Mesoporous TiO2 films doped with Ca2+, W6+ and nitrogen were obtained by sol-gel method using dip-coating procedure onto glass-slides in order to study the influence of dopants in their textural properties and photocatalytic activity. Titania sols were synthesized with and without dopants using titanium isopropoxide as titanium precursor, two complexing agents, acetic acid and acetyl acetone, and two pore generating agents, Pluronic F-127 (F127) and polyethylene glycol hexadecyl ether (Brij58). Films were characterised by Fourier Transform Infrared Spectroscopy (FTIR), Grazing X-ray diffraction (GXRD) and Transmission Electron Microscopy (TEM). Environmental Ellipsometric Porosimetry (EEP) permitted to obtain the adsorption/desorption isotherms and total pore volume, and to determine the porous size distribution and specific surface area (Ss) of the films. The photocatalytic activity was evaluated through the degradation of methyl orange (MO) in aqueous solution under UV light exposure. The photocatalytic activity depends on the nature of dopants, which affect the TiO2-anatase crystallite size and the textural properties of the final material. The best results of MO degradation were obtained for the films doped with Ca2+, this being correlated with the pore size and specific surface area of the films besides the dopant-effect on the photocatalytic mechanisms.
Se prepararon recubrimientos de TiO2 mesoporosos dopados con Ca2+, W6+ y nitrógeno por el método sol-gel sobre porta objetos de vidrio utilizando la técnica de inmersión, con objeto de estudiar la influencia de los dopantes en las propiedades texturales y fotocatalíticas. Se prepararon soles de titania con y sin dopantes utilizando isopropóxido de titanio como precursor de titanio, dos agentes complejantes, ácido acético y acetona acetilo, y dos surfactantes, Pluronic F-127 (F127) y éter hexadecil polietilenglicol (Brij58). Los recubrimientos se caracterizan por Espectroscopia Infrarroja de Transformada de Fourier (FTIR), Difracción de Rayos X a bajo ángulo (GXRD) y Microscopía Electrónica de Transmisión (MET). Mediante estudios de Elipsometría Espectral Porosimetrica (EEP) se obtuvieron las isotermas de adsorción/desorción y el volumen total de poro, junto con la distribución del tamaño de poro y la superficie específica (Ss) de los recubrimientos. Los estudios de actividad fotocatalítica se realizaron a través de la descomposición de naranja de metilo bajo irradiación con luz UV/vis. La actividad fotocatalítica depende de la naturaleza del dopante, el cual afecta al tamaño de cristal de TiO2 anatasa y a las propiedades texturales del material final. Los mejores resultados de la degradación de MO se obtuvieron para los recubrimientos dopadas con Ca2+, que corresponde con un adecuado tamaño de poro y superficie específica, además de las características específicas del dopante.
Titanium dioxide in its anatase phase is widely used as a semiconductor photo-catalyst because of its long-term stability, no toxicity and good photocatalytic activity.1 For an effective photoexcitation of TiO2 semiconductor it is necessary the illumination with light with energy higher than the titania-anatase band gap (Eband-gap), around 3.2 eV; therefore the absorption threshold corresponds to 380nm. Consequently, only the ultraviolet fraction of the solar irradiation, about 5%, is active in the photoexcitation processes.2 On the other hand, the recombination of photogenerated electron-hole pairs results in low photoquantum efficiency.3
In recent years, the doping of TiO2 with transition metal ions (Cr, V, Fe, W, etc.)4 and non-metallic elements (N, F, C, S, B, etc.) has been extensively investigated with the aim of increasing the photocatalytic activity under visible light and full solar light irradiation by introducing additional energy levels in the band gap of TiO2. Choi et al.5 carried out a systematic investigation of the photocatalytic efficiency of TiO2 doped with 21 different metal ions. They report that the efficiency of a dopant depends on different parameters such as the concentration and distribution of the dopant in the matrix of TiO2, the creation of additional energy levels in the band gap, the electron donor concentration, etc. For example, an excess of dopant concentration could produce a detrimental effect decreasing the photocatalytic activity, because the charge recombination is favoured.5,6
The most popular transition metals used as dopants are W, Cr, Fe, Co, Mn and Cu5 which modify the optical and photo-electrochemical properties of TiO2, shifting the light absorption of TiO2 to the visible region and prolonging the lifetime of electron and holes, thus increasing the photocatalytic activity.7 Particularly, tungsten (W) is found to inhibit the recombination of photoinduced e–/h+.8 Rampaul et al.9 and Yang et al.8 evidenced the efficiency of photocatalytic oxidation through W-TiO2 doped films. The effect of dopant is to create additional intermediate energy levels that modify the electrical and optical properties of the TiO2 matrix.
On the other hand, the photocatalytic properties of TiO2 are modified when doped with alkaline and alkaline-earth ions. Al-Salim et al.10 considered that the photocatalytic activity of TiO2 films doped with Ca2+ increases due to Ca2+ ions could be isomorphously substituted or interstitially introduced into the matrix producing oxygen vacant matrix or interstitial Ti3+ deforming the TiO2. The concentration of active sites improves and the recombination of h+/e– pairs is avoided. This is the most plausible explanation to the photocatalytic enhance produced by Ca, although we have not found a clear justification in the consulted literature.
Asahi et al.11 showed that the doping with anionic species (C, N, S, P and F) could replace O in the TiO2 matrix and thus narrow the band gap. In particular, doping with nitrogen (N-doped) may affect the band-gap structure by mixing N-2p and O-2p states.12 Kitazawa13 and Martínez-Ferrero14 have studied N-doped mesoporous and mesostructured TiO2 films after ammonia vapour treatment. For both, TiO2 films treated in ammonia at 500°C/2-4 h show enhanced photocatalytic activity, getting the optimum N substitution and maintaining the porous structure.
A large variety of methods have been developed to prepare TiO2 films but sol-gel is the most widely used due to its facility to prepare films onto wide-area bodies at low temperature. The use of templates allows the self-assembly of organized micelles, and creates mesoporous films with high specific surface area. The textural and morphological properties greatly enhance the photocatalytic efficiency properties.15,16 Different studies indicate that photocatalytic activity depends mainly on the total surface effectively irradiated with UV photons.17
The aim of this work was to prepare mesoporous nanocrystalline TiO2-anatase films doped with Ca, W and N, using F127 and Brij58 as surfactants, to analyse the effect of dopants in the photocatalytic activity. Three dopants were selected: an anionic specie, N, that produces substitution of N at O sites, W as transition metal and Ca as alkaline-earth ions, both substituting Ti sites. The effect of dopants in the different parameters such as film thickness, total pore volume, specific surface area and total surface exposed to illumination was evaluated together with the photocatalytic activity through the degradation of methyl orange. The analysis of textural properties permits to advance in the relationship between photocatalytic properties and film structure. The influence of dopants is important to explain the photocatalytic mechanisms inducing enhanced efficiency.
ExperimentalSynthesis and characterisation of doped and un-doped TiO2 solsDoped TiO2 sols were prepared using titanium isopropoxide (TISP, Aldrich, 284.22 g/mol, 97%) as precursor via acid catalysis. TISP was chemically modified by mixing with acetic acid (AcOH, Aldrich, 60.05 g/mol, 99.99%) or acetyl-acetone (AcAc, Merk, 100.12 g/mol, 99.5%), and absolute ethanol, in order to control the hydrolysis and condensation reactions. After 1 h of stirring, polyethylene glycol hexadecyl ether P5884 (Brij58, Aldrich, 1124 g/mol) was added to TISP/EtOH/AcOH solution, and Pluronic F-127 (F127, Aldrich, 12600 g/mol) to TISP/EtOH/AcAc one. Then, Ca(NO3)2 (Aldrich, 236.15 g/mol, 99%) was incorporated to TISP/EtOH/AcAc/F127 sol, while WCl6 (Aldrich, 396.56 g/mol, 99.9%) was added to TISP/EtOH/AcOH/Brij58 sol with molar ratios Ca(NO3)2/TISP=0.03 and WCl6/TISP=0.01. Finally, a mixture of ethanol and acidified water (0.1M HCl) was added drop by drop onto the solutions, up to reach a final oxide concentration of 30 g/L.
The final molar ratio was fixed to 1 TISP: 1 AcOH: 40 EtOH: 0.07 Brij58: 2 H2O: (0.01-0.03) W/Ca, and 1 TISP: 1 AcAc: 40 EtOH: 5*10–4 F127: 2 H2O: (0.01-0.03) W/Ca. All the sols were aged for 2 days before coating deposition.
A reference TiO2-AcOH-Brij58 and TiO2-AcAc-F127 sols were prepared following the same process but without dop-ants addition.
The stability of the sols was studied through the evolution of viscosity with time, using an Ostwald viscometer (Pobel, 0c model, viscosity range 0.6-3 mPas).
Deposition of doped and un-doped TiO2 filmsDoped and un-doped TiO2 thin films were deposited by dip-coating combined with Evaporation-Induced Self-Assembly (EISA) method onto glass-slides and silicon wafers. The films were obtained at 35 cm/min and 20% RH, and heat-treated in air at 450°C for 1 h using a heating ramp of 10°C/min. In the case of multilayer coatings, the same withdrawal rate was used along with an intermediate heat treatment of 350°C/1 h between coatings followed by a final treatment of 450°C for 1 h.
The glass-slides used to measure the photocatalytic activity were coated with one first layer of SiO2, using a SiO2 sol prepared in a two-step using TEOS (tetraethoxysilane).18 SiO2 coatings were sintered at 450°C for 30min, obtaining a thickness of ∼210nm, and a refractive index of ∼1.44, corresponding to 98% of theoretical density of SiO2. The quite dense SiO2 coating avoids the diffusion of Na+ cations from the glass substrate to the TiO2 coating during firing and the possible inhibition of photocatalytic activity.19,20
Nitridation of TiO2 filmsTwo layer TiO2-AcOH-Brij58 films were nitrogen doped (N-doped) through nitridation treatments carried out in a tubular furnace using an anhydrous ammonia flow at 500°C during 2 h. The treatment was performed increasing the temperature up to 500°C in N2 flow, then maintaining the sample in NH3 flow for 2 h, and finally decreasing the temperature in N2 atmosphere.
Characterisation of doped filmsThe coatings were characterised by optical microscopy (Zeiss, HP1, Germany) to detect the presence of precipitates, impurities, bubbles or cracks, and studied by transmission electronic microscopy (TEM) (Hitachi H-7100, Japan) to confirm the homogeneity and porous structure of the films. TEM samples were obtained by scratching the films and depositing the scaled fragments onto carbon-coated copper grids.
Ellipsometry and Environmental Ellipsometric Porosimetry (EEP) measurements were performed using a spectral Ellipsometer (M-2000UTM, J.A. Co., Woollam) modified with a system that allows controlling the relative humidity (RH) (Humidity Generator HG-1, Michel Instruments) to characterize films deposited onto glass-slides. The spectra were taken in the visible region, between 250 and 900nm at a fixed incident angle of 70°. The data were fitted using the WVASE32 software with Cauchy model. From the fitting data, the refractive index (n) (taken at λ=700 nm) and the thickness (e) of the films were obtained as a function of relative humidity (from 0% to 100%). The total pore volume and the adsorption-desorption isotherms were further obtained by considering the Bruggeman Effective Medium Approximation model (BEMA). The pore size distributions were calculated utilizing a modified Kelvin equation taking into account ellipsoidal pore geometry.21 Finally, an estimation of the specific surface area and the exposed surface area per cm2 of sample were calculated.
Coatings deposited onto silicon wafers were analyzed by Fourier transform infrared spectroscopy (FTIR) to follow the elimination of surfactants and the crystallization of anatase. FTIR spectra were recorded in transmission mode in the frequency range 4000-400 cm–1 with a resolution of 2 cm–1 using a Perkin Elmer FTIR Spectrum 100 equipment.
The hydrophobic/hydrophilic character of coatings was evaluated through the contact angle using an Easy Drop equipment (“Drop Shape Analysis System” Kruss DSA 100).
Grazing incidence X-ray diffraction (GXRD) studies were performed on films deposited onto silicon wafers using CuKα radiation in a Panalytical diffractometer (X’Pert PRO theta/theta) for analysing the crystallisation of TiO2-anatase. The diffractrograms were recorded in the range of 2θ=20-70°, using a fixed counting time of 20 s/step and an increment of 0.05°.
Finally, the photocatalytic activity was evaluated by the degradation of methyl orange (MO) in aqueous solution using the films deposited onto glass-slides on top of a first layer of SiO2. The light sources system consists of three UV-lamps of 6W with a maximum emission at 365nm wavelength (Philips F/TL/6W/08, Holland).
The measurements were performed using 50mL of an aqueous solution of methyl orange (MO) with a concentration c=3 mg/L. The pH of the solution was adjusted to 2 using HCl22 and the total surface tested was equal to 50 cm2. The reactor vessel containing the solution was covered with window-glass to prevent solution evaporation. First, photolysis and dark tests (adsorption) were performed to confirm that the degradation of MO is associated to the TiO2 film and not to light irradiation and/or adsorption.
Photocatalytic measurements were performed introducing TiO2 samples in the MO solution and maintained under continuous stirring and irradiation. Aliquots of 1mL were collected every 10min and analysed by spectrophotometry UV/vis (UV-vis, Perkin Elmer, Lambda 950), evaluating the degradation of MO through the diminution of the absorption band at 508nm.
Results and discussionDoped TiO2 sols and films characterisationHomogeneous and transparent doped and un-doped sols were obtained for both compositions TiO2-AcAc-F127 and TiO2-AcOH-Brij58. The sols prepared with AcAc are yellow, the rest being colourless.
The stability of doped and un-doped TiO2 sols was evaluated through viscosity measurements at 25°C as a function of aging time. In all the cases, a Newtonian behaviour was observed with initial viscosities between 1.5 and 2 mPa.s, not changing with the aging time for at least 1 month, thus revealing an excellent stability. The doping does not affect the stability of TiO2 sols.
TiO2 films were deposited onto glass-slides and Si-wafers from doped and un-doped TiO2-AcAc-F127 and TiO2-AcOH-Brij58 sols. After the heat-treatment good optical quality, transparency without precipitates and excellent adherence and mechanical stability were observed for all the coatings.
