High affinity and adhesion capacity for Gram-positive bacteria on minerals has been widely studied. In this work the adhesion of bacteria on synthesized zeolite has been studied. The Zeolite Linde Type A (LTA) has been synthesized using hydrothermal route using processing parameters to obtain low cost materials. For adhesion studies Staphylococcus aureus and Bacillus subtilis were used as Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa are used as Gram-negative bacteria.
X-ray diffraction, environmental scanning electron microscope and attenuated total reflection-Fourier transform infrared spectroscopy were used to characterize the synthesized zeolite. To evaluate the bacterial adhesion to zeolite LTA the hydrophobicity and surface properties are examined using contact angle measurement.
La afinidad y capacidad de adhesión de las bacterias grampositivas sobre minerales ha sido ampliamente estudiada. En este trabajo se ha estudiado la adhesión de bacterias sobre una zeolita sintética. Se ha sintetizado la zeolita Linde tipo A (LTA) mediante la via hidrotermal utilizando parámetros de síntesis con objeto de obtener materiales de bajo costo. Para los estudios de adhesión se han empleado Staphylococcus aureus y Bacillus subtilis como bacterias grampositivas y Escherichia coli y Pseudomonas aeruginosa como bacterias gram-negativas.
Para la caracterización de la zeolita sintetizada se ha empleado la difracción de rayos X, la microscopia electrónica de barrido ambiental y la espectroscopia infrarroja mediante transformada de Fourier en el modo de reflexión total atenuada. Para la evaluación de la adhesión de las bacterias se ha estudiado la hidrofobicidad utilizando la medida del ángulo de contacto.
The bacterial adhesion to different surfaces has received considerable attention [1–4]. It was studied in many applications, such as the infection of biomaterials, ship fouling, and wastewater treatment. In recent years, bacterial adhesion to minerals has been widely investigated. Most of the works focused on study of bacterial adhesion on granular activated carbon, kaolin, montmorillonite, goethite, iron oxide, clinoptilolite and NaY [1,2,5]. Moreover, several studies have been done on the characterization of different materials after adhesion of bacteria on surface [3,6]. As an example: Scanning Electron Microscopy, Fourier Transform Infrared and Isothermal Titration Calorimetry techniques were used to explore the interaction of Pseudomonas puptida with goethite [6].
Zeolites are crystalline aluminosilicate or silicate materials with regular and open microporous structure created by a three-dimensional network of SiO4 and AlO4 tetrahedral. Zeolite LTA (Linde Type A) is one of the most representative synthetic zeolites, which were firstly obtained by the hydrothermal crystallization method. It has been widely used in adsorption, ion exchange, zeolite membranes and catalyze due to their mesoporous and microporous structures [7]. The bacterial adhesion to zeolite is extensively explored in environmental application. The bacteria adhered to zeolite proved a high efficiency on heavy metal removal from wastewater especially chromium using zeolite Y and natural zeolites [2,5]. The nitrate is removed using bacterial cells adhered to zeolite [8]. Bai et al. proved that modified zeolite has a high efficiency on pyridine and quinolone removal using bacteria adhered to zeolite [9], also their capacity to adsorb heavy metals and purifying industrial products have been evaluated [10]. In contrast, the study of zeolite characterization after bacterial adhesion is scarce.
To the best of our knowledge, the modifications of structural and morphological characteristics of zeolite LTA after bacterial adhesion have not been reported in the literature. Therefore, the understanding of morphological and structural characteristic of zeolite after its adhesion to bacterial cell is of great significance. Thus, the main objectives of this work have been the synthesis of LTA zeolite and study the characteristics of zeolite before and after bacterial adhesion. For this purpose the X-ray diffraction (XRD), environmental scanning electron microscope (ESEM) and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) were used as characterization methods. The Staphylococcus aureus and Bacillus subtilis were used as Gram-positive bacteria, Escherichia coli and Pseudomonas aeruginosa are used as Gram-negative bacteria. This study can be useful later for wastewater treatment using bacteria-zeolite composite for adsorption of heavy metal.
Materials and methodsZeolite synthesisSodium silicate (Na2O·SiO2·5H2O, Sigma-Aldrich) and sodium aluminate (Na2O·Al2O3 anhydrous, Sigma-Aldrich) were employed as silica and alumina source respectively. A hydrogel with the molar ratio of 3Na2O:Al2O3:1.9SiO2:128H2O was used for the synthesis of zeolite by conventional heating.
Two solutions are prepared. Solution A was made by dissolving sodium aluminate in deionized water and subsequent addition of sodium hydroxide pellets (NaOH 99%, Sigma-Aldrich) with vigorous stirring. A second solution, designated B, was obtained by dissolving silicate aluminate in deionized water and final addition of sodium hydroxide pellets. The two solutions are maintained under stirring until obtaining a clear solution, then solution B is added to solution A. The overall gel is mixed until homogenized and hydrothermally treated for 4h at temperature of 100°C without stirring. Then, the solid product is filtered off, washed with deionized water until the neutral pH and dried in air at 80°C overnight [11].
