This paper provides direct evidence to support the role of capping agents in controlling the evolution of TiO2 seeds into nanocrystals with a specific shape. Starting with Ti(OBut)4 and using oleid acid, oleylamine, dioleamide, 11-aminoundecanoic acid, arginine, trifluroacetic acid or HF as capping agents, mainly TiO2 truncated octahedrons enclosed by {101} and {001} facets were obtained. We could also selectively obtain square, rods and rounded rhombic-shaped nanoparticles by growing of {010} facets by adding oleic acid and oleylamine in ratio 6:4, respectively, while all other parameters were kept the same. This research not only offers new insights into the role played by a capping agent in shape-controlled synthesis but also provides, a versatile approach to controlling the shape of metal oxide nanocrystals.
El presente trabajo proporciona una evidencia directa del papel de los surfactantes en el control de la cristalización de nanopartículas de TiO2 con una determinada morfología. Empleando Ti(OBut)4 como producto de partida y usando ácido oleico, oleilamina, dioleilamida, ácido 11-aminoundecanoico, arginina, ácido trifluoroacético o HF como surfactantes se han obtenido, principalmente y de un modo selectivo, octaedros truncados de TiO2, formados por caras {101} y {001}. También se han obtenido de modo selectivo cubos, varillas y romboedros-redondeados, por crecimiento de las caras {010}, mediante la adición de ácido oleico y oleilamina en relación de 6:4, respectivamente, mientras que el resto de parámetros se mantuvieron iguales. Esta investigación no sólo ofrece nuevas perspectivas sobre el papel desempeñado por un surfactante en la obtención de nanocristales con una morfología específica, sino que también ofrece un enfoque versátil para controlar la forma final de nanopartículas de óxidos metálicos.
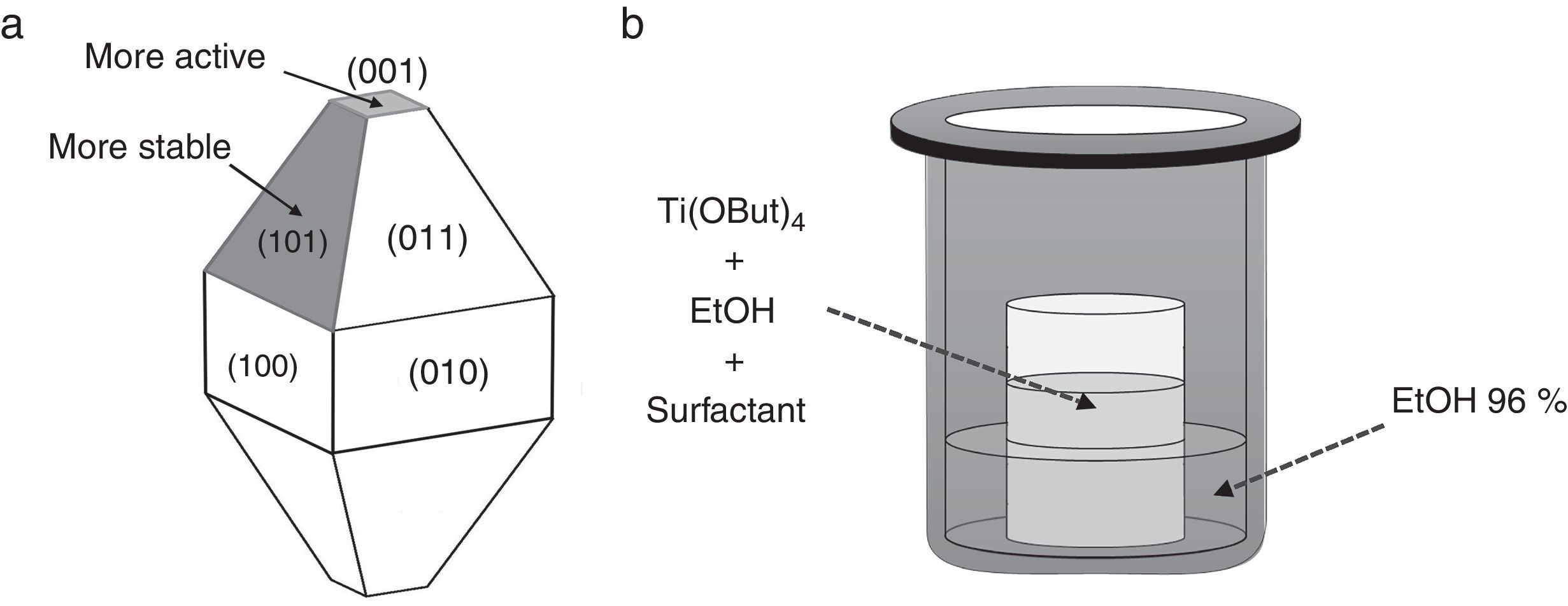
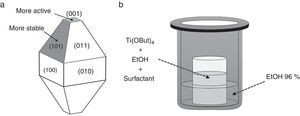
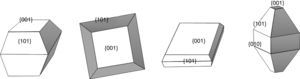
Photocatalyst materials based on semiconductors have been widely studied in the last decades due to their potential application in diverse fields, such as pollutants elimination or water splitting by photocatalytic processes. Among these materials, titanium dioxide has attracted much attention in recent years due to its exceptional optical and electronic properties, high efficiency, high photo-stability, strong oxidizing power, non-toxicity, chemical stability, and low cost [1–4]. It has been reported that the photoactivity of TiO2 anatase, the most active photocatalyst [5–11], is influenced by different properties such as surface area, crystallinity, crystallite size and crystal structure [12,13]. But, this photoactivity depends not only on these properties, but also on the specific morphology of the particles, which determines which crystal facets are exposed, having a strong influence on the photocatalytic performance, e.g. they can lead to a reduction and the formation of electron traps and thus facilitate the electron transfer in the semiconductor structure [14–21]. Both theoretical and experimental studies have shown that the (001) surface of anatase TiO2 with 100% Ti five-fold coordinated (Ti5c) is much more reactive than the thermodynamically more stable {101} facets with 50% Ti5c and 50% Ti6c (Fig. 1a) [22–26]. Unfortunately {001} facets are energetically unfavorable, this means that under normal conditions the synthesized TiO2 crystals consist mainly of poorly reactive {101} facets. Accordingly, the synthesis of well-crystallized and nanostructured TiO2 particles with tailored morphology represents a current major challenge. In order to reverse this situation, i.e. to stabilize the more reactive facets, one effective strategy is choosing the most convenient working conditions. In this sense, the use of morphological agents that cap the growth in one or more facets, plays a critical role controlling the ratio of growth for specific facets, making the crystal develop only in certain directions and hence altering/controlling the morphology and size distribution of the nanoparticles [27].
To address this issue, in this work we report a set of experiments based on seeded growth to single out the role of different compounds, used as morphological control agents during the synthesis of TiO2 anatase nanoparticles. The use of several capping agents with different functional groups, carboxylic, amine or amide groups, has been evaluated. Diverse morphologies have been obtained, with different percentages of {001} facets, depending on the used control agent and the working conditions. The synthetic approach used is based on a non-aqueous technique and the controlled addition of water to the media, in order to accelerate the hydrolysis of the titanium precursor, Ti(ButO)4. This water comes from an azeotropic mixture of ethanol-water or a controlled addition, so that the grown kinetics of the particles is slowed down. Different capping agents (oleid acid, oleylamine, dioleamide, 11-aminoundecanoic acid, arginine and trifluroacetic acid) have been added to control the crystal growth in one or more facets, obtaining TiO2 nanocrystals with a controlled and tailored morphology. In addition, the synthesis of TiO2 nanoparticles was also carried out by a hydrothermal procedure using HF as morphological control agent. This work provides a versatile approach to controlling the shape of metal oxide nanocrystals by varying the capping agent while other parameters are kept the same.
ExperimentalChemicalsThe chemicals titanium(IV) tetrabutoxide (Ti(OC4H9)4, Fluka, 98%), ethanol (EtOH, Merck, analytically pure), hydrofluoric acid (HF, 48%, JT Baker), oleic acid ((Z)-octadec-9-enoicacid, CH3(CH2)7CHCH(CH2)7COOH, Fluka, analytically pure, OA), oleylamine ((Z)-octadec-9-en-1-amine, CH3(CH2)7CHCH(CH2)7CH2NH2, Aldrich, 70%, OM), trifluoroacetic acid (CF3COOH, Aldrich, 70%, TFAA), arginine (2-amino-5-carbamimidamidopentanoic acid, H2NC(NH)NH(CH2)3CH(NH2)CO2H, AR) and 11-aminoundecanoic acid (NH2(CH2)10CO2H, UDA) were used without further purification.
Synthesis of TiO2 nanoparticles in presence of OA and OM (Ti-OAOM)The synthesis was carried out on the basis of a previously reported methodology [28], but introducing some modifications. In a typical procedure, Ti(OBut)4 (5mmol) was added to a mixture of X mmol OA, Y mmol OM, in 5:5 or 6:4 ratios, and 6ml of dry ethanol in a Teflon beaker. The Teflon beaker containing the obtained mixture is placed into a 50ml Teflon-lined stainless steel autoclave (see Fig. 1b). The system is then heated at 180°C for 72h. The obtained precipitate was washed with dry ethanol and deionized water several times and then dried at 105°C.
Synthesis of TiO2 nanoparticles in presence of AR or UDA (Ti-AR or Ti-UDA)In a typical procedure, Ti(OBut)4 (5mmol) is dissolved into 6ml of dry ethanol in a Teflon beaker together with 10mmol the surfactant. The Teflon beaker containing the obtained mixture is placed into a 50ml Teflon-lined stainless steel autoclave (see Fig. 1b). The system is then heated at 180°C for 72h. The obtained precipitate is washed several times with water and ethanol (96%) and then dried at 105°C.
Synthesis of TiO2 nanoparticles in presence of TFAA (Ti-TFAA)They were synthesized based on a previously reported procedure [29]. 5ml of Ti(OBut)4 are introduced in a 50ml Teflon-lined stainless steel autoclave, together with 1.9g of TFAA. A small amount of deionized water (0.4ml) is added to accelerate the hydrolysis reaction. The system is then heated at 200°C for 72h. The obtained white-brown precipitate is washed several times with water and ethanol (96%) and then dried at 105°C. The washed solid is completely cleaned by irradiation under UV–vis light of a suspension in water for 6h.
Synthesis of TiO2 nanoparticles with HF (Ti-HF)5ml of Ti(OBut)4 are introduced in a 50ml Teflon-lined stainless steel autoclave, together with 0.6ml of HF. The system is then heated at 180°C for 72h. The obtained white precipitate is washed several times with water and ethanol (96%) and then dried at 105°C.
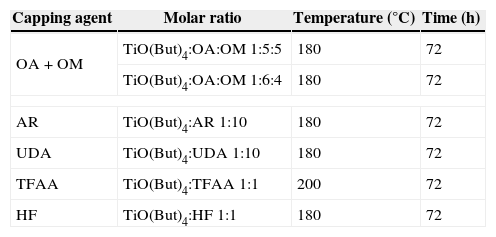
Table 1 summarizes reaction conditions for each morphological control agent.
Reaction conditions used in the synthesis of TiO2 nanoparticles.
| Capping agent | Molar ratio | Temperature (°C) | Time (h) |
|---|---|---|---|
| OA+OM | TiO(But)4:OA:OM 1:5:5 | 180 | 72 |
| TiO(But)4:OA:OM 1:6:4 | 180 | 72 | |
| AR | TiO(But)4:AR 1:10 | 180 | 72 |
| UDA | TiO(But)4:UDA 1:10 | 180 | 72 |
| TFAA | TiO(But)4:TFAA 1:1 | 200 | 72 |
| HF | TiO(But)4:HF 1:1 | 180 | 72 |
The analyses of the crystalline structure and the phase identification were performed by X-ray diffraction (XRD Bruker D8 ADVANCE, Madison, WI, USA) with a monochromatized source of Cu-Kα1 radiation (λ=1.5406Å) at 1.6kW (40kV, 40mA); samples were prepared by placing a drop of a concentrated ethanol dispersion of particles onto a single crystal silicon plate. Transmission electron microscopy (TEM) images were obtained on a JEOL 2100 F TEM/STEM (Tokyo, Japan) operating at 200kV and equipped with a field emission electron gun providing a point resolution of 0.19nm; samples were prepared by placing a drop of a dilute ethanol dispersion of nanoparticles onto a 300-mesh carbon-coated copper grid and evaporated immediately at 60°C.
Results and discussionTiO2 particles with distinct morphologies have been synthesized using different capping agents under a non-aqueous technique and the controlled addition of water to the media, or through a hydrothermal method using HF as the morphological control agent. All reactions were carried out at using Ti(ButO)4 as the starting reagent because it presents a slow rate of hydrolysis, attributable to the butoxide group which slows down the process of diffusion and polymerization compared to other alkyl groups.
To obtain the TiO2 nanoparticles a semi-solvothermal synthesis methodology was used, with EtOH (96%) providing the water necessary to accelerate the hydrolysis reaction, and oleic acid (OA) and oleylamine (OM), 11-aminoundecanoic acid, arginine or trifluroacetic acid as capping agents. In the case of the reaction with HF, a hydrothermal method was used from the basis of that reported by Li et al. [30]. In those experiments involving OA, OM, AR and UDA, the Ti(OBut)4: surfactant molar ratio was kept constant at 1:10 and in the case of using TFAA and HF as the capping agents the molar ratio was 1:1.
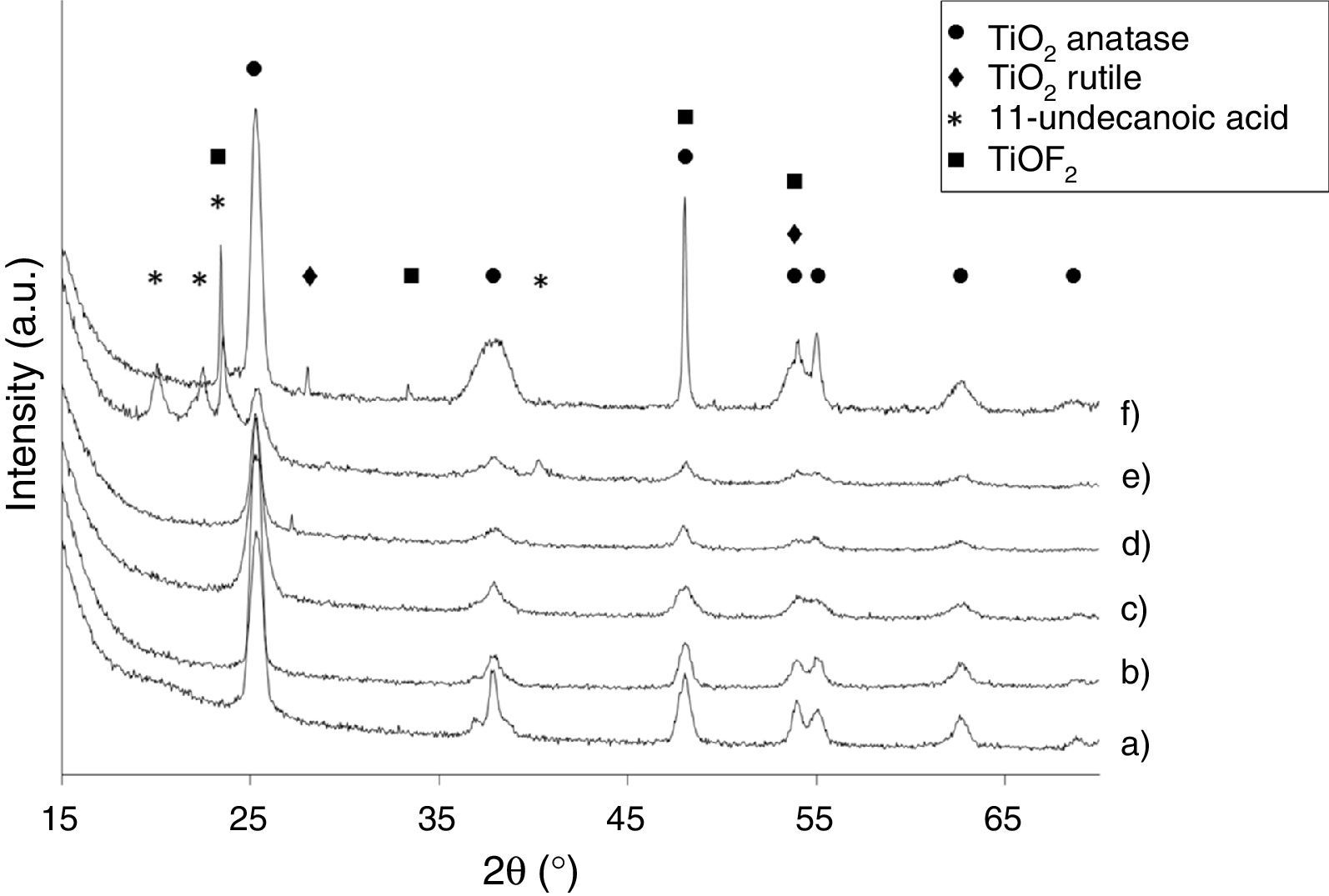
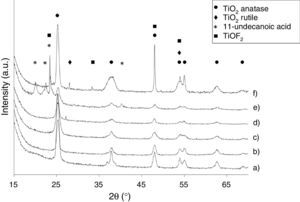
Effect of surfactants and surfactants ratioThe crystallinity of the synthesized samples was verified by selected area electron diffraction (SAED) and X-ray powder diffraction. As shown in Fig. 2, the crystallization of pure anatase phase (JCPDS File no. 21-1272) is observed for Ti-OAOM5, Ti-OAOM6, and Ti-TFAA (Fig. 2a–c). In the case of the reaction with TFAA the reaction is carried out at 200°C in order to improve the crystallinity of the TiO2 nanoparticles, because at 180°C the particles do not show almost crystallinity. The X-ray difractogram of Ti-AR shows the presence of an additional phase corresponding to rutile TiO2 (JCPDS file no. 21–1276) (Fig. 2d). The formation of a rutile TiO2 in the reaction with AR suggests that this molecule stabilizes the anatase phase less effectively and this partially evolves to the highest temperature phase during synthesis, the rutile phase. For the case of Ti-UDA, the X-ray difractogram reveals the presence of peaks corresponding to 11-undecanoic acid (Fig. 2e), probably physic-chemically adsorbed on the surface of the particles. Finally, X-ray data for Ti-HF product indicates the formation of a TiOF2 phase, probably due to the formation of an interphase between the TiO2 anatase phase and the F− adsorbed over the surface of the TiO2 nanoparticles (Fig. 2f). The morphological changes of the particles are not appreciated in the XRD peak area ratios corresponding to the {101} and {004} facets; this must be attributed to the way the samples are prepared for the XRD measurements, which favors the orientation of the nanoparticles with the {101} facets parallel to the substrate, the most entropic orientation in most of the cases [29].
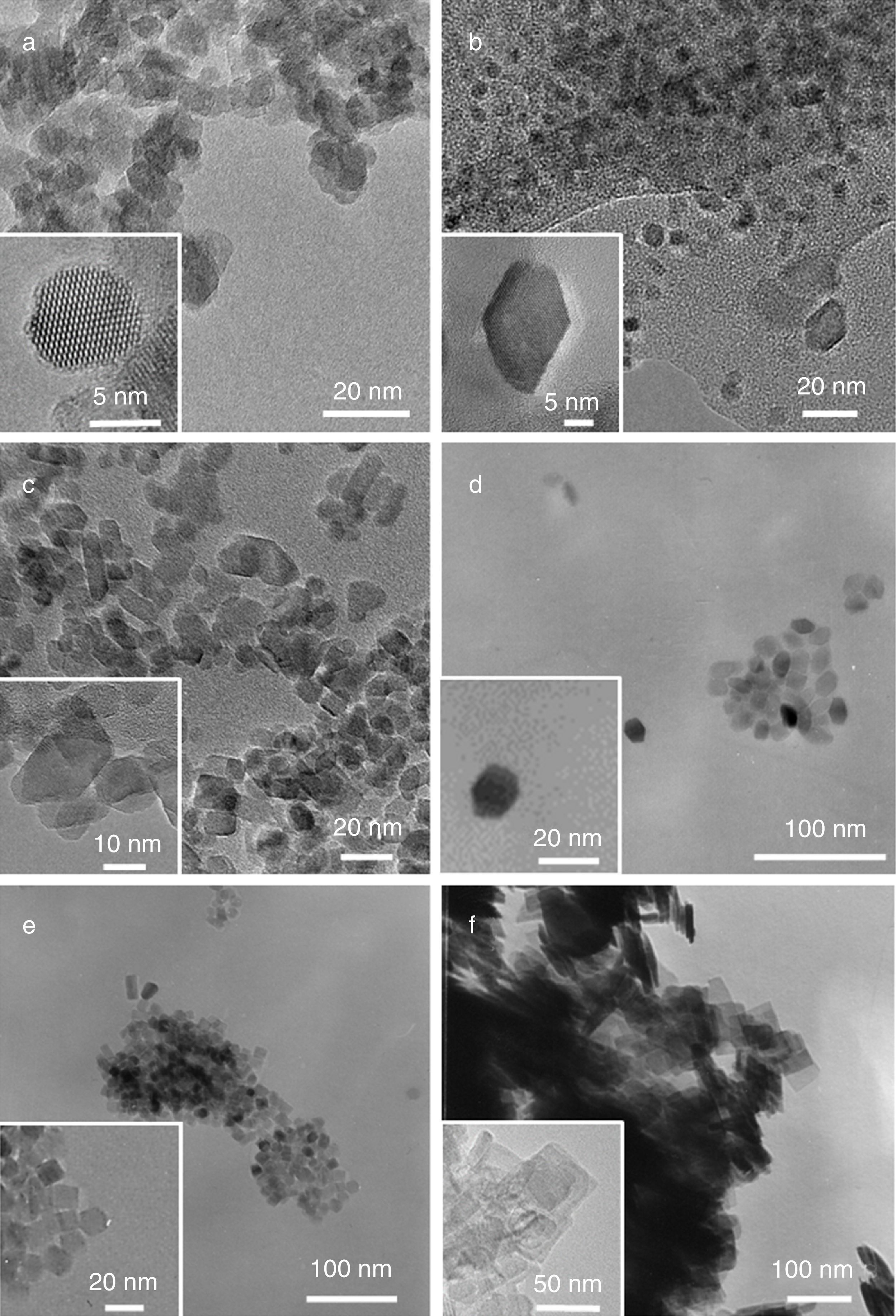
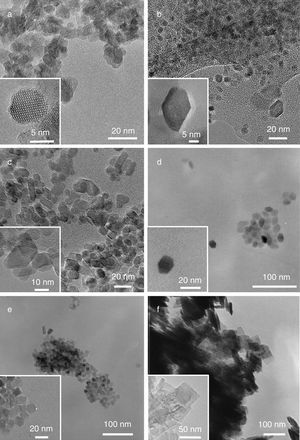
In all the reaction conditions TiO2 well faceted nanoparticles are obtained with different morphologies depending on the surfactant and the experimental conditions, except in the presence of AR, in which non-well defined morphology is obtained (Fig. 3a).