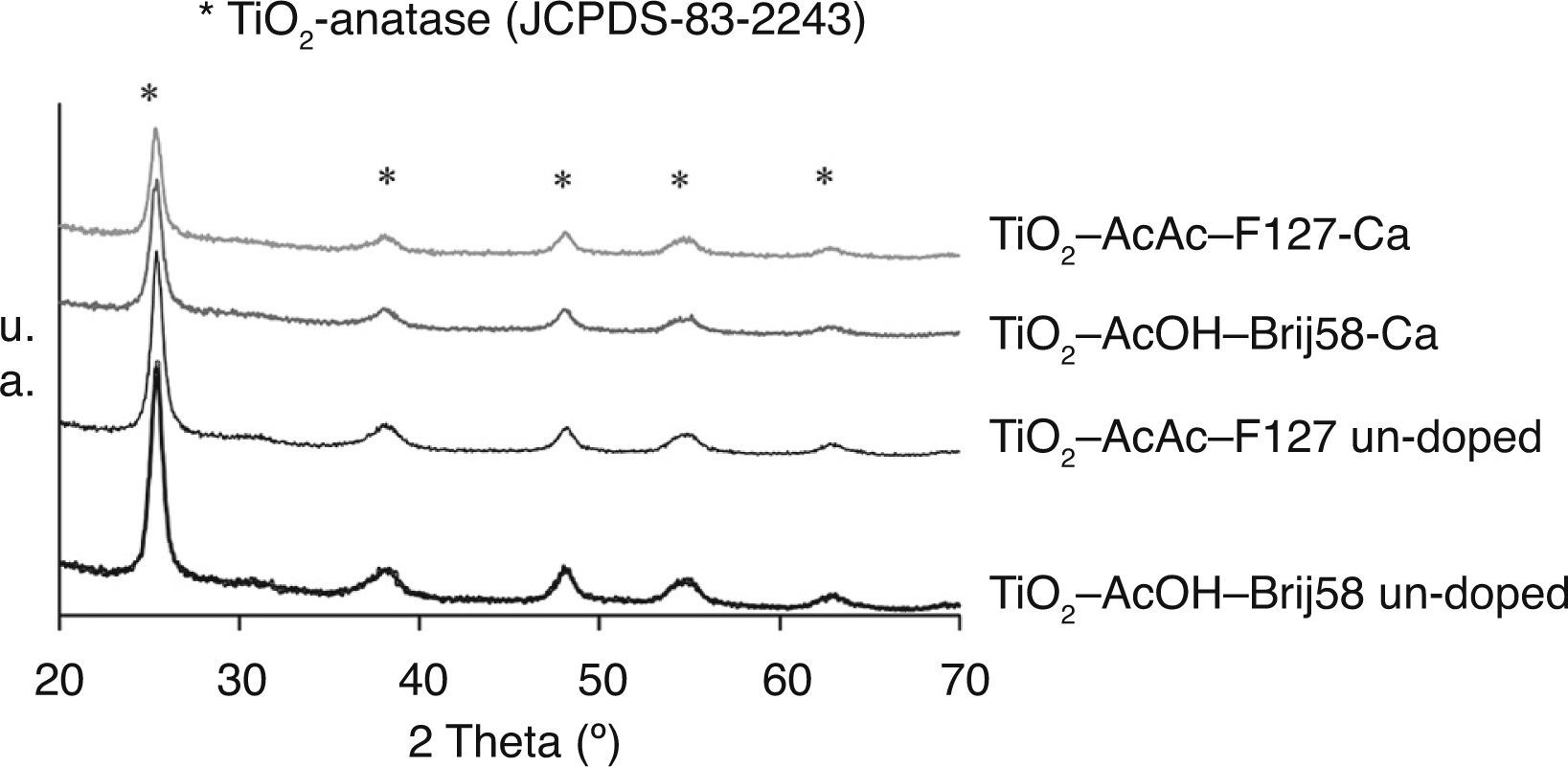
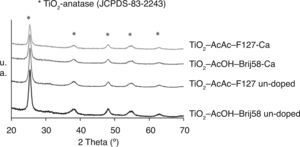
The crystallisation of the inorganic network as TiO2 anatase was confirmed by GXRD. Figure 1 shows the GXRD patterns of TiO2-AcAc-F127, TiO2-AcOH-Brij58 and the same compositions doped with Ca.
For all the coatings, tetragonal TiO2-anatase phase (JCPDS-00-083-2243) was identified as only phase, rutile or brookite phases not appearing. For Ca-TiO2-coatings, no calcium oxide impurity phases were detected likely due to the small concentration of Ca2+ incorporated. For the films doped with W and N the same effect was observed (not shown). However, the doping of TiO2 affects the crystallinity, decreasing the intensity peaks. Using the Scherrer's equation the particle size (D) of TiO2 coatings were calculated obtaining values between 9.6 and 10nm. These results indicate that the dopant does not affect the crystal size but the crystal fraction tends to decrease.
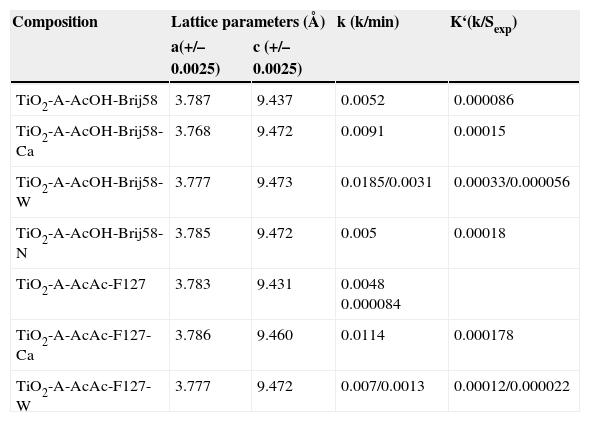
The crystal parameters have been calculated considering a tetrahedral crystal structure (Table 1). The doping of TiO2 produced some distortion in the lattice of TiO2. For Ca and W doped TiO2 the lattice parameter “a” decreases, increasing the “c” parameter. Depending on the ionic radius of the dopant, it can be introduced substitutionally or interstitially in the matrix. In all the cases, a deformation of the tetrahedron is observed. R. Rodriguez-Talavera et al.23 report that if the size of the dopant is larger than titanium but smaller than oxygen, such as Ca2+ (0.99 Å), the dopant is introduced substitutionally in the matrix, producing an oxygen deficiency in the crystal and stabilizing the anatase phase. Thus, the elongation of the tetrahedron obtained in our case could be explained considering the introduction of Ca2+ in substitution Ti4+ sites in the TiO2 lattice. For W6+, the ionic radius (0.64 Å) is very similar to Ti4+ (0.68 Å) whereas the charge of the ion dopant is higher. In this case, the excess of charge produces a similar effect that the ion size. The W6+ is introduced substitutionally in the TiO2 matrix, deforming the structure due to the electric field created between the dopant and the surrounding ions.23
– Crystal parameters and kinetics constants of un-doped and doped photocatalysts.
| Composition | Lattice parameters (Å) | k (k/min) | K‘(k/Sexp) | |
|---|---|---|---|---|
| a(+/–0.0025) | c (+/–0.0025) | |||
| TiO2-A-AcOH-Brij58 | 3.787 | 9.437 | 0.0052 | 0.000086 |
| TiO2-A-AcOH-Brij58-Ca | 3.768 | 9.472 | 0.0091 | 0.00015 |
| TiO2-A-AcOH-Brij58-W | 3.777 | 9.473 | 0.0185/0.0031 | 0.00033/0.000056 |
| TiO2-A-AcOH-Brij58-N | 3.785 | 9.472 | 0.005 | 0.00018 |
| TiO2-A-AcAc-F127 | 3.783 | 9.431 | 0.0048 0.000084 | |
| TiO2-A-AcAc-F127-Ca | 3.786 | 9.460 | 0.0114 | 0.000178 |
| TiO2-A-AcAc-F127-W | 3.777 | 9.472 | 0.007/0.0013 | 0.00012/0.000022 |
The FTIR spectra (not shown), revealed that C-H and C-O vibrations of Brij58 and F-127 did not appear, confirming that templates have been totally removed and TiO2 matrix is fully crystallized in anatase form after the heat treatment.
Nitrided TiO2-AcOH-Brij58 films were homogeneous, transparent, crack free and slightly yellowish. The crystallisation of TiO2 in anatase phase was confirmed by XRD and crystal sizes around 10nm were determined, indicating that nitridation treatment does not affect the crystal size compared with un-doped films. The lattice parameter “a” does not change with the nitridation however the “c” axis increases (Table 1). The defect of charge of N3– respecting to the substituted O2– in the anatase network likely explained the deformation of the structure.
