Preventing the formation of the pyrochlore phase in the synthesis of PZN-PT powders requires controlling calcination parameters such as temperature, soaking time and atmosphere. These parameters were examined extensively to determine the time and temperature at which the perovskite phase is the majority phase, as well as the atmosphere that facilitates the formation of this phase. A maximum of 74% of perovskite phase was obtained under the following conditions: 1000°C, 4h in nitrogen atmosphere. In this work, we studied the influence of these parameters, which were optimized, on the formation the perovskite phase in PZN-10PT powders synthesized by the conventional solid state method.
Evitar la formación de la fase pirocloro en la síntesis de polvos de PZN-PT requiere el control de parámetros en el proceso de calcinación tales como temperatura, tiempo y atmósfera utilizada en el tratamiento térmico. Estos parámetros fueron examinados extensamente para determinar el tiempo y la temperatura a la que la fase perovskita es la fase mayoritaria, así como la atmósfera que facilita la formación de esta fase. Se obtuvo un 74% de la fase perovskita bajo las siguientes condiciones: temperatura de 1.000°C por un tiempo de 4h en atmósfera de nitrógeno. En este trabajo se estudió la influencia de estos parámetros, que fueron optimizados, en la formación de la fase perovskita en polvos cerámicos de PZN-10PT sintetizados por el método de estado sólido convencional.
Lead zinc niobate, Pb(Zn1/3Nb2/3)O3 (PZN), is a very interesting ferroelectric material because of its excellent dielectric and electrostrictive properties [1]. Solid solutions of PZN with rhombohedral symmetry and PbTiO3 (PT) with tetragonal symmetry have a morphotropic phase boundary (MPB) of nearly 10mol% PT [2,3]. Single crystals whose composition is close to the MPB show an extremely high dielectric constant (k∼22,000) and piezoelectric coefficients (Kp∼92%, d33∼1500pC/N) [2,3]; maximum d33 values of ∼2500pC/N were determined in rhombohedral PZN-8% PT crystals oriented along 〈001〉 [4]. However, it has been reported that perovskite PZN or 0.90PZN-0.10PT (PZN-10PT) crystals are thermodynamically unstable over a wide range of temperatures (600–1400°C), rapidly yielding pyrochlore phase, PbO and ZnO as products of decomposition [5,6].
It is well known that PZN and PZN-PT ceramics (in MPB compositions) with single-phase perovskite are difficult to obtain by conventional ceramic processing under environmental conditions of pressure and temperature without the application of mechanical pressure. This difficulty is due to the inherent instability of PZN at different temperatures. In Pb-based complex perovskites, PbO evaporates easily at high temperatures and forms undesirable phases. The presence of an undesirable phase such as cubic pyrochlore, even in small amounts, is detrimental to both dielectric and piezoelectric properties. Much effort has focused on eliminating the cubic pyrochlore phase and on stabilizing the perovskite phase in the PZN-PT system. These attempts include various ceramic and chemistry-based processing routes such as oxide mixtures, columbite and sol-gel methods, or even novel ones such as combustion [7], Pechini [8], mechanochemical [9] methods and forming solid solutions with more stable perovskites [10]. Ceramics were obtained by the combination of mechanosynthesis and spark-plasma sintering [11]. However, the single crystal growth technique is the only method that has succeeded in yielding PZN-PT material with feasible perovskite phase. Processing routes that involve polycrystalline forms produce a pyrochlore phase with or without a small amount of perovskite phase in the resulting material.
In this work, we studied the influence of those parameters, which were optimized, on the formation the perovskite phase in PZN-10PT powders synthesized by the conventional solid state method. The calcination process was carried out at temperatures ranging from 850°C to 1050°C, applying different soaking times of 1 to 6h. Four different atmospheres were used in this process: oxygen, air, argon and nitrogen. The powders obtained at different temperatures, soaking times and atmospheres were analyzed by X-ray diffraction (XRD). In addition, the powders calcined at 1000°C for 4h in each atmosphere were characterized by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) to detect phases that were not observed by XRD.
