Proteomics is the study of the expression of changes and post-translational modifications (PTM) of proteins along a metabolic condition either normal or pathological. In the field of health, proteomics allows obtaining valuable data for treatment, diagnosis or pathophysiological mechanisms of different illnesses. To illustrate the aforementioned, we describe two projects currently being performed at the Instituto Nacional de Pediatría: The immuno-proteomic study of cow milk allergy and the Proteomic study of childhood cataract.
Cow's milk proteins (CMP) are the first antigens to which infants are exposed and generate allergy in some of them. In Mexico, the incidence of CMP allergy has been estimated at 5-7%. Clinical manifestations include both gastrointestinal and extra-gastrointestinal symptoms, making its diagnosis extremely difficult. An inappropriate diagnosis affects the development and growth of children. The goals of the study are to identify the main immune-reactive CMP in Mexican pediatric population and to design more accurate diagnostic tools for this disease.
Childhood cataract is a major ocular disease representing one of the main causes of blindness in infants; in developing countries, this disease promotes up to 27% of cases related to visual loss. From this group, it has been estimated that close to 60% of children do not survive beyond two years after vision lost. PTM have been pointed out as the main cause of protein precipitation at the crystalline and, consequently, clouding of this tissue. The study of childhood cataract represents an outstanding opportunity to identify the PTM associated to the cataract-genesis process.
La proteómica estudia los cambios de expresión y post-traduccionales (PTM) de las proteínas durante una condición metabólica normal o patológica. En el campo de la salud, la proteómica permite obtener datos útiles para el tratamiento, diagnóstico o en la fisiopatología de diferentes enfermedades. Para ilustrar lo anterior, describimos dos proyectos realizados en el Instituto Nacional de Pediatría: El estudio inmunoproteómico de la alergia a la leche y el estudio proteómico de la catarata infantil.
Las proteínas de leche bovina (PLB) son los primeros antígenos a los que se exponen los infantes y un porcentaje de ellos generará alergias. En México, se estima que la incidencia de alergias a las PLB es del 5-7%. Las manifestaciones clínicas incluyen tanto síntomas gastrointestinales como extra-gastrointestinales, dificultando su diagnóstico. Un mal diagnóstico afecta el desarrollo y crecimiento del infante. Los objetivos del estudio son identificar las principales PLB inmunoreactivas en población infantil mexicana y diseñar herramientas diagnósticas más precisas para esta patología.
La catarata infantil es una enfermedad ocular que representa una de las causas principales de ceguera infantil; en países subdesarrollados genera cerca del 27% de casos relacionados con pérdida visual. De este grupo, se estima que cerca del 60% de los infantes no sobreviven más allá de los dos años después de perder la visión. Se señala a las PTM como las responsables de la precipitación de proteínas del cristalino y, por tanto, de su opacidad. El estudio de la catarata infantil representa una oportunidad para identificar las PTM vinculadas con la cataratogénesis.
Proteomics can be defined as the study of expression changes and post-translational modifications (PTM) of proteins along a metabolic condition either in normal or pathological tissue, cell culture or microorganisms. Proteomics allows obtaining basic information for treatment assistance, diagnosis or pathophysiological mechanisms of different illnesses. To illustrate this point, we describe two research projects that have been performed at the Instituto Nacional de Pediatría: The immuno-proteomic study of cow milk allergy and the Proteomics study of childhood cataract.
Cow milk proteins (CMP) are the first antigens to which infants are exposed; fortunately, most kids tolerate such antigens. However, a percentage of children develop allergy. In Mexico, the incidence of CMP allergy has been estimated at 5-7%. A broad diversity of clinical manifestations appears during the first months of life including both gastrointestinal and extra-gastrointestinal symptoms making diagnosis extremely difficult. An inappropriate diagnosis promotes food rejection, fail to thrive syndrome, vomiting and persistent diarrhea; all of them affect the development and growth of children. The first work has the objective to identify the main immune-reactive CMP in Mexican pediatric population as well as the search for more accurate diagnostic tools for this disease.
On the other hand, childhood cataract is a major ocular disease and represents one of the main causes of blindness in the pediatric group; in developing countries, this disease promotes up to 27% of cases related to visual loss. From this group, 60% of children have been estimated do not survive beyond two years after vision lost. PTM have been pointed out as the main cause of protein precipitation in the crystalline and, consequently, clouding of this tissue. The study of childhood cataract represents an outstanding opportunity to identify the PTM associated to the cataract-genesis process.
2Immunoproteomics to CMP allergy2.1OverviewThe CMP allergy is the most common alimentary hypersensitivity in infants under three years of age. In the last decades, the world incidence of this disease has increased in developed countries due to a decreasing of breastfeeding practice. As a consequence, there is an early exposition of potential immune-reactive proteins in infants. In Mexico, the incidence of CMP allergy is unknown. However, it has been calculated that affects 5-7% of infants.1,2
There are no pathognomonic symptoms of CMP allergy; however, patients can exhibit gastrointestinal symptoms, extra-gastrointestinal symptoms (dermatologic and respiratory), and nonspecific clinical manifestations, which can occur either in isolated or combined forms.3 In general, the symptoms are linked to different immunological mediators, such as IgE, IgG, IgM antibodies and T cells (Th1 or Th2). These factors are related to several hypersensitivity mechanisms:
- o
Type I or immediate hypersensitivity, mediated by IgE.
- o
Type II or cytotoxic reaction, mainly mediated by IgM, IgG and complement factors.
- o
Type III or immune complexes, formed by IgG, IgM and complement factors (C3a, 4a, and 5a) that bind to circulating antigens.
- o
Type IV or cell-mediated reaction, like T lymphocytes, monocytes and macrophages.4–6
Currently, diagnosis of CMP allergy is based on a detailed clinical history (including atopy antecedents), detailed physical examination and eventually in vitro or in vivo tests to detect immunoreactivity against some specific allergens. Most of the in vitro tests evaluate few milk proteins (casein, β-lactoglobulin, and α-lactalbumin), by quantification of total or specific IgE present in serum. However, some patients with CMP allergy, and showing acute clinical manifestations, do not exhibit detectable levels of specific IgE against the evaluated allergens. Therefore, the gold standard for diagnosis is still the double-blind, placebo-controlled study of oral food challenge. It is established that the response of patients with CMP allergy mediated by IgE against milk is immediate.7–10 Together, these studies allow the diagnosis in affected children; however, individual data do not provide prognostic information or distinguish between the different phenotypes of the disease. It is important to underline that challenge trials must be performed with medical staff and properly equipped areas to take care of patients susceptible to develop anaphylactic shock.
Cow milk (Bos taurus) contains nearly 200 proteins,11 which may be potentially immuno-reactive. The main allergens include lactalbumin, bovine serum albumin, and caseins; the latter are located in micellar complexes conferring its milky appearance. Conventional treatments during current pasteurization do not secure the loss of protein immunoreactivity. Indeed, it has been demonstrated that their partial denaturalization induces exposition of new regions potentially allergenic, as reported for β-lactoglobulin.12
A common procedure for partial separation of milk proteins is the acidification method. Thus, upon milk acidification at pH 4.6, two main fractions can be obtained:
- i.
A clot mainly constituted of casein isoforms that conform 80% of total milk proteins.
- ii.
Proteins of whey that represents the remaining 20% of total milk proteins.