Textural characterisation of TiO2 filmsSpectral Ellipsometry and Environmental Ellipsometric Porosimetry (EEP) measurements were used to determine the thickness, refractive index and porosity properties of the films.
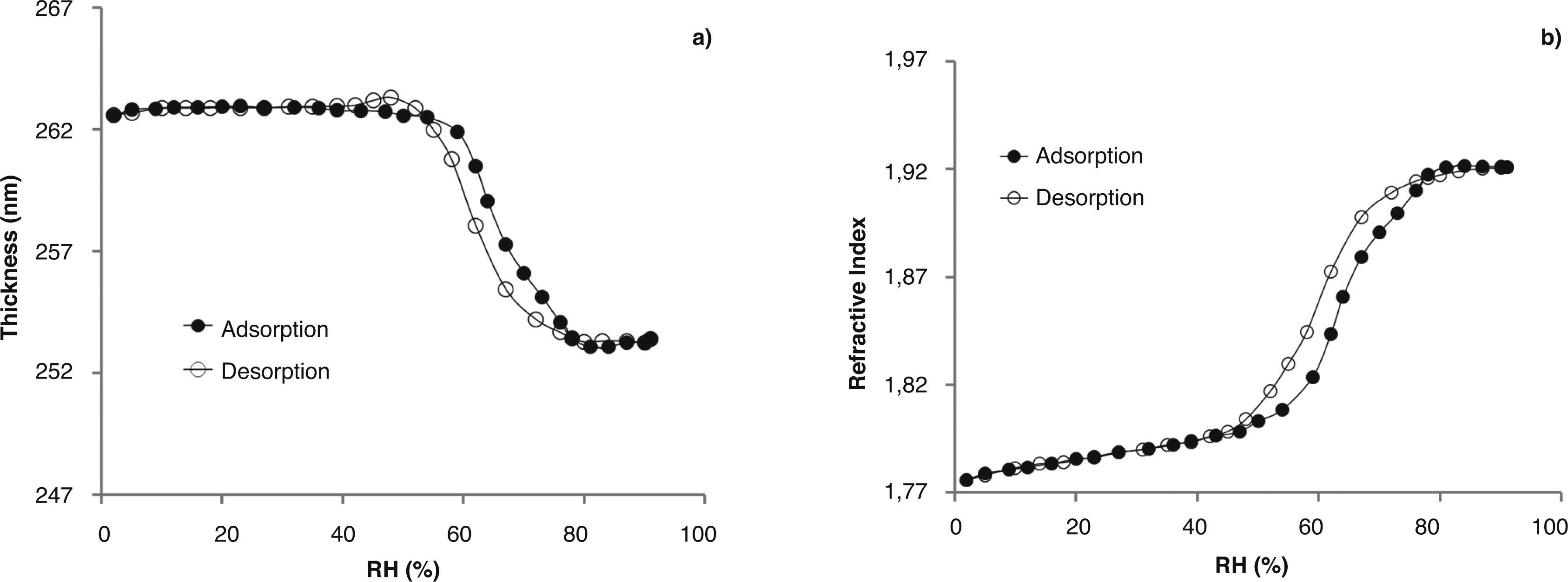
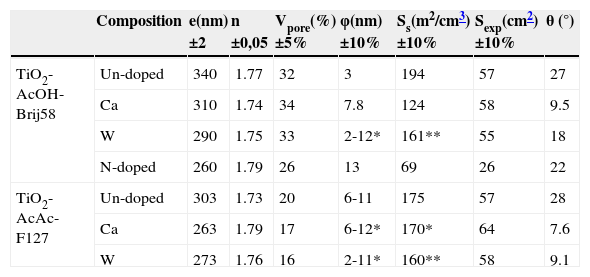
Table 2 summarizes the thickness (e) and refractive index (n) of the coatings. Doped TiO2 films show lower thickness and similar refractive index than those of un-doped films, except to N-doped film in which the nitridation treatment slightly increases the refractive index and decreases the thickness because of an extra thermal treatment. Figure 2a and 2b shows the thickness and the refractive index of a TiO2-AcAc-F127-Ca film at λ=700nm, as a function of RH during adsorption and desorption measurements.
– EEP characterisation for doped and un-doped TiO2 films.
| Composition | e(nm) ±2 | n ±0,05 | Vpore(%) ±5% | φ(nm) ±10% | Ss(m2/cm3) ±10% | Sexp(cm2) ±10% | θ (°) | |
|---|---|---|---|---|---|---|---|---|
| TiO2-AcOH-Brij58 | Un-doped | 340 | 1.77 | 32 | 3 | 194 | 57 | 27 |
| Ca | 310 | 1.74 | 34 | 7.8 | 124 | 58 | 9.5 | |
| W | 290 | 1.75 | 33 | 2-12* | 161** | 55 | 18 | |
| N-doped | 260 | 1.79 | 26 | 13 | 69 | 26 | 22 | |
| TiO2-AcAc-F127 | Un-doped | 303 | 1.73 | 20 | 6-11 | 175 | 57 | 28 |
| Ca | 263 | 1.79 | 17 | 6-12* | 170* | 64 | 7.6 | |
| W | 273 | 1.76 | 16 | 2-11* | 160** | 58 | 9.1 |
* Bimodal distribution
** Ss medium
At low RH, low values of refractive index (n=1.73) were obtained, which is characteristic of the presence of high porosity. When RH increases, the thickness and the refractive index remain near constant up to 50%. At this humidity, a sharp increase of refractive index and a simultaneous decrease in thickness is observed, indicating the capillary water condensation into the pores and the corresponding filling of them.21 After the first adsorption/desorption cycle, the refractive index and the thickness reached the original values indicating that the film is stable during all the adsorption and desorption process.
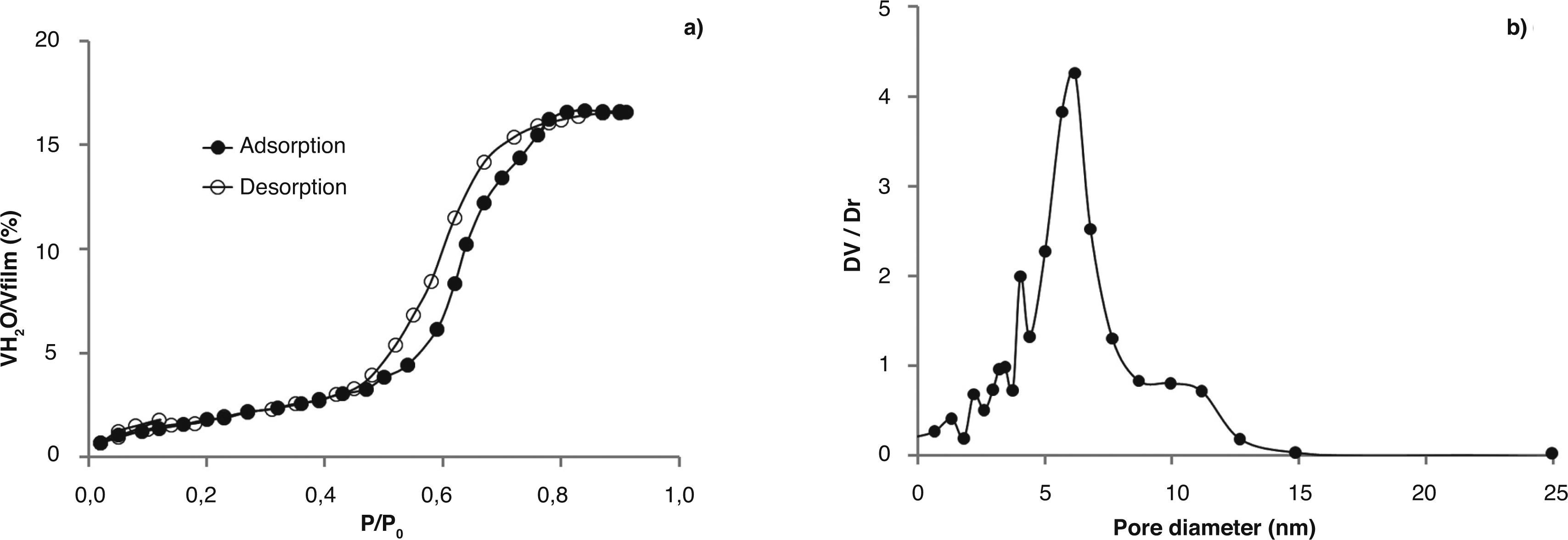
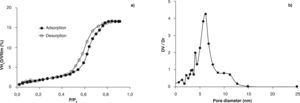
The total pore volume in dry air and the full isotherms were calculated by using BEMA model and the pore size distribution using the Kelvin's equation modified. Figure 3a and 3b shows the water adsorption/desorption isotherms and the pore size distribution for a TiO2-AcAc-F127-Ca film. A reversible type IV-adsorption/desorption isotherm with hysteresis loop is observed (Fig. 3a), indicating the presence of pores in the mesoporous range (2-50 nm). TiO2-AcAc-F127-Ca films present a total pore volume of 17% (Fig. 3a) and a bimodal pore size distribution between 6.2 and 10nm (Fig. 3b).