Experimental procedureCeramic powders of 0.90Pb(Zn1/3Nb2/3)O3–0.10PbTiO3 (PZN-10PT) composition were obtained by means of a conventional solid state reaction, following the columbite precursor method [12]. The columbite precursor was first prepared by mixing stoichiometric amounts of high purity of ZnO (99.9%) and Nb2O5 (99.8%) powders. These starting chemicals were ball milled together in isopropyl alcohol for 20h, using zirconia balls as milling media. The resulting slurry was dried at 60°C and calcined for 4h at 1000°C to form ZnNb2O6. The columbite precursor was then mixed and ball-milled with pre-determined amounts of litharge PbO (99.9+%) and rutile TiO2 (95+%). The powders were then ball milled again for 20h using the same conditions, after which a sample of 5g these powders were calcined in different conditions of temperature, soaking time and atmosphere, and the resulting phases were analyzed by XRD. The powders calcined at 1000°C for 4h in the four atmospheres were characterized by SEM (Philips XL30-FEG) and EDS (Philips XL30-FEG). XRD patterns were recorded in a Siemens 5100 diffractometer, using CuKα radiation in the 2θ range from 20° to 60° to determine the relative amounts of pyrochlore and perovskite phases. The percentage of perovskite phase was calculated using the following equation [12]:
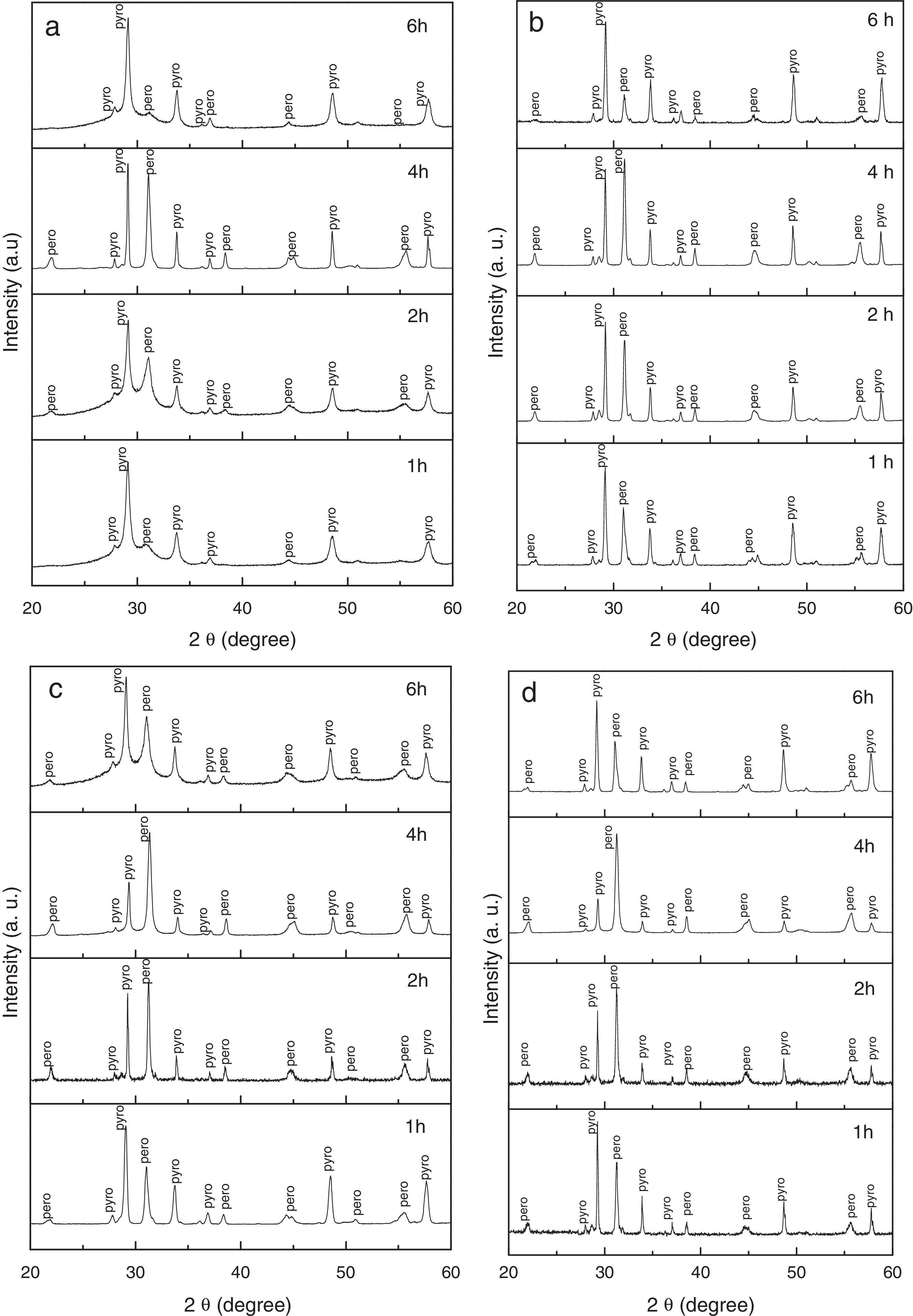