In B. taurus milk, the casein fraction consists of four isoforms contained in different amounts: αS1-casein (32%), α-S2-casein (10%), β-casein (28%) and κ-casein (10%); αS1-casein is considered the most relevant allergen from this group. Likewise, the reported allergens contained in whey are α-lactalbumin (5%), β-lactoglobulin (10%), immunoglobulins, bovine serum albumin and lactoferrin traces; α-lactalbumin and β-lactoglobulin are considered the main allergens from the whey fraction.13
In another prevalence study of immunoreactive CMP, in which assays like RAST and 2D-immunoblot were implemented, specific IgE levels against several proteins exhibiting different percentage of allergenic capacity were detected: IgG heavy chain (95%), α-S2-casein (90%), α-S1-casein (50%), lactoferrin (50%), β-lactoglobulin (45%), bovine serum albumin (45%) and β-casein (15%).14 Interestingly, none of the studied patients recognized α-lactalbumin as an allergen.
D’Amato et al. performed low-abundance protein enrichment from whey milk by using combinatorial peptide ligand library method.15 Along with 2D electrophoresis, the study allowed the identification of 149 different proteins, from which four were clearly identified as allergens: β-lactoglobulin, α-lactoalbumin, immunoglobulins, and lactoferrin.
On the other hand, Gasilova et al. conducted an analysis of allergens in whey milk; this team applied immunoaffinity capillary electrophoresis coupled to MALDI-TOF mass spectrometry to identify allergen traces.16 They identified two main proteins: β-lactoglobulin and α-lactalbumin.
The studies mentioned above indicate the existence of proteins of very low abundance with high allergenic capacity that can be identified by the implementation of high sensitivity techniques.
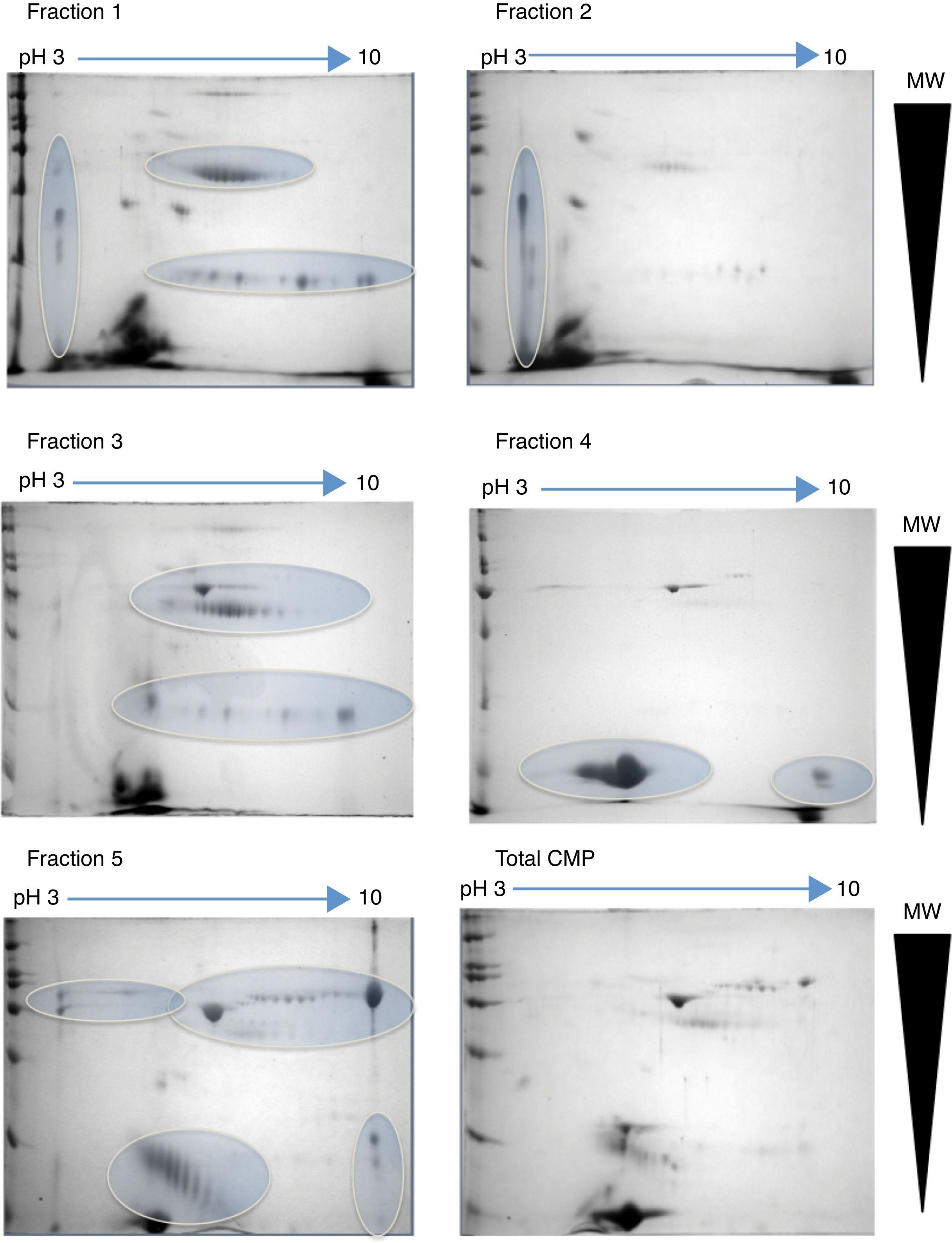
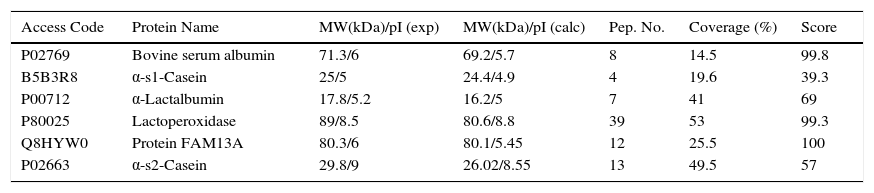
