In 1889, the pancreatectomy performed on a dog by Joseph von Mehring and Oskar Minkowski led to the discovery of the pancreatic origin of diabetes disease. Already 200 years before, Johann Conrad Brunner (fig. 1) had successfully performed eight pancreatectomies on dogs and had precisely described the symptoms of polyphagia, polyuria, and polydipsia. He did not, however, recognise the association with the diabetes disease and thus missed an opportunity to accelerate the course of diabetes research by 200 years1.
Claude Bernard, in 1865, made it possible to develop the incipient Physiology and the birth of Endocrinology. He explored the significance of the pancreas in diabetes, and observed that pancreatic atrophy, induced by ligature of pancreatic ducts, was not associated to diabetes in experimental animals2. In 1877, Etiénne Lancereaux introduced the concept of pancreatic diabetes (fig. 2) after describing two young severe diabetic subjects who died and showed advanced atrophy of the pancreas3. In his doctoral thesis of just 31 pages, dedicated to Rudolf Virchow, Paul Langerhans described that there was no direct transition of the secretory elements in the pancreas into the epithelia of the ducts4. The relevance of this observation was not taken into consideration until that Edouard Laguese described the cell clusters as the islet of Langerhans in 18835.
In 1889, Oskar Minkowski and Joseph F. von Mering, when working in the Department of Medicine, University of Strasbourg (directed by Bernard Nauynin) confirmed the original observation of Brunner, demonstