study and those reported by Zhang can be caused by the load applied during measurements (HV0.5 and HV1 respectively). Musa et al. [10] densified TaB2 to ∼96% relative density through the application of two-stage pressure conditions during Reactive Spark Plasma Sintering (first 20MPa and then, immediately after the occurrence of the synthesis reaction, 60MPa). There is no data about the values of Young's modulus in the in Musa's paper. Licheri et al. [11] reported that tantalum diboride materials obtained through SHS-SPS and those obtained through RSPS are characterized by the relative density of 94–95%, the Vickers hardness of 17.5–18.4GPa and the fracture toughness of 3.2–4.8MPa·m1/2.
ConclusionsThe SPS heating of tantalum and boron mixture prepared according to the reaction (1) Ta+2B leads to the formation of tantalum diboride. Carrying out the sintering processes in graphite components causes the formation of tantalum carbide traces (up to 2%).
The heating rate influences the progress of the synthesis reaction. Depending on the heating rates applied, different values of initiation temperature, temperature increase and change of samples height were recorded.
The sintering temperature and the duration of annealing at a maximum temperature determines the phase composition and properties of the obtained materials. All the materials were sintered at the temperature of 2200°C during 5min, which is above the melting point of boron. XRD analysis of the sintered materials demonstrated the presence of stoichiometric tantalum diboride. The densification curves indicate that TaB2 could be formed in two ways: (i) as the result of the synthesis reaction (for the heating rates of 200°C/min, 300°C/min, 400°C/min) and (ii) in two steps: by synthesis reaction followed by the diffusion of boron (for the heating rates of 50°C/min and 100°C/min).
The materials obtained through Reactive Spark Plasma Sintering processes were characterized by the relative density of 91–95% and the Young's modulus of 530GPa up to 580GPa. The Vickers hardness of the best materials was above 20GPa.
This study was supported by the statutory activity of The Institute of Advanced Manufacturing Technology, Cracow, Poland.