2.2Project progressAt the Instituto Nacional de Pediatría, we aimed to identify CMP with potential immunoreactivity to implement more precise diagnostic tests against allergy. In this scenario, the percentage of CMP allergic patients in the Mexican pediatric population is currently unknown. Hence, a combination of biochemical, immunological and proteomic techniques allowed us to make progress in this field. First, we enriched low abundance proteins from whey milk by ionic-exchange chromatographic methods, obtaining different protein fractions; these fractions were used to implement Western blot in 2D-electrophoresis by using samples of sera from both cow milk allergy patients and healthy children, to identify the immuno-reactive proteins (Figure 1). These latter were extracted from gels and processed for their identification through fingerprint method in MALDI-TOF mass spectrometry.17 The mass spectrometry data were processed in Protein-Lynx software in which we used a database of bovine and bovine milk proteins (downloaded from UniProt) for the search engine; each database contained respectively 117,686 and 3,472 proteins.18 The results from the latter analysis were correlated with those of molecular weight (MW) and isoelectric point (pI) calculated from 2D-electrophoresis. From the spots observed with immunoreactivity, we identified 11 proteins (Table 1). As previously reported α-S1-casein, α-S2-casein, bovine serum albumin, and α-lactalbumin were identified as allergens; however, interestingly we found two novel allergenic proteins that have not been reported before: lactoperoxidase and protein FAM13A.
2D-electrophoresis of CMP. Pattern of proteins from each fraction obtained by ion-exchange chromatography separation. The gels were stained with colloidal Coomassie. Horizontal arrows indicate de pH interval used for isoelectrofocusing separation, whereas right triangles indicate the molecular weight range. The ovals show the regions where there was a high protein enrichment obtained from each fraction.