The rest of samples exhibit isotherms with similar shapes. All the films presented total pore volume (Vpore) between 33 and 16%, and pore size between 3 y 13nm (Table 2). TiO2-A-AcOH-Brij58-W, TiO2-A-AcAc-F127 and TiO2-A-AcAc-F127-Ca films present bimodal pore size distributions with values around 2 and 12nm.
The specific surface area (Ss) together with the exposed area (Sexp) was calculated,17,21,24 (Table 2). This last parameter represents the total area exposed to irradiation, thus being relevant for photocatalytic behaviour.
All the doped TiO2 films present lower values of contact angle (θ) than un-doped coating, showing that dopants increase the hydrophilicity of the films; this behaviour agrees with the results reported by Yuan et al.25
The textural properties are summarised for doped and undoped TiO2 films in Table 2 together with the contact angle.
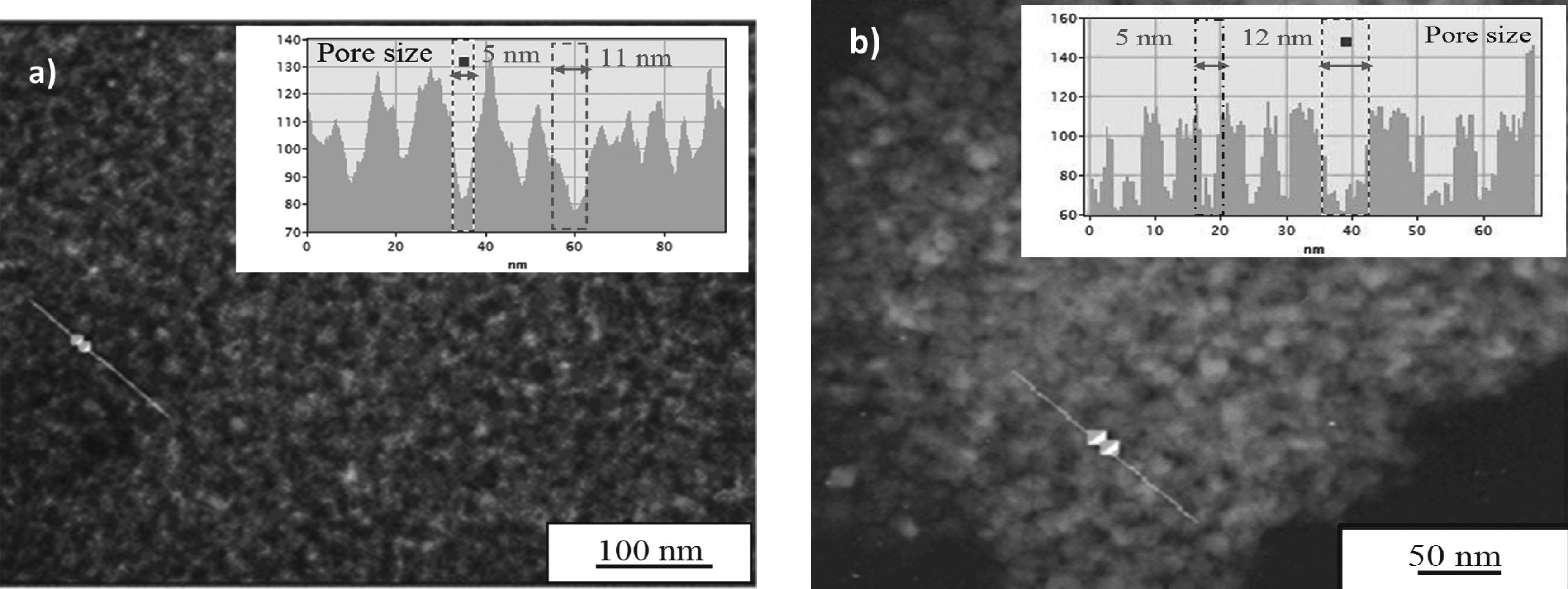
Transmission electronic microscopy (TEM) was used to confirm the porous structure of the coatings. Figure 4 shows the micrographs of TiO2-AcAc-F127 un-doped and TiO2-AcAc-F127-Ca films. TiO2-AcAc-F127 un-doped film shows a high porosity with bimodal pore size distribution around 5 and 11nm, but without any order. On the other hand, TiO2-AcAc-F127-Ca film presents a similar structure, with slightly higher bimodal pore size around 5 and 12nm. These results confirm the porosity characterisation obtained by EEP.
UV-visible spectra were used to determine the band-gap of TiO2 films. The Eband-gap can be calculated through equation 1 by fitting the linear relation of (α E)1/m versus E plot.
α is the absorption coefficient, Eg is the energy of a photon, A is a constant, and m is the parameter that depends on the electronic transition of the semiconductor; for indirect transition semiconductor such as TiO2-anatase phase m=2.26 The calculated band gap of undoped-TiO2 film is 3.5eV shifting to 3.4 or 3.3eV for doped TiO2 films.
The incorporation of Ca or W dopants produces a slight displacement of the band gap likely to due intermedium states created within the band gap according to data observed by R. Long and N. J. English.27
Photocatalytic activity of TiO2 filmsThe photocatalytic activity of doped and un-doped TiO2 films was evaluated by studying the degradation of methyl orange (MO).22 The preliminary tests show that neither photolysis nor adsorption process occur.