Bacteria and culture preparationE. coli (Al52) and P. aeruginosa (ATCC-27853) were used as model for Gram-negative bacteria, whereas B. subtilis (CIP-B-4378) and S. aureus (ATCC-25923) were chosen as model for Gram-positive bacteria.
Stock bacteria cultures were stored at −80°C. Bacterial cell cultures were growing on 100ml LB medium (at 37°C for 24h). After 24h, bacterial cell cultures were centrifuged at 7000rpm for 10min. After centrifugation, LB was removed from cell cultures and the cell cultures were washed twice with phosphate buffer solution PBS, suspended in PBS solution and adjusted to obtain an optical density o.d. 600nm of 0.5.
Bacterial adhesion on zeoliteThe bacterial cells are suspended in Phosphate Buffered Saline PBS (pH 7), aliquots of 100mg of zeolite are incubated with 10ml of each bacterial cell suspension for 1h at 37°. The amount of bacterial cells adsorbed on zeolite is measured using the absorbance of the supernatant at 600nm using V-1200 spectrophotometer (MAPADA) [12].
CharacterizationCrystallinity and purity of the synthesized zeolite phases and zeolite adhered to bacterial cells were evaluated by XRD using an X-ray Diffractometer X’PERT PRO (PANalytical) with CuKα radiation.
The morphology was examined using ESEM using a Quanta-200 microscope (FEI Co.). The infrared spectrum was recorded using ATR-FTIR spectrometer Vertex 70 (Bruker Optic).