Antigenic proteins from bovine milk identified by Protein Lynx.
| Access Code | Protein Name | MW(kDa)/pI (exp) | MW(kDa)/pI (calc) | Pep. No. | Coverage (%) | Score |
|---|---|---|---|---|---|---|
| P02769 | Bovine serum albumin | 71.3/6 | 69.2/5.7 | 8 | 14.5 | 99.8 |
| B5B3R8 | α-s1-Casein | 25/5 | 24.4/4.9 | 4 | 19.6 | 39.3 |
| P00712 | α-Lactalbumin | 17.8/5.2 | 16.2/5 | 7 | 41 | 69 |
| P80025 | Lactoperoxidase | 89/8.5 | 80.6/8.8 | 39 | 53 | 99.3 |
| Q8HYW0 | Protein FAM13A | 80.3/6 | 80.1/5.45 | 12 | 25.5 | 100 |
| P02663 | α-s2-Casein | 29.8/9 | 26.02/8.55 | 13 | 49.5 | 57 |
MW, molecular weight; pI, isoelectric point; exp, experimental; calc, calculated; Pep. No., number of peptides.
Hence, from the data above, we can conclude that working with proteomic and immunological techniques along with protein identification by mass spectrometry opens new opportunities for the discovery of novel allergens. The enrichment of low abundance proteins was fundamental to reveal novel relevant allergens difficult to detect, mainly because of the strong signals of more abundant immuno-reactive proteins in the samples. On the other hand, application of these techniques may shed light on the understanding of the acuteness of symptoms and relate those to the allergens in each patient.
3Proteomic study of childhood cataract3.1OverviewIn general, a cataract can be defined as any opacity of the crystalline lens. The origin of such opacities can correlate with the natural processes of ageing but can also be associated with pathologic processes derived from environmental, nutritional, genetics, infectious or traumatic factors. Cataracts are classified as congenital whether observed at birth; infantile if develop during the first decade of life; pre-senile, if are present before 45 years of age; and senile when they appear >45 years of age. The estimated incidence of pediatric cataract is 1-6 per 10,000 live births, and it is considered one of the main causes of infantile blindness. It has been estimated that this disease is responsible for 15% of the cases of visual loss in childhood. It has an extreme relevance as high mortality associated with infantile blindness is observed; for instance, in the United Kingdom, it has been described that after vision loss, 10% of children do not survive beyond one year. This problem is largely magnified in developing countries, in which until 60% of blind children die within the first or second year of vision loss.19
At the physiological level, it is known that both the crystalline and the cornea work together to transmit and refract the light on the retina. Whereas the cornea has additional protecting purposes, the central function of the crystalline is to transmit and focus the light. Besides, the crystalline in mammals has the ability to focus the light correctly onto the retina by a process known as accommodation.20,21 The transparency of the crystalline is the result of the appropriate architecture of the lens cells and the tightly packing of the proteins contained in it.22 In this context, it is important that the crystalline shows the highest protein concentration compared to any other tissue, around 60% of its wet weight is protein. From the total protein of the crystalline, 80-90% is constituted of structural proteins called α, β, and γ crystallins; those proteins have a central role in the transparency and correct diffraction of light.23 The rest of proteins (10-20%) are called taxon-specific crystallins since they are only found in few species.24
At the molecular level, the pathophysiology of the cataract can be explained as the result of structural modifications in the crystalline proteins that promote their aggregation, crosslinking and insolubilization. Hence, the disruption of the fine balance of the protein structure affects the transparency of the crystalline provoking the anomalous dispersion of light. The fundamental question concerning the molecular processes driving the development of cataracts is to understand the factors that perturb the protein structure, leading to their aggregation in the crystalline. In this sense, PTM have received special attention. Owing to the practically null exchange of proteins in the crystalline, PTM accumulates during the lifespan, probably playing a fundamental role in the development of opacities linked to age. However, many PTM have also been found in soluble proteins of crystalline as part of the senescence process.25
It is noteworthy that PTM associated with the ageing process are evident since early stages of life development. Studies of normal crystalline from different age groups showed that PTM is present since the age of three years, reaching a maximum level at 17; at this early age, the PTM pattern is quite similar to those lenses from 50 or 60-year-old people.26–28 Therefore, the study of childhood cataracts from two-year-old patients represents a unique opportunity to identify the PTM associated to cataract-genesis, independently of those derived from the aging process.