where Ipero and Ipyro are the intensities of the highest peaks corresponding to perovskite (110) and pyrochlore phase (220), respectively.Results and discussionAn in-depth study of the influence of temperature on perovskite phase formation and pyrochlore-perovskite phase transformation led to the conclusion that a temperature of 1000°C allows for the kinetic reconstruction of the cations and anion sublattices involved in this transformation. Based on the above, we examined the influence of time on the formation of perovskite phase and the pyrochlore-perovskite phase transformation at this temperature. The mixture of PbO, ZnNb2O6 and TiO2 powders was subjected to calcination tests as a function of atmosphere, applying soaking times of 1h to 6h at 1000°C. Fig. 1 shows a series of XRD patterns of these powders calcined in (a) oxygen, (b) air, (c) argon, and (d) nitrogen atmospheres. All the diffraction patterns confirmed that 1hour suffices to complete the reaction between the precursors, with pyrochlore and perovskite phases coexisting in all the atmospheres. An analysis of the effect of the calcination atmosphere at each soaking time indicated that the percentage of perovskite phase showed a tendency to increase in the following order: oxygen, air, argon and nitrogen. Regardless of the atmosphere, however, the highest concentrations of perovskite phase occurred within a 4-hour time frame, and were 47%, 52%, 66% and 74%, respectively, in the aforementioned atmospheres.