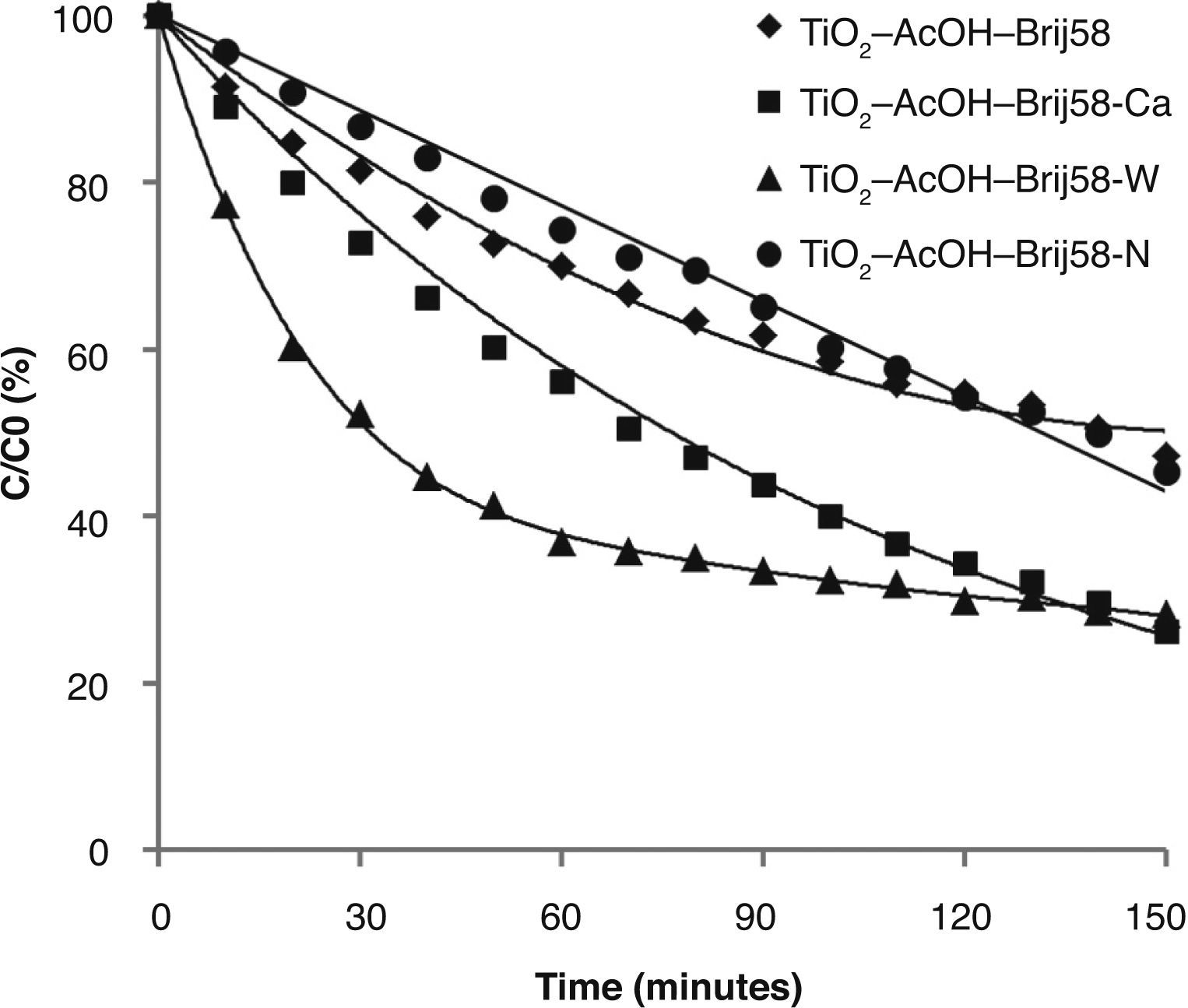
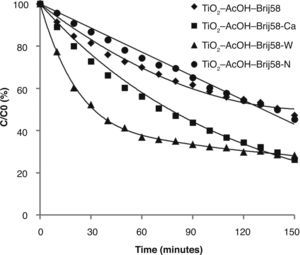
The photocatalytic behaviour of TiO2-AcOH-Brij58 films with and without dopant was first analysed (Fig. 5).
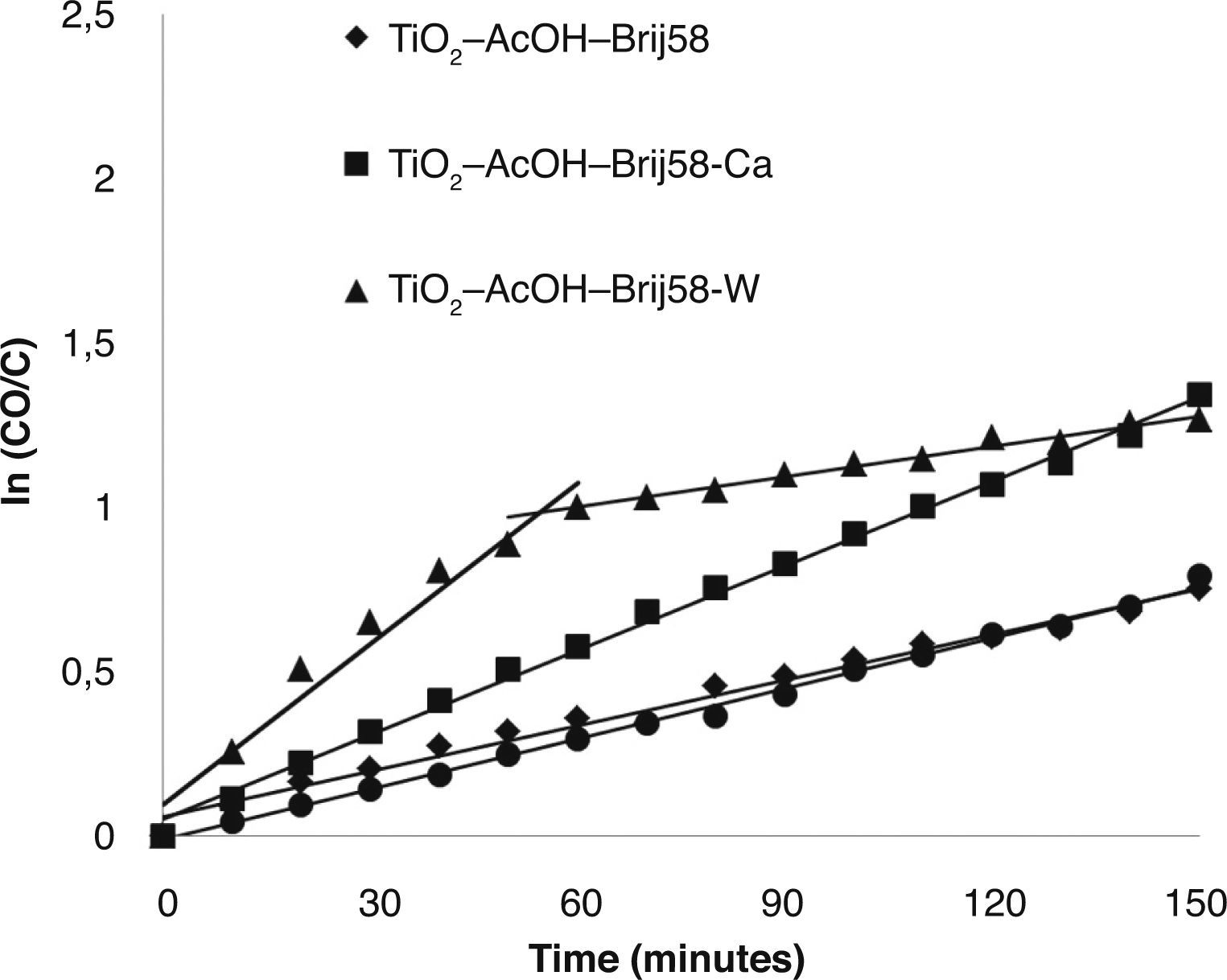
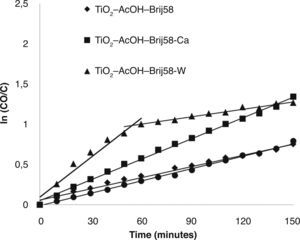
The study revealed that the photocatalytic activity of TiO2 films was greatly enhanced by Ca2+ and W6+ doping. TiO2-AcOH-Brij58-Ca and TiO2-AcOH-Brij58-W films show a decomposing of 72% of MO after 2.5 h under UV irradiation. Even though the final degradation is similar for both dopants, the kinetic behaviour is different. W-doped-TiO2 degrades up to 60% of MO after 50min of irradiation whereas TiO2-AcOHBrij58- Ca reaches only 40%. This indicates that the TiO2-AcOH-Brij58-W film is initially more active but the active sites could be further blocked by dye ions, thus decreasing the degradation rate. In this case, the kinetic process can be divided in two steps. For obtaining the kinetic parameters, the photocatalytic decomposition of MO was described by a first order kinetic model.28 ln (C0/C)=kt, where C0 and C is the concentration at t=0 and at time t. The plots of ln (C0/C) vs t show a straight line the slope k (Fig. 6). Table 2 shows the first order rate constants, k, together with the normalized rate constants, K’ defined as K’=k/(Sexp) for the different TiO2 films used in this study.
On the other side, k value parameter of TiO2-AcOH-Brij58-Ca film was the maximum even after it was normalized per surface area. Un-doped and TiO2-AcOH-Brij58-W films show similar degradation rate, 0.005 k/min, but TiO2-AcOH-Brij58-W film shows two different behaviours. For degradation times lower than 50min, a high kinetic rate (0.0185 k/min) is obtained, even higher than that of TiO2-AcOH-Brij58-Ca film. However, the photocatalytic process drops sharply from this time and the reaction rate decreases up to 0.0031 k/min, which is associated with the blocking or saturation of the active sites.
In order to explain the photocatalytic behaviour, two aspects should be considered, one associated with the nature of the dopant and other with the textural and porous structure of the coatings. In the case of Ca2+ ion, the dopant enters in the lattice substituting Ti4+ and induces oxygen vacancies, increasing the active sites. Furthermore, W6+ ions could easily replace Ti4+ (similar ionic radii) but causing an excess of positive charge on the network. Both effects can reduce the recombination e–/h+ pairs, acting as bridge for electron transition and increasing the photocatalytic activity.29,30
The other aspect affecting the photocatalytic activity is associated with the porosity (Table 2) of Ca and W doped films. TiO2-AcOH-Brij58-Ca films present a small pore size associated with high specific surface area (Ss). Chen and Dionysiou31 demonstrated that photocatalysts with pore size around 5nm show higher photocatalytic degradation rates. On the other hand, TiO2–AcOH-Brij58-W coatings show a bimodal pore size distribution. The smaller pores are initially more active, but will rapidly saturate by adsorption of MO molecules thus causing the decrease in photocatalytic activity.
Considering the first aspect (nature of dopant), TiO2-AcOH-Brij58-W film should have high photocatalytic activity, but the textural properties reduce this activity. However, for Ca-doped TiO2 films both factors affect positively promoting enhanced photocatalytic performance.
Finally, TiO2-AcOH-Brij58-N films show similar photocatalytic efficiency that un-doped TiO2-AcOH-Brij58 film because the nitridation treatment provokes the partial collapse of the porosity structure of the coatings, Ss of 69 m2/cm2 versus 194 m2/cm2, this reducing drastically the efficiency. Thus the effect of N as dopant is counteracted by the reduction of porosity and Ss.
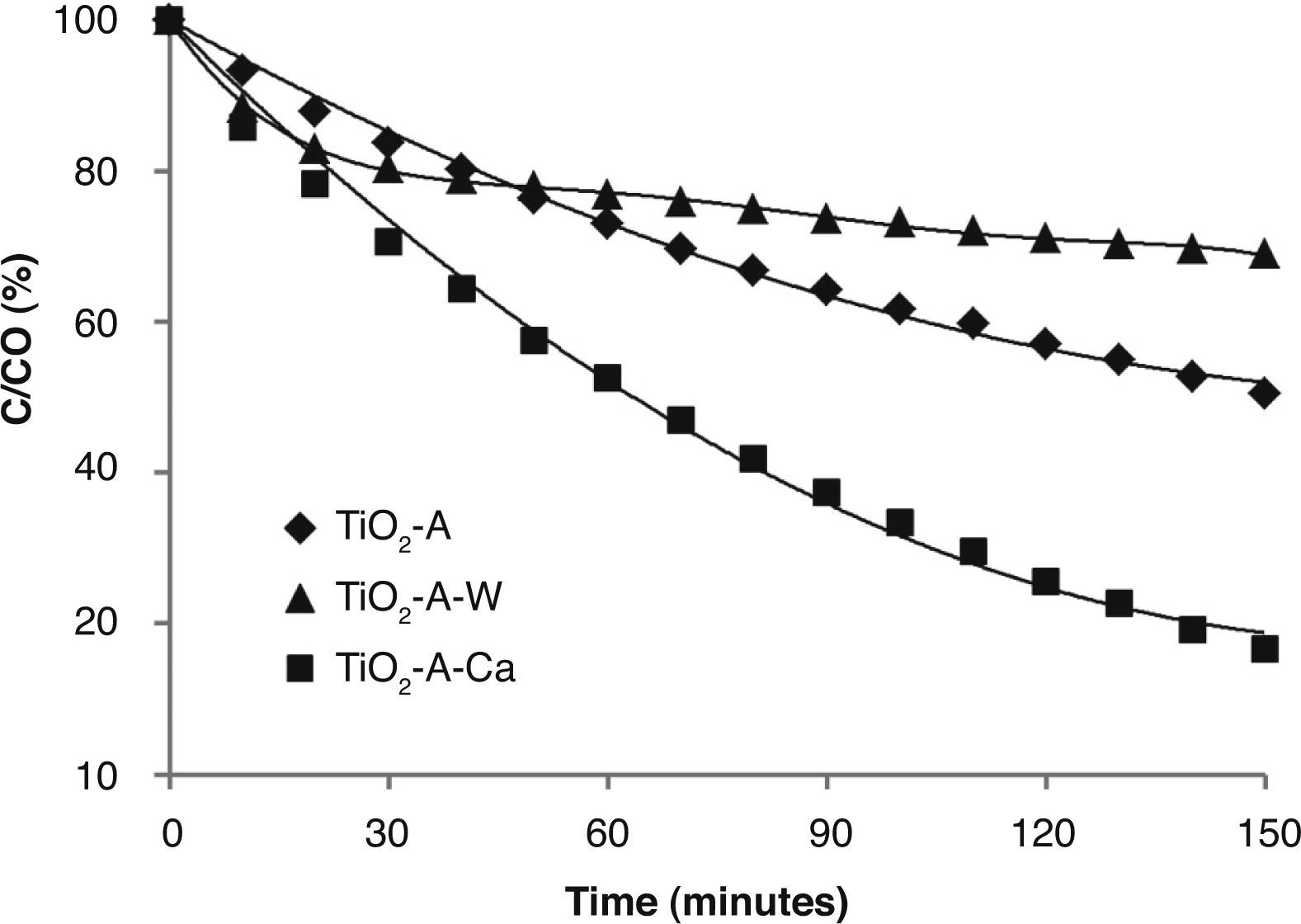
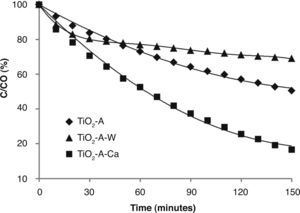
TiO2-AcAc-F127 films were also evaluated (Fig. 7). In this case, Ca-doped TiO2-AcAc-F127 film shows the highest photocatalytic efficiency, decomposing 83% of MO after 2.5 h of UV irradiation, followed by un-doped film. The photocatalytic behaviour of Ca as dopant is similar to that of TiO2-AcOH-Brij58-Ca film with the highest k constant rate, 0.0114 k/min. As in the previous case, W-doped presents two different steps during the irradiation time. The results reveal a rapid degradation of MO at initial radiation time, with a k value of 0.007 k/min, followed by a sharp decrease of the degradation rate down to 0.0013 k/min. Analogous arguments can explain the best photocatalytic activity of the catalyst. Ca-doped films present a pore size around 6nm and high Ss (m2/cm3) that, together with the introduction of the Ca2+ ions in the TiO2 matrix, contributes to obtain the best photocatalytic behaviour, avoiding the recombination of e–/h+ pairs.