TEM micrographs of: (a) the rounded crystals of Ti-AR, (b) truncated rhombic-shaped nanoparticles of Ti-UDA, (c) truncated rhombic-shaped nanoparticles of Ti-TFAA, (d) TiO2 truncated octahedrons obtained by using Ti:OA:OM in a 1:5:5 ratio (Ti-OAOM5), (e) mixture of square, rods and rounded rhombic-shaped TiO2 nanoparticles prepared under a Ti:OA:OM in a 1:6:4 ratio (Ti-OAOM6), (f) nanosheets of TiO2 obtained using HF as capping agent (Ti-HF).
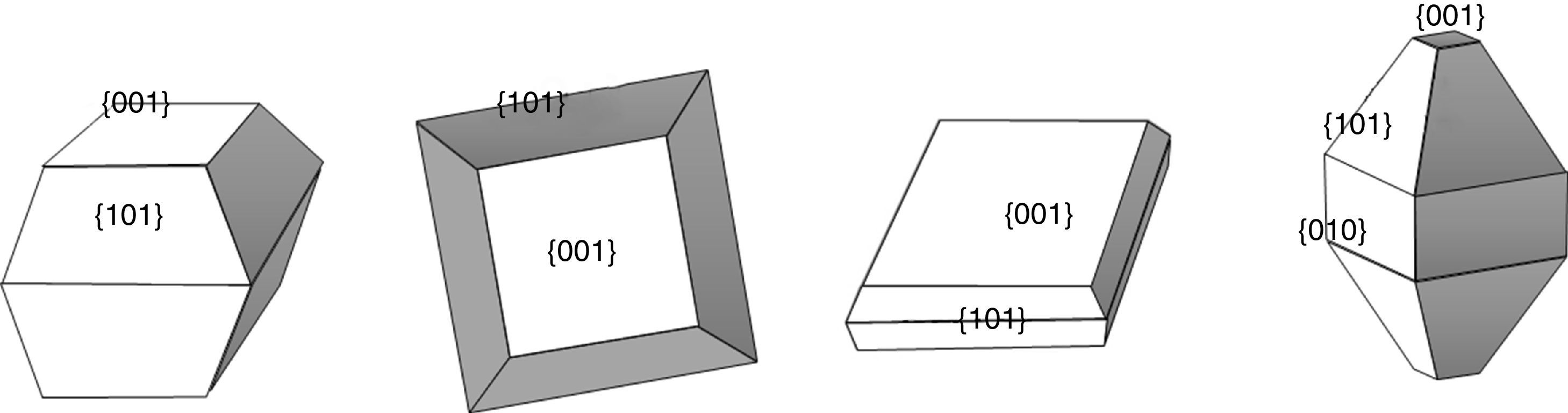
As it can be observed, when UDA, TFAA or OA/OM in a 5:5 ratio were used as capping agents, truncated rhombic-shaped TiO2 nanoparticles are obtained, with a size of about 20nm (Figs. 3b–d). To evaluate the effect of OA/OM ratio, the ratio was modified to 6:4, which leads to the formation of TiO2 nanoparticles with similar size but a mixture of square, rods and rounded rhombic-shaped morphologies (Fig. 3e). Finally, when HF was introduced as the morphological control agent nanosheets of TiO2 were obtained (Fig. 3f). These morphologies can be explained by the different degree of truncation of the particles and the observation direction. Thus if the octahedron has a high degree of truncation square plates are observed, whereas a lower level of truncation leads to hexagonal shapes (Fig. 4). In the case of Ti-OAOM6 the nanorods morphology is probably due to an elongation of truncated octahedra along the [001] direction by the growth of new facets {010}.
The changes in the growth habit of the TiO2 nanoparticles obtained must be interpreted as an effect of two main processes which control the formation of TiO2, i.e. hydrolysis of the titanium precursor and the subsequent condensation reactions to form a Ti-O-Ti network [28,31]. By using surfactants with different functional groups and distinct binding strengths, the morphology of resulting particles can be controlled [32–34]. In our case and as it has been previously reported, carboxylic groups [28] and fluorine groups binds to the TiO2 {001} facets and stabilize them [16,22], whereas amine groups tends to adhere on the {101} ones [20]. These preferences of the functional groups for one facet or another is caused by the {001} facets having 100% of five-coordinate Ti (Ti5c) in contrast to {101} facets with only 50% Tic atoms, which results in a different surface chemistry [35].
Therefore, the Ti-HF composition has the highest percentage of {001} facets, leading to the formation of nanosheets where this facet is predominant. This is probably because the F atoms exert a stronger stabilizing effect than the O atoms on {001} facets [16]. In the case of Ti-TFAA a higher stabilization of this facet with respect to the other products is also observed, due to TFAA being degraded during the synthesis releasing F ions, and therefore truncated octahedrons and nanosheets are observed. If AO or UDA are used as capping agents the stabilization of {001} facets is exerted through the O from the carboxylic groups, whose stabilizing effect is not as drastic as the fluorine one, and therefore morphologies where the facet {001} is stabilized but not predominant are observed, leading mainly to truncated octahedrons. Thus, varying the characteristics of the surfactants it is possible to modulate the rate of hydrolysis and control the formation of TiO2 with specific shape and size. In the case of the use of OA/OM as capping agents, there is an additional factor that must be taken into account: oleylamine and oleic acid condense exothermically in situ forming dioleamide (DO) [28]. This molecule also acts a surfactant binding more selectively to the {001} facets through the carboxylic group and in a weaker way to the {101} facets through the NH group. So, when a Ti:OA:OM 1:5:5 ratio is used the DO is the only surfactant but when the ratio is changed to 1:6:4 there is an equilibrium between DO and the excess of OA reacted, which explains the change in the growth habit of the TiO2 nanoparticles leading to obtaining of a mixture of square, rods and rounded rhombic-shaped TiO2 nanoparticles in the case of 1:6:4 ratio. These morphologies can be also explained by the different degree of truncation of the particles and the observation direction together with an elongation, in this case, of truncated octahedra along the [001] direction due to the growth of new facets {010} (Fig. 3). This situation can be ascribed to a kinetic regime [36]. Actually, our experiments with 6:4 OA/OM molar ratio suggest that the growth of the {001} facets is initially faster than that of the {010} ones, since for shorter reaction times (not show here) only the formation of these facets is observed [28], but beyond a certain point which we achieve by increasing the reaction time, the preferential growth switches to the {010} facets [28]. Furthermore, Barnard et al. [37] also found that the formation of these new {010} facets is encouraged by conditions in which oxygenated surfacets are favored, something that we have in this conditions samples with a higher amount of OA (6:4M ratio) in the reaction medium.
ConclusionsIn summary, we have demonstrated the use of controlled growth for directly comparing, the effects of capping agents on shape control for crystalline TiO2 nanocrystals. Choosing carefully the working conditions and using different morphological control agents are crucial factors to control the morphology of the obtained particles. Different capping agents have been studied, both fluorine based compounds and compounds with carboxylic and amine functional groups, which selectively stabilize particular facets of the anatase phase. We found that rounded, truncated rhombic-shaped, square, rounded rhombic-shaped and nanosheets morphologies of TiO2 could be selectively obtained by controlling the working conditions and introducing different molecules with different functional groups and distinct binding strengths as the capping agent. We expect this method could be further extended to quickly screen and evaluate the facet selectivity of a capping agent.
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) through the projects IPT-120000-2010-033 (GESHTOS), IPT-2011-1113-310000 (NANOBAC) and MAT2013-40722-R (SCOBA) and by the Comunidad de Madrid through the project MULTIMAT-CHALLENGE P2013/MIT-2862..