Contact angle measurements and surface propertiesThe contact angle was measured using goniometer (GBX Instruments) by sessile drop method. Three liquids with different polarities were used: water, formamide and di-iodomethane (Table 1).
The contact angles of bacterial cells were measured on layers of cells deposited on membrane filters prepared following the procedure described by Van Oss [13]. A defined volume of bacteria suspended in PBS solution (0.1M) was vacuum-filtered on a cellulose acetate membrane filter (pore diameter: 0.45μm) to form a uniform cell layer. The filters with bacterial layers were then mounted on a metal sample disc with double sided sticky tape and air-dried for approximately 30–60min in order to obtain stable, so-called “plateau” water contact angles. For each strain, three independently grown cultures were used, from which three filters of each were prepared and measured.
The preparation of zeolite for contact angle measurement was done following next procedure [14].
Concentrated stock suspension was dispersed in deionized water to a concentration of about 1–2%wt/vol and stirred with a magnetic stir bar for several hours. Then, 1.5ml suspension was placed on the microscope slide (the microscope slide was cleaned with acetone and deionized water) evaporated for two days under laminar air flow, and finally dried in an oven at 105°C for 12h. Glass slides were kept horizontal during the drying process. It has been reported that the value of the contact angle of water determines the hydrophobicity or hydrophilicity of surface, the water contact angle greater than 65° reflects a hydrophobic surface, while water contact angle less than 65° reflects a hydrophilic surface.
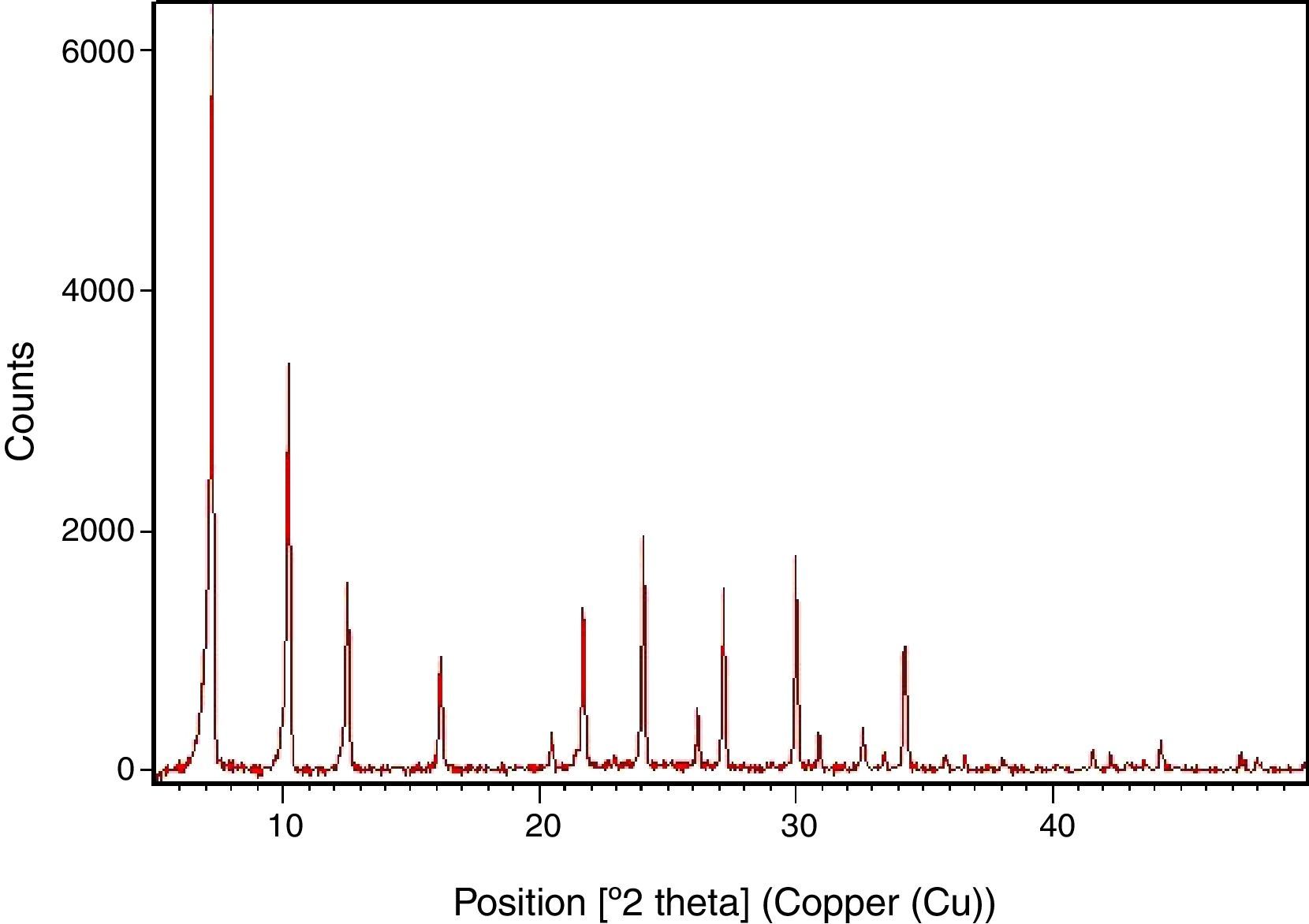
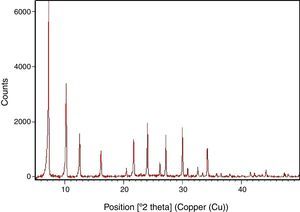
Results and discussionSynthesis of LTA zeoliteXRD pattern of the synthesized zeolite is reported in Fig. 1. The powder XRD profile is characteristic of highly crystalline material. It shows the principal reflections, at 2θ: 7.2°, 10.2°, 12.5°, 16.2°, 21.7°, 24°, corresponding to LTA zeolite [15].
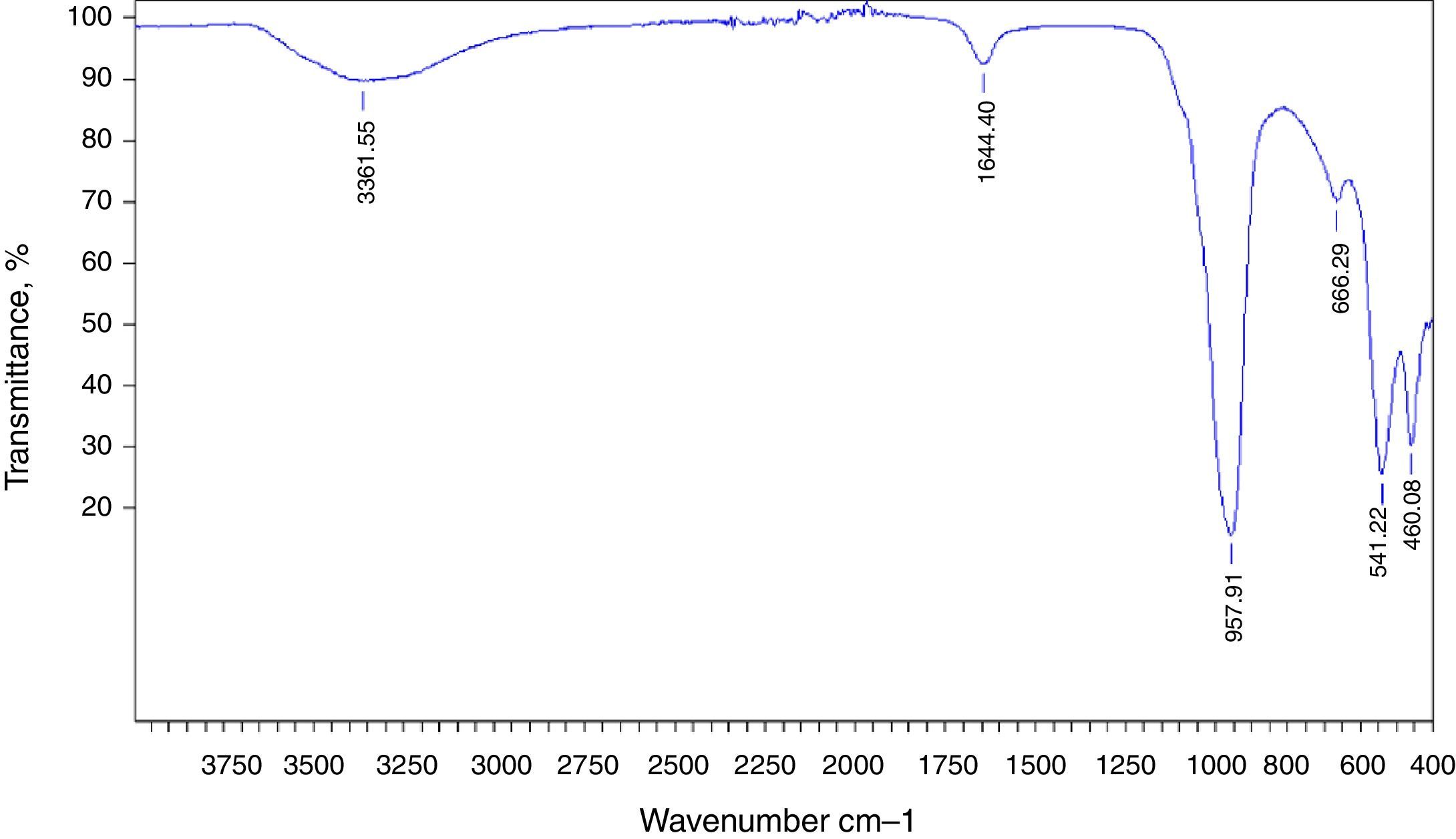
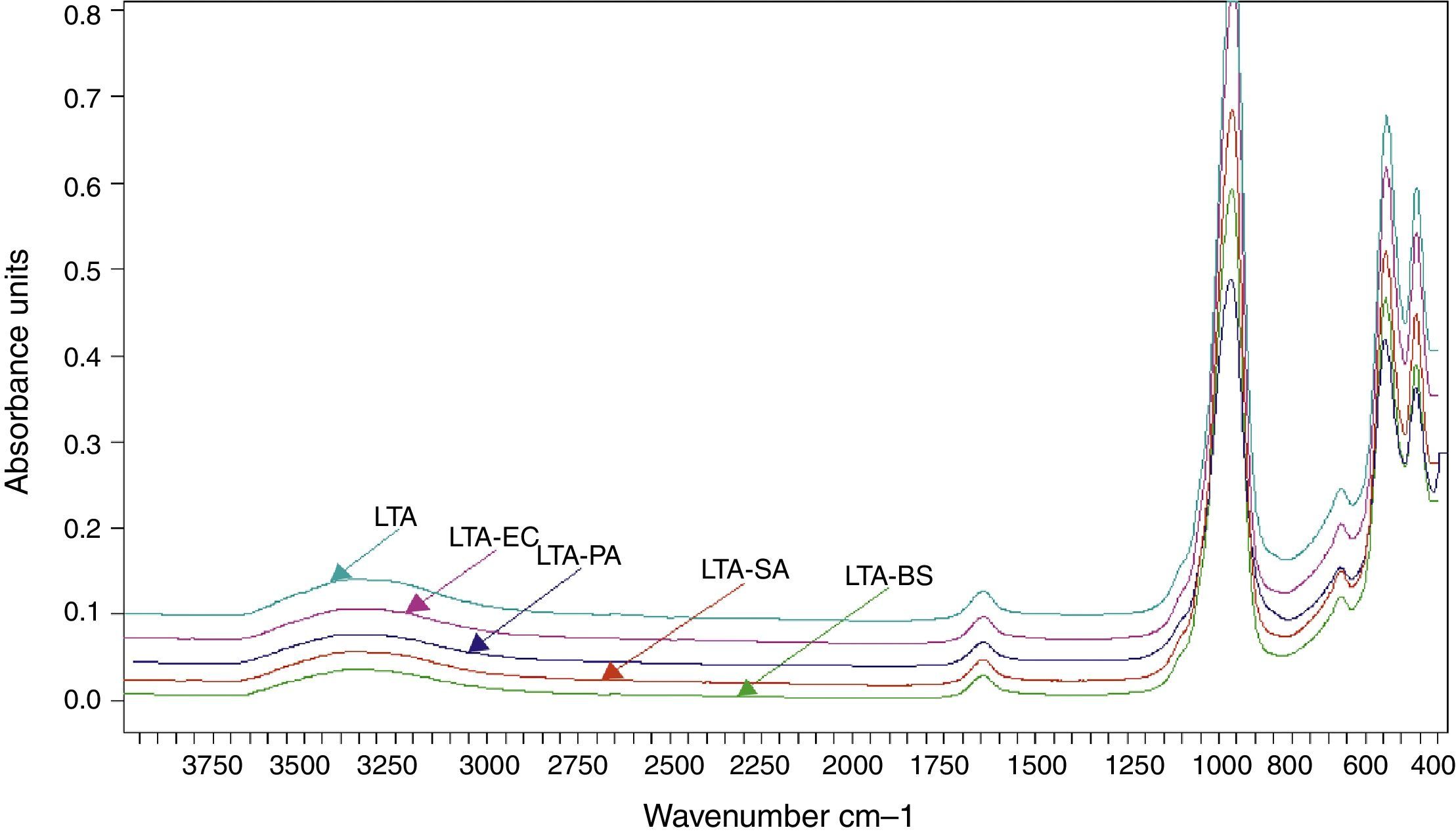
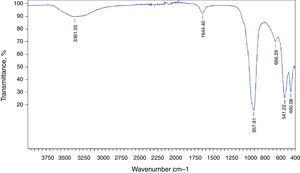
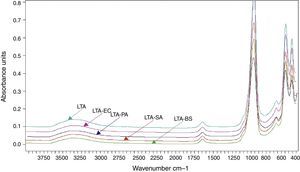
The preparation of LTA zeolite was also confirmed by ATR- FTIR spectroscopy in the mid-infrared region. Fig. 2 shows the ATR-FTIR spectrum of the synthesized LTA zeolite. The 968, 666 and 460cm−1 are close to the bands 1003, 668 and 462cm−1 assigned to the asymmetric stretching vibration of internal tetrahedral, the symmetric stretching vibration and the bending vibration modes of T–O bonds in TO4 tetrahedra (where T=Si or Al), respectively. The 541cm−1 band is due to D4R which is the major secondary building unit in LTA zeolite and the band at 1650cm−1 is attributed to the flexion vibration of OH group in adsorbed water [16]. Finally a typical stretching broad band of water is present around 3361cm−1[17].