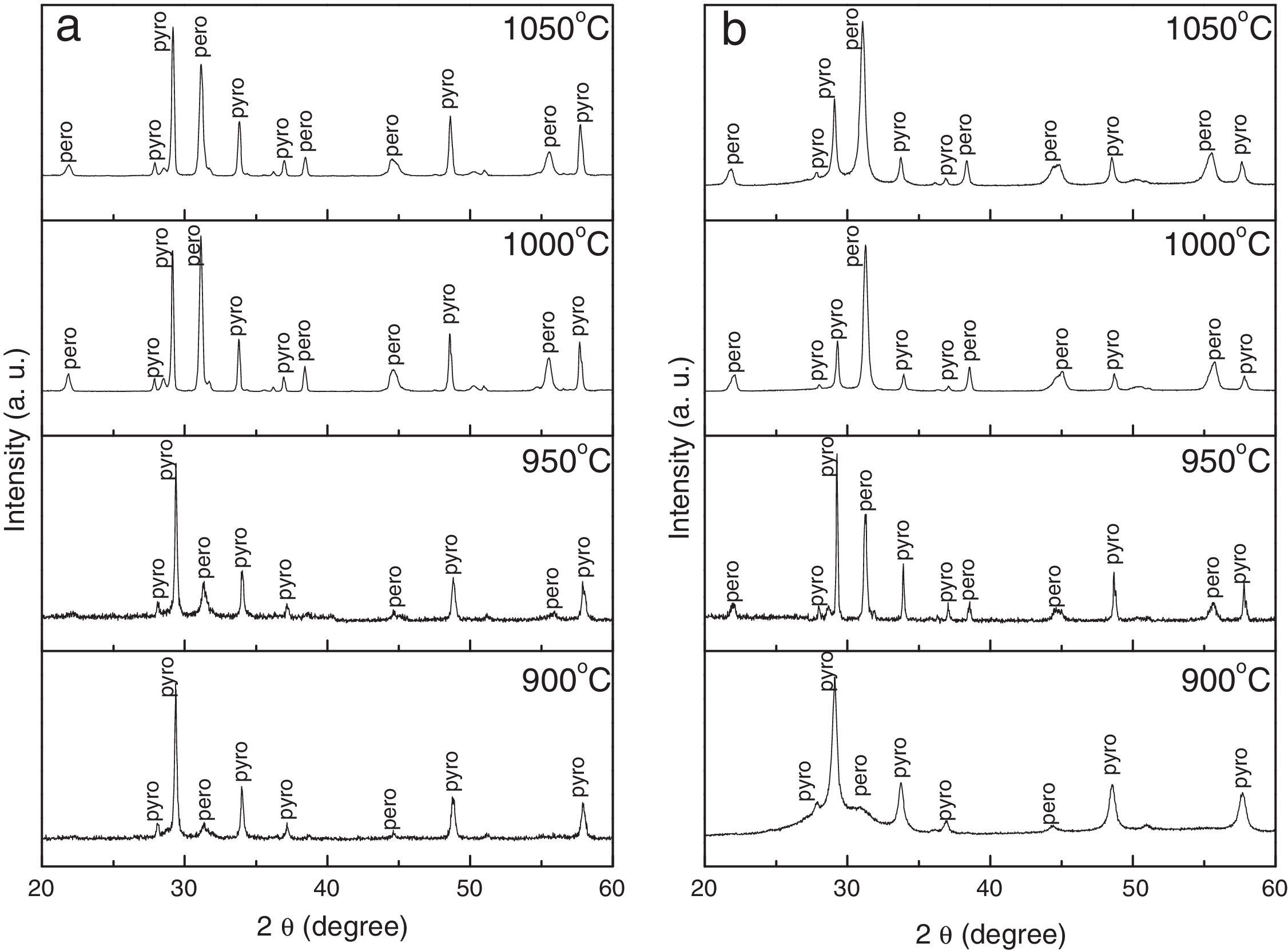
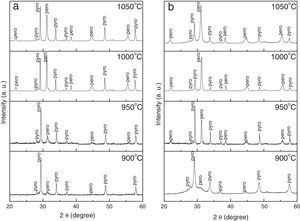
Fig. 2 shows X-ray diffraction patterns of mixture of PbO, ZnNb2O6 and TiO2 precursor powders calcined at 900°C, 950°C, 1000°C and 1050°C for 4h, at a heating/cooling rate of 5°C/min, in (a) air and (b) nitrogen atmospheres. Intensity of the peaks in all XRD spectra is increased as the calcination temperatures increase. However, when the calcination temperature increases over the optimum condition (1000°C), the perovskite phase is easily decomposes. As can be seen in Fig. 2, the precursor powders reacted initially to form the pyrochlore phase, after which the perovskite phase was formed, nucleated and began to grow starting from the pyrochlore-perovskite phase transformation. This process took place at temperatures above 850°C, and since this is a diffusive process, the phase transformation kinetics was probably accelerated at higher temperatures. However, higher temperatures and longer processing times compete with the stability of the perovskite phase due to the volatilization of PbO and/or ZnO; hence, the maximum concentration is determined by specific temperature and time processing conditions. Although the atmosphere affects the percentage of perovskite phase considerably, the transformation and growth mechanisms are expected to be the same under all the aforementioned atmospheres, since time and temperature conditions do not change as a function of atmosphere.