3.2Project progressIn our Institute, we are focused on the molecular study of childhood cataract using a proteomics approach. Although there is a broad set of cataract studies related to ageing, only a few studies are dedicated to this pathology in a pediatric population. In contrast to that observed in the lens of adults, the proteins from crystallines in children <2 years of age almost do not exhibit PTM associated with senescence progression. Therefore, it seems to be clear that those PTM detected in childhood cataracts may be associated with the cataract-genesis process.
So far, data indicate that the proposed study is feasible; we collected cataract samples during the standard procedure of phacoemulsification, which does not represent additional risks during surgery. The crystalline control samples were obtained from enucleation surgeries performed for retinoblastoma treatment. The selected crystalline controls satisfied the following criteria: a) the enucleated eyes did not receive previous treatments (thermotherapy, chemotherapy or radiotherapy); b) no retinoblastoma infiltration toward the crystalline; and c) no detectable signs of eye degeneration (ptisis bulbi). The next step was the technical standardization of the 2D electrophoresis, which was the selected method to detect changes in the cataract proteome. The standardization included the optimal protein concentration for electrophoresis, optimal conditions for both dimensions, as well as the most appropriate staining procedure.
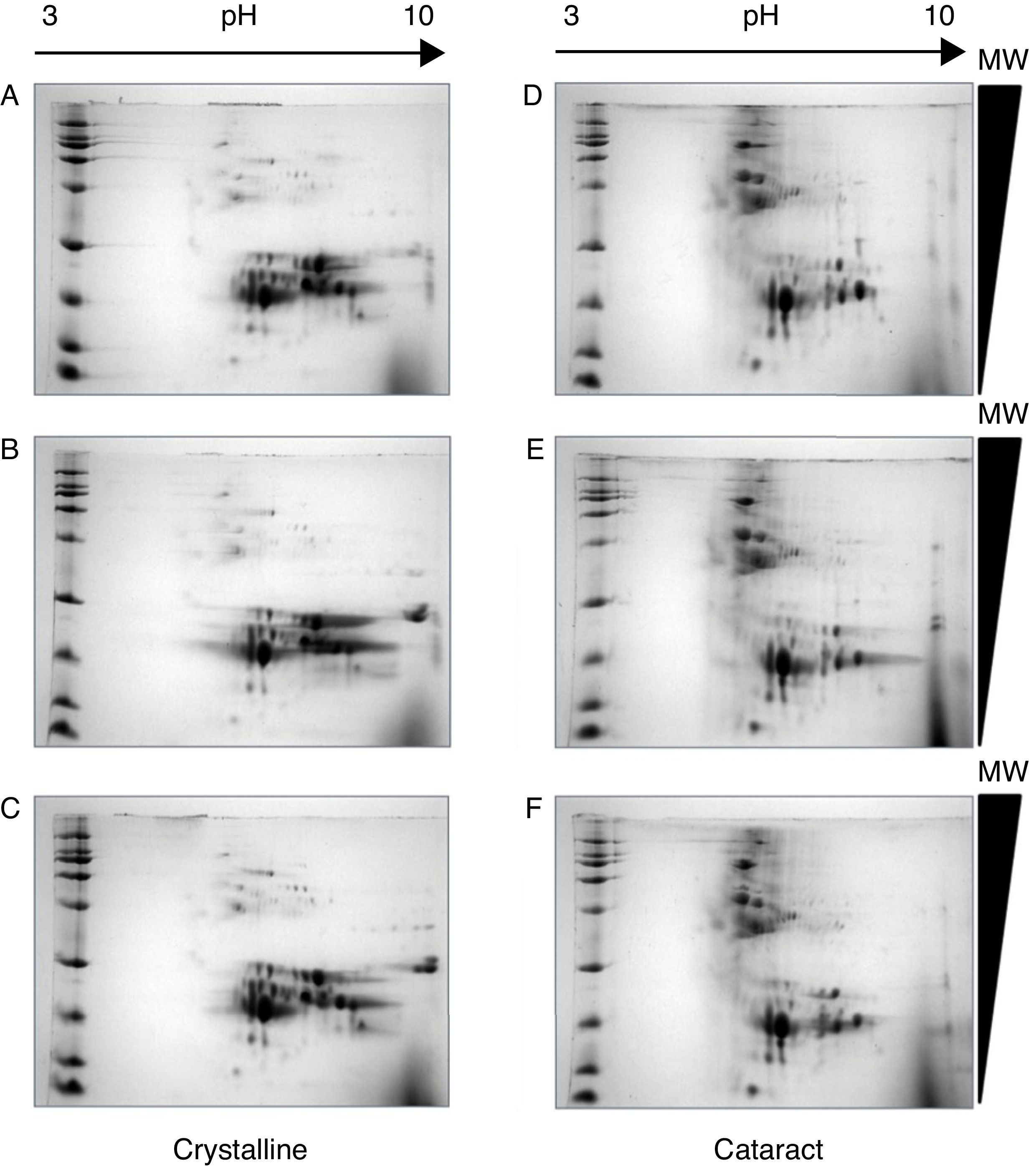
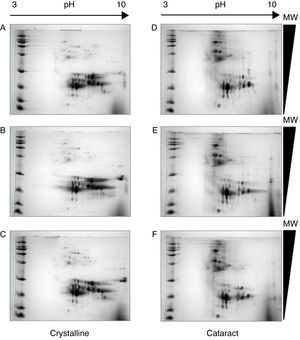
After the methodology had been optimized, the next step was the reproducibility of the methods. Since the comparison of proteomic patterns between control and cataract lens requires the statistical analysis of the total spots composing the samples, it is a compulsory requisite to perform replicates of each condition. Hereafter, the individual images of each sample were digitally averaged and compared with the average image of the control samples. Figure 2 shows triplicate experiments of each condition exhibiting high qualities, and high reproducibility is observed between replicates of both control and cataract lens.
Quality and reproducibility of 2D electrophoresis from samples of both control crystalline and cataract lens of pediatric patients. A sample of normal crystalline (A, B, C) and cataract (D, E, F) lens were applied by triplicate to 2D-SDS-PAGE. Arrows represent the pH interval used for isoelectrofocusing interval, whereas right triangles indicate the molecular weight range of separation.
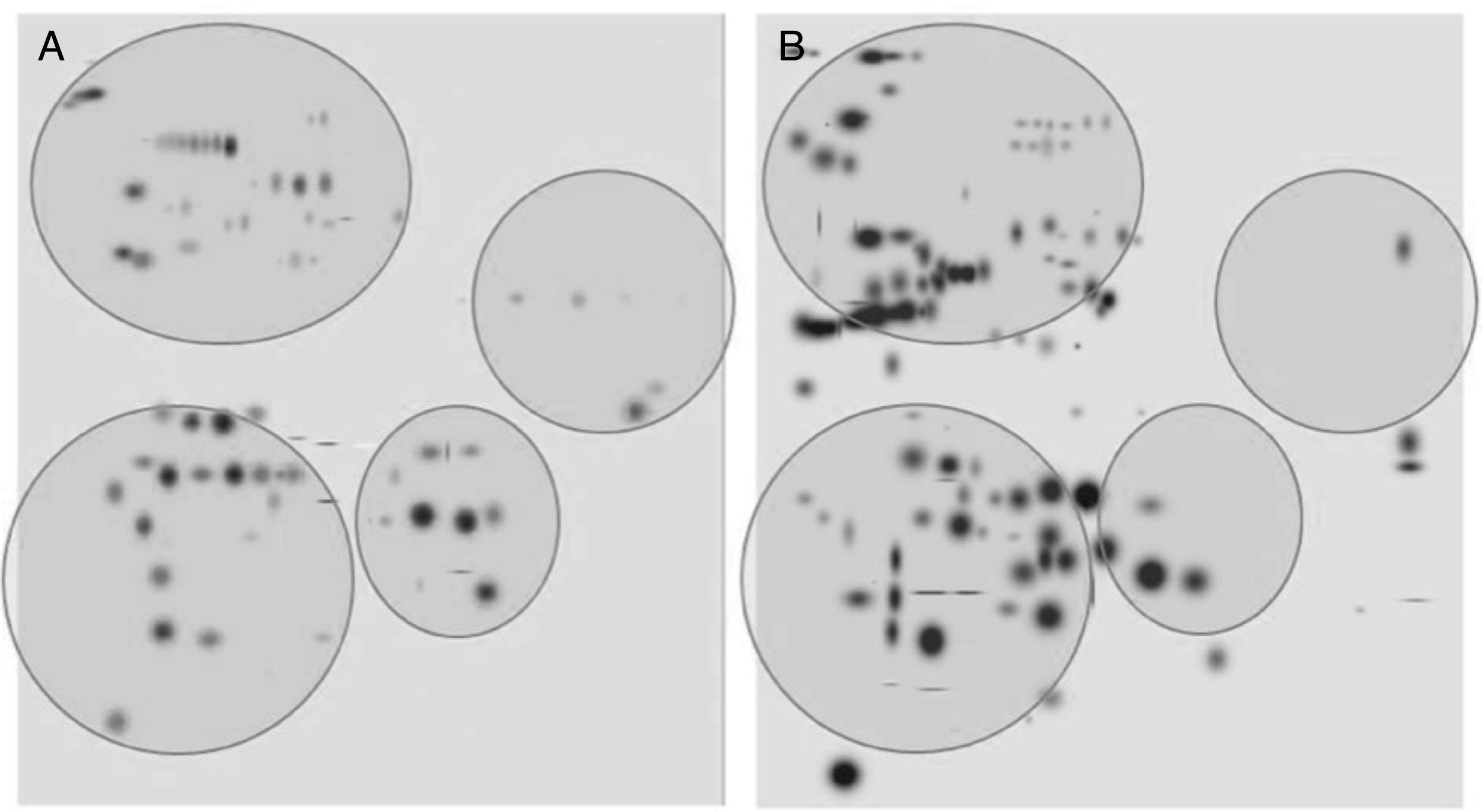
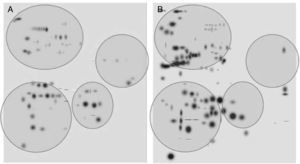
After digitalization of 2D gels, three images of each condition were integrated into a virtual master gel (Figure 3). The comparative analysis of master gels showed that 84 spots were detected in the control crystalline samples, while the master gel of cataract displayed 123 spots. Only 27 spots were shared between crystalline and cataract master gels, whereas 96 spots were different. From crystalline samples, all spots were located in one of four clearly identified regions in the master gel; in contrast, the spot distribution of cataract samples was markedly disorganized (Figure 3).
2D-master gels from samples of crystalline (A) and cataract (B). Three samples of each condition were integrated by using a PDQuest software. The analysis allowed to obtain the number of spots from each condition, those spots present in both conditions and those exhibited in each condition. The circles show four regions containing most of the spots in the control crystalline. The same regions are depicted in the cataract image.
So far, differences in proteomic profiles were identified, and we are working on the identification of those proteins; later on, PTM will be identified using MALDI-TOF mass spectrometry and the fingerprint method. The identification of the proteins forming the cataract as well as their PTM will contribute to a better understanding of this disease at a molecular level.
Conflict of interestThe authors declare no conflicts of interest of any nature.
H R-V and J O-H received grants supported from Instituto Nacional de Pediatría, No. 074/2012 and 047/2011, respectively.