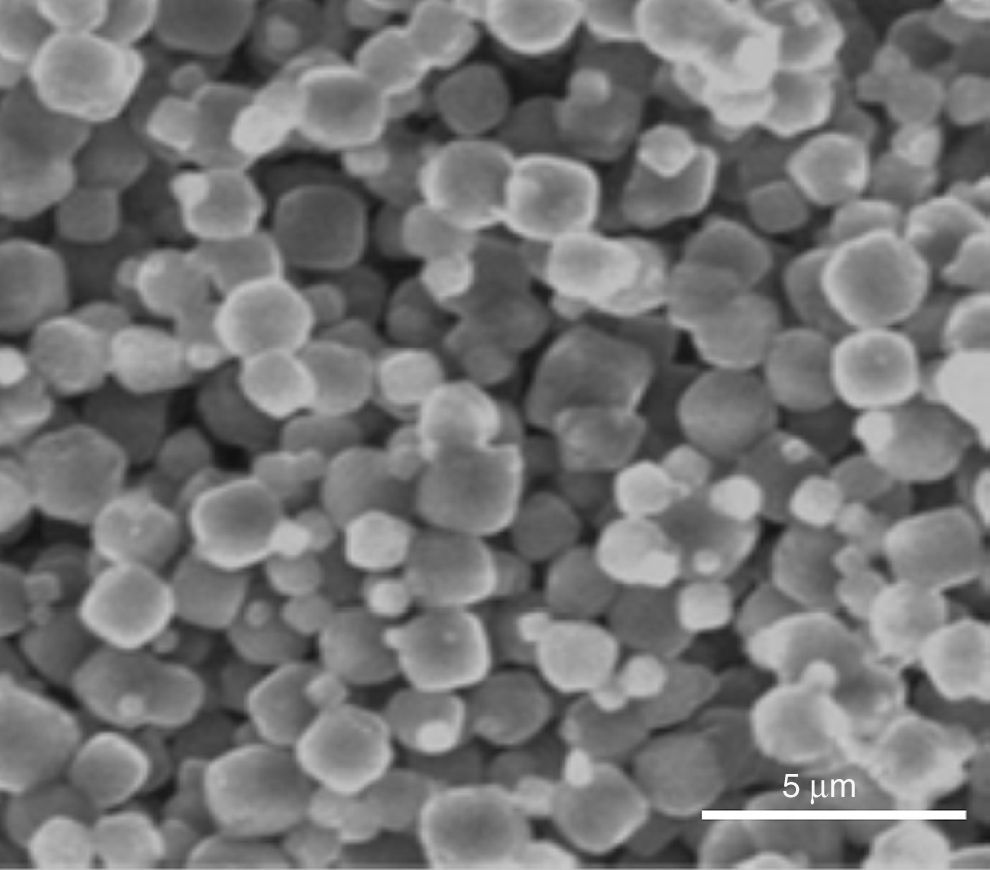
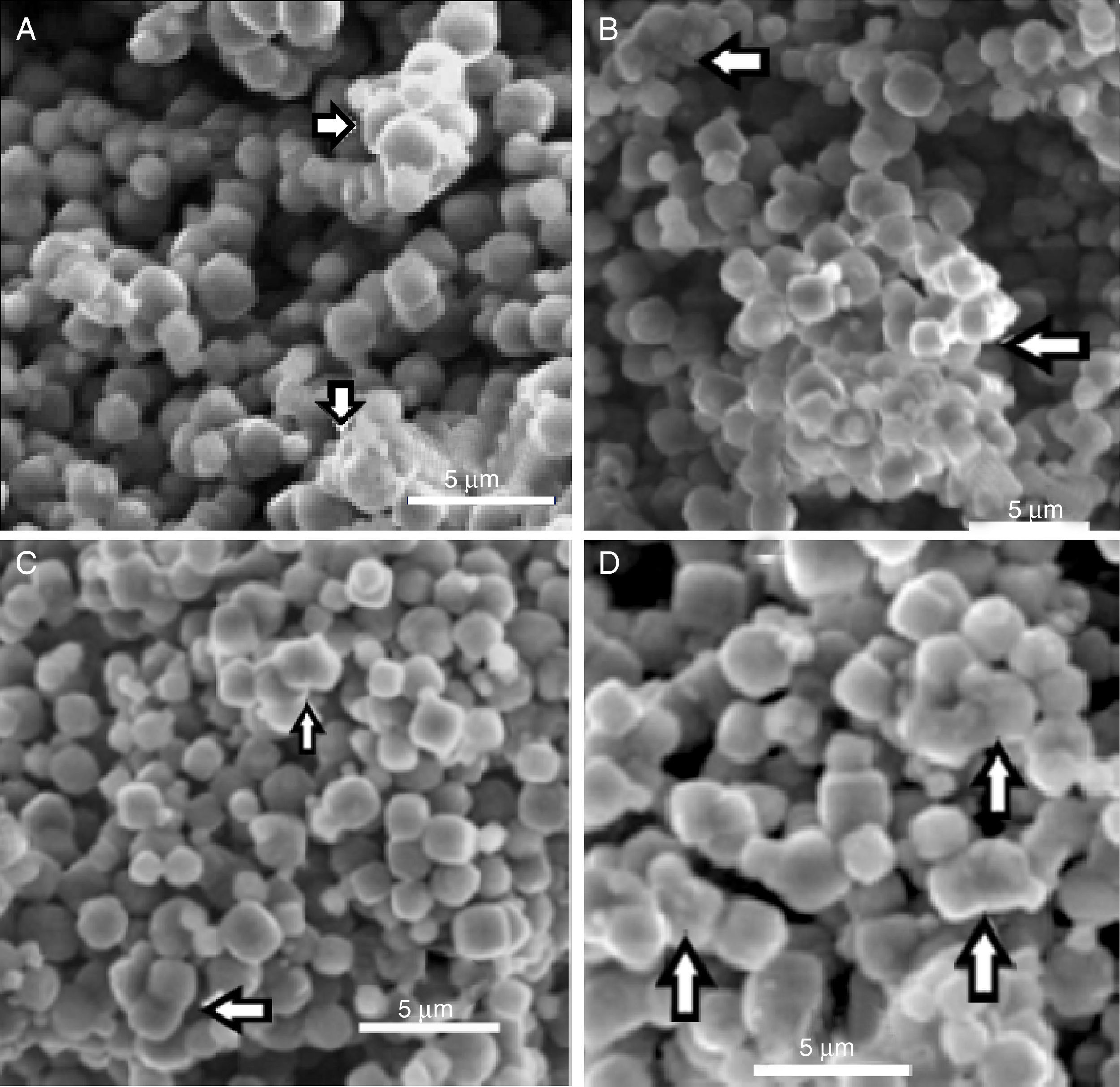
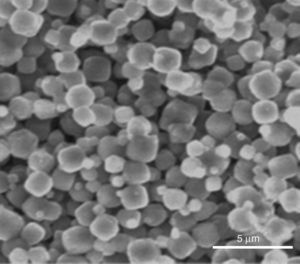
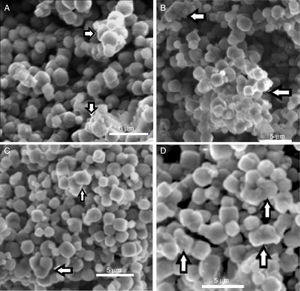
Indeed, the ESEM image of zeolite sample (Fig. 3) reveals a particle size less than 5μm and a characteristic cube shape of the LTA zeolites [18]. Bacterial adhesion on Zeolite LTA materials.
Table 2 shows the percentage of adhesion of bacterial cells onto zeolite. B. subtilis and S. aureus Gram-positive bacteria adhere to zeolite more than P. aeruginosa and E. coli Gram-negative bacteria. Many investigators showed that Gram-positive bacteria adhered to different type of materials more than Gram-negative bacteria. [19,20]. Kubota et al. proved that Gram-negative bacteria did not adsorb well onto different type of zeolites such as Na-BEA, X, H-Y, Na-Y, LTA, H-USY 330, H-MOR versus Gram-positive bacteria, because the surface structures of Gram-negative and positive cells differ [12]. From the other hand, comparing our results with Kubota et al. finding [12], they cited that there is no adhesion of majority of the studied bacteria on zeolite A. This discrepancy between our study and Kubota's et al. [12] could be explained by the difference in zeolite's characteristic witch may be due to the difference in synthesis protocol (Kubota et al. used Charnel protocol [21]). The zeolite synthetized in this study reveal a large cubic crystal (more than 30μm) with some twinning.
As mentioned in literature, bacterial adhesion is a complicated phenomenon influenced by many factors; hydrophobicity, shape, zeta potential, roughness, surface area, pH, size, etc. [22,23] and governed by both characteristics of bacteria and surface.
Analyzing the morphology of zeolite used in our work and that used by Kubota et al. [12], it is clear that they differ in size and shape. This difference may be explained by difference in synthesis parameters as it was described before in the literature [7,24,25]. Jafari et al. proved that the choice of chemical precursors and synthesis temperature affects significantly the crystal size and morphology [24]. Zhang et al. showed that the crystallization time have a considerable effect on the crystal size and morphology [7].
As it has been reported in literature, the particle size has an important effect on the bacterial adhesion [26,27]. Javed et al. showed that small grain size materials are more susceptible to bacterial attachment [28].
There is a correlation between the particle size and the external surface area. It has been reported that by decreasing the particle size, external surface area will be increased [29,30] and as it has been described by W. Zhao et al. the surface area has an effect on bacterial adhesion [23]. This effect has been shown in Sirikamon et al. results, they exhibit that efficient antimicrobial properties is shown using silver in the form of small-sized particles due to their extremely large surface area, producing an effective contact with microorganisms [27].
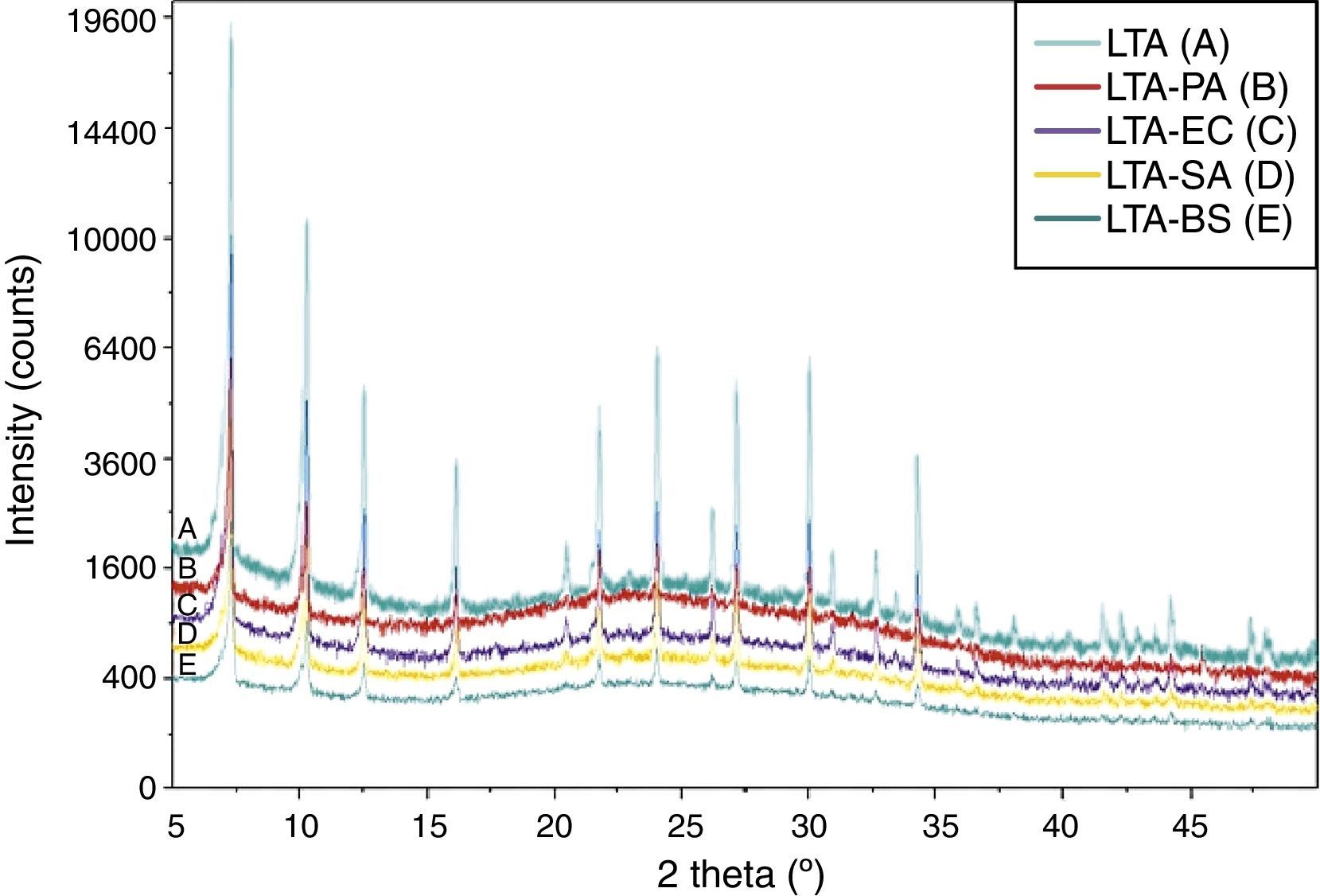
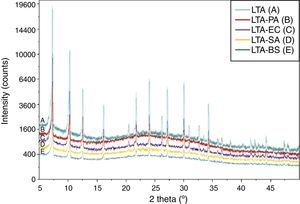
Fig. 4 shows the XRD pattern for zeolite and bacteria adhered to zeolite. There are no new reflections on the bacteria-zeolite complex compared to those of zeolite samples. This finding corroborates with the result of I.A. Vasiliadou et al., they found out that no new reflections were found on the bacteria–kaolinite complexes compared to those that appeared on the standard kaolinite samples [3].
In Fig. 5 can be observed the ESEM images of zeolite and bacteria–zeolite complex. The micrographs A, B, C and D show that bacteria adhered to zeolite have a tendency to aggregate and form larger particles. The bacterial cells were partially covered or buried by zeolite aggregate. No such aggregates could be found in the ESEM image of pure zeolite (E). It is reasonable that these aggregates are bacteria-zeolite complex. The arrows in figure A, B, C and D indicate the aggregation of zeolite particles due of the bacterial adhesion.
The FTIR spectra of zeolite LTA and bacteria adhered to zeolite (Fig. 6). No new absorption band was found on the bacteria-zeolite complex comparing with these appeared at zeolite. However, the vibrations of water molecules and the water sorbet on zeolite shifted, respectively, from 3362 and 1644cm−1 for zeolite to 3349 and 1644cm−1 for B. subtilis, to 3354 and 1646cm−1 for S. aureus cm−1, to 3350 and 1644cm−1 for E. coli and to 3353 and 1643cm−1 for P. aeruginosa. These shifts suggest that the water molecule on the zeolite is involved in bacterial sorption.
Rong et al. displayed that the vibrations of water molecules sorbet on goethite shifted from 3438cm−1 to 3432cm−1 and from 1637cm−1 to 1639cm−1 after Pseudomonas putida adhesion [6].
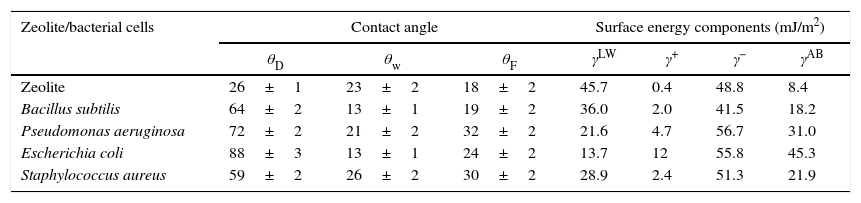
Table 3 lists the measured contact angles for Diiodomethane (θD) water (θw) formamide (θF), the physic-chemical properties for zeolite LTA and bacterial cells as measured by contact angle. It has been reported that the value of the contact angle of water determines the hydrophilicity or hydrophobicity of the surface, the value of contact angle (Table 3) shows that all bacterial cells and zeolite are hydrophilic.
Contact angles of diiodomethane (θD) water (θw) formamide (θF) and surface energy components of zeolite and bacterial cells (mJ/m2).
| Zeolite/bacterial cells | Contact angle | Surface energy components (mJ/m2) | |||||
|---|---|---|---|---|---|---|---|
| θD | θw | θF | γLW | γ+ | γ− | γAB | |
| Zeolite | 26±1 | 23±2 | 18±2 | 45.7 | 0.4 | 48.8 | 8.4 |
| Bacillus subtilis | 64±2 | 13±1 | 19±2 | 36.0 | 2.0 | 41.5 | 18.2 |
| Pseudomonas aeruginosa | 72±2 | 21±2 | 32±2 | 21.6 | 4.7 | 56.7 | 31.0 |
| Escherichia coli | 88±3 | 13±1 | 24±2 | 13.7 | 12 | 55.8 | 45.3 |
| Staphylococcus aureus | 59±2 | 26±2 | 30±2 | 28.9 | 2.4 | 51.3 | 21.9 |
It is generally accepted that hydrophobic cells adhere to hydrophobic surfaces, and the same is true for hydrophilic cells with hydrophilic surfaces. In our study, all bacterial cell surfaces and zeolite are hydrophilic; accordingly, it can be concluded that the hydrophobicity is involved in the adhesion of bacterial cells on zeolite. On the other hand, bacterial cells show a strong electron donor property and a weak electron acceptor property. Thus, the acid–base interactions between the electrons donor character of bacterial cells B. subtilis (γ−=41.54mJ/m2), P. aeruginosa (γ−=56.67mJ/m2), E. coli (γ−=55.77mJ/m2) and S. aureus (γ−=51.3mJ/m2) and electrons acceptor of zeolite (γ+=0.4mJ/m2) could contribute to the adhesion zeolite surface.
ConclusionsThe LTA zeolite was synthesized successfully via hydrothermal synthesis method, using relatively low temperature, in a pure phase with homogeneous cubic shape.
Zeolite LTA presented a higher affinity and adhesion capacity for Gram-positive bacteria.
The ESEM images indicated that the bacteria adhered to zeolite has a tendency to aggregate and form larger particles. Finally, the ATR-FTIR showed that the vibrations of water molecules sorbet on zeolite shifted in bacteria adhered to zeolite comparing with zeolite. Therefore, it is suggested that the water molecule on the zeolite is involved in bacterial sorption.
It can be concluded also that the hydrophobicity and acid-basic properties could be involved in the adhesion of bacterial cells on zeolite.
It would be possible to think of a LTA zeolite-Bacteria system for use in removing contaminants in wastewater.