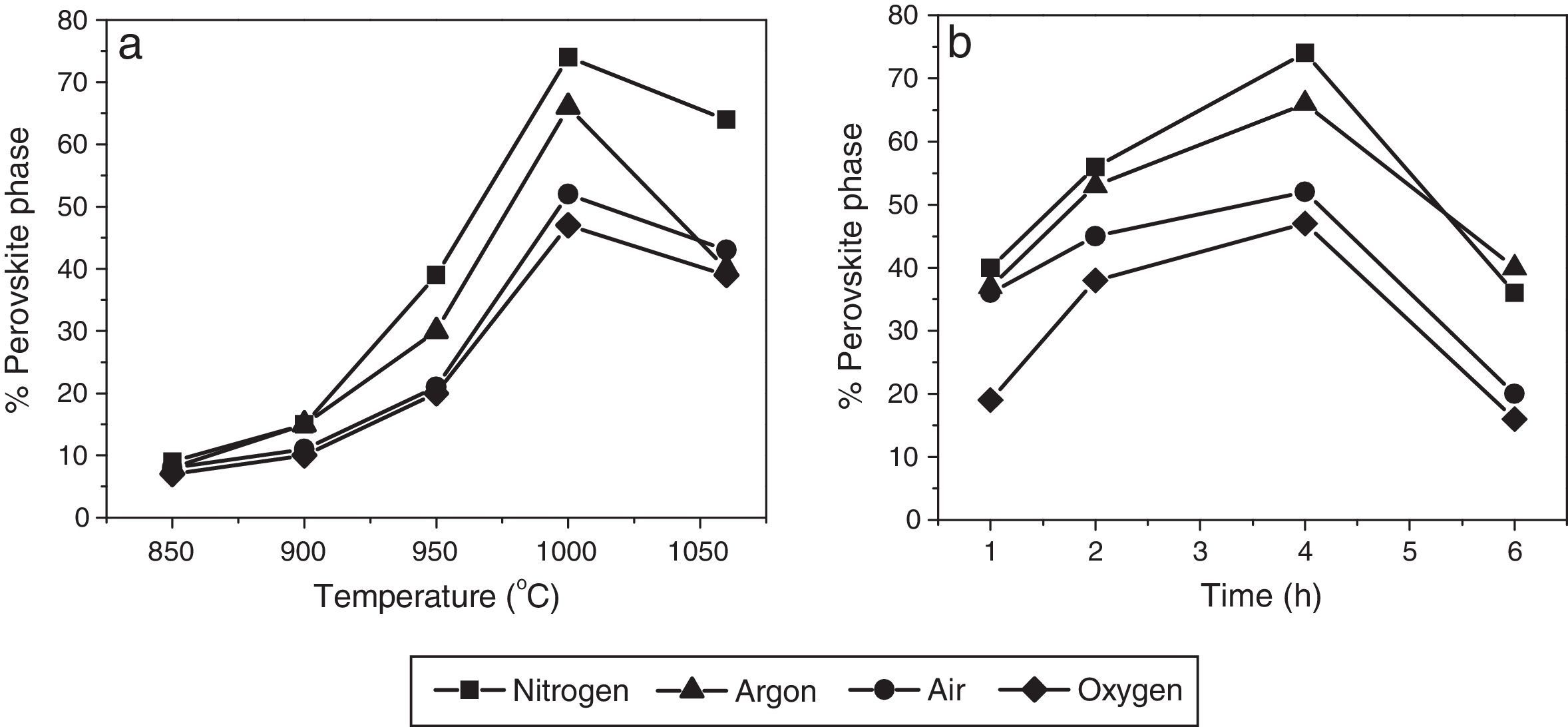
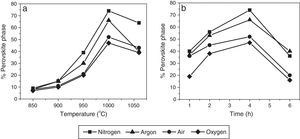
Fig. 3a shows the percentages of perovskite phase corresponding to powder obtained by the mixed oxides method and calcined under the four aforementioned atmospheres for 4h, from 850°C to 1050°C. The influence of the soaking time on the pyrochlore-perovskite phase transformation was analyzed by calcining the powders for 1–6h in the same atmospheres, at a constant temperature of 1000°C, as illustrated in Fig. 3b.