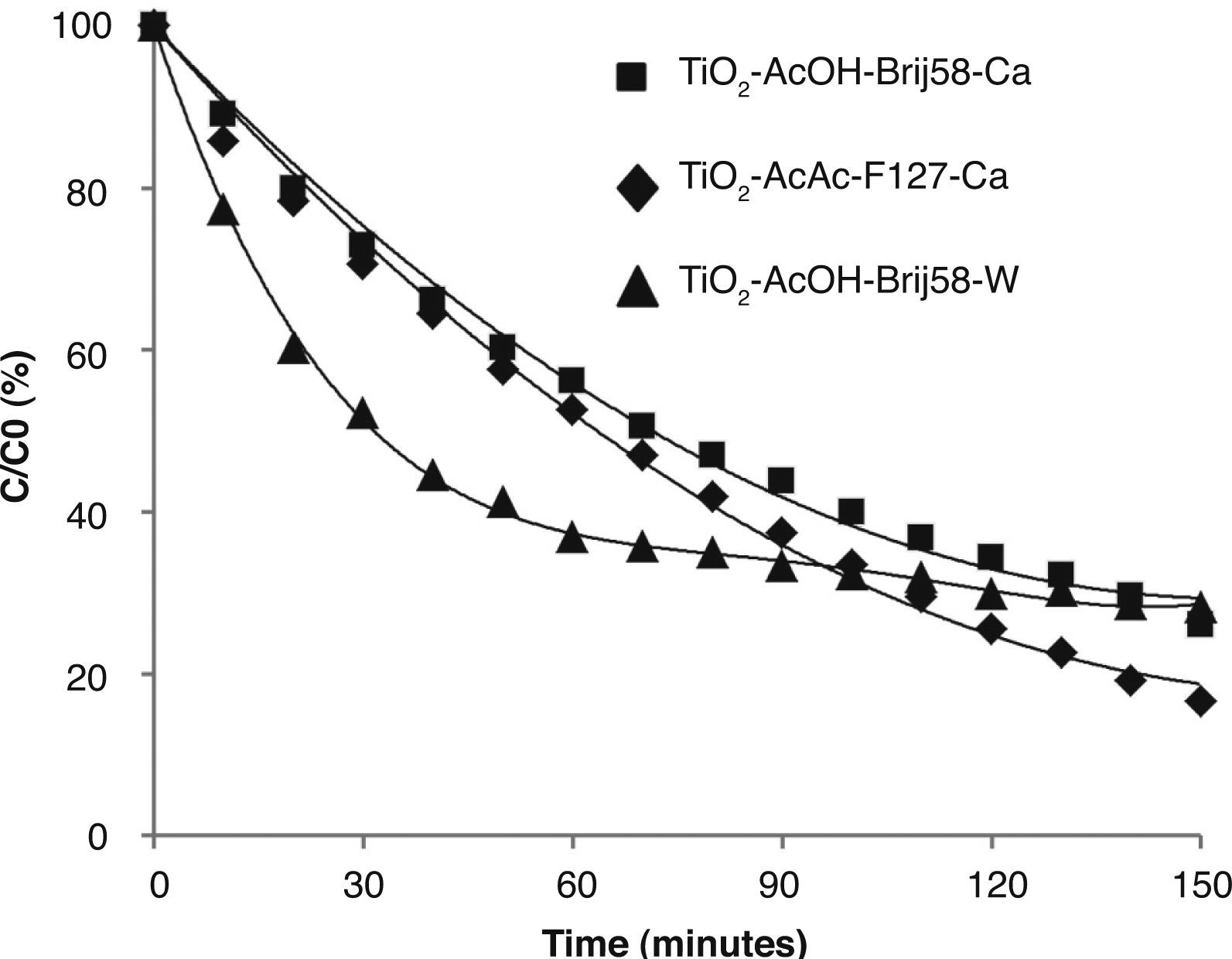
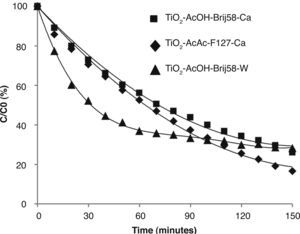
Figure 8 compares the best photocatalytic results obtained for TiO2 films. The highest photocatalytic efficiencies were obtained for films doped with Ca and W associated with their textural properties: small pore size (6 nm) and high specific surface area, together with the effect of the incorporation of the dopant into the TiO2 matrix substituting Ti4+ ions in the anatase network.
The textural properties, particle size and specific surface area of the TiO2 films, as well as the chemical nature, size and charge of the dopants, are all key factors affecting the photocatalytic properties and determining the efficiency of the catalysts.
The much enhanced efficiency of Ca2+ and W6+ doped mesoporous titania films convert these materials in appropriated candidates for in-door and out-door applications.
ConclusionsTiO2 mesoporous films with much enhanced photocatalytic activity were prepared by doping with Ca and W ions. Anatase was the only identified phase in all the photocatalysts, whether doped or un-doped.
The doping of TiO2 affects the crystallinity provoking the decrease of the peak intensity. The calculated crystal size of TiO2-anatase is around 10nm in all the coatings, indicating that the dopant does not affect the crystal size but the crystal fraction tends to decrease.
The introduction of Ca2+ or W6+ ions produces some distortion in the unit cell dimension of TiO2. The lattice parameter “a” decreases, and the “c” parameter increases for doped TiO2 films.
The pore volume and pore size values vary between 33 and 16% and 3 y 13nm, respectively. Moreover, TiO2-AcAc-F127 films with and without dopants present bimodal porosity greatly affects the photocatalytic performance.
TiO2-AcOH-Brij58-W films show higher photocatalytic activity at initial UV radiation time; however, the saturation of the smaller porosity reduces the activity. For Ca-doped films, the adequate textural properties (pore size and high Ss) and the introduction of Ca2+ ions in substitutional positions of Ti4+ in the TiO2 matrix contributes positively to obtain a much enhanced photocatalytic activity.
Ca2+ ions enter in the lattice substituting Ti4+ and inducing oxygen vacancies that increase the active sites. Furthermore, W6+ ions could easily replace Ti4+ (similar ionic radii) but causing an excess of positive charge on the network. Both effects can reduce the recombination e–/h+ pairs, acting as bridge for electron transition and increasing the photocatalytic activity.
On the other hand, the nitridation process produces the partial collapse of the TiO2 matrix being detrimental for the photocatalytic activity.
Textural properties, particle size and specific surface area, as well as chemical nature, size and charge of the dopants are key factors affecting the photocatalytic properties.
The much enhanced efficiency of Ca2+ and W6+ doped mesoporous titania films convert these materials in appropriated candidates for in-door and out-door applications.
The work was supported by PIE-CSIC-2004-60E637. The authors also acknowledge Laura Peláez and Aritz Iglesias for the experimental work.