An analysis of the effect of atmosphere at each of the analyzed temperatures (Fig. 3a) revealed that the percentage of perovskite phase tended to increase in the following order: oxygen, air, argon and nitrogen. However, the maximum concentration of perovskite phase was reached at a temperature of 1000°C, regardless of the atmosphere, and was 74% for nitrogen and 47% for oxygen. The analysis as a function of soaking time (Fig. 3b), also regardless of the atmosphere, indicated that the maximum concentration of perovskite phase was reached in 4h. The decrease in perovskite phase at temperatures above 1000°C and calcination times of more than 4h was likely due to the volatilization of PbO and/or ZnO, which would explain the decomposition of the pyrochlore phase.
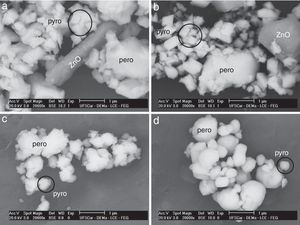
In these powders, the perovskite and pyrochlore phases and a zinc oxide-rich phase (not detected in the XRD patterns) were identified by SEM (Fig. 4) coupled with EDS. The perovskite phase was particularly visible in the powders processed in oxygen and air atmospheres. The presence of zinc oxide-rich phase appears to be due to its precipitation during the decomposition of perovskite in the pyrochlore phase, since the former contains more zinc than the latter. Since pyrochlore phase is formed at lower temperatures than perovskite phase, the latter is formed by diffusive processes occurring in the former in response to increasing temperature. These processes generate oxygen vacancies and cause mainly zinc diffusion, which in turn determines the formation of perovskite phase, since the segregation of this element of the structure through evaporation or oxidation prevents the formation of ferroelectric phase. Thus, although the atmosphere does not affect the pyrochlore-perovskite phase transformation mechanisms, it influences the concentration of perovskite phase by facilitating or hindering the decomposition process, i.e., it enables the metallic oxides in the structure to become volatilized.
The transformation of the pyrochlore-perovskite phase in PZN-PT was also limited by the presence of oxygen at 1000°C. This atmosphere released the ZnO from the newly formed crystalline structure, a phenomenon that was confirmed by the EDS analysis. The ZnO-rich phase appeared in the powders calcined in oxygen and air atmospheres, but not in those calcined in argon or nitrogen atmospheres under the previously described conditions.
ConclusionsThe XRD patterns confirmed that 0.9PZN-0.1PT powders with large amounts of perovskite phase can be synthesized by the conventional method. The highest percentage of perovskite phase was obtained under the following conditions: a temperature of 1000°C, calcination time of 4h, and nitrogen atmosphere. The nitrogen atmosphere favored the pyrochlore-perovskite phase transformation by inhibiting of the PbO and/or ZnO volatilization of the crystalline structure. The presence of oxygen prevents the pyrochlore-perovskite phase transformation because it inhibited the release of oxygen from the structure, causing the precipitation of ZnO and the formation of pyrochlore phase under the conditions employed in this study.
The authors gratefully acknowledge the financial support of FAPESP and CNPq Brazilian agencies for financial support, and the Universidad del Cauca (Colombia) for its support of Professor Claudia Fernanda Villaquiran Raigoza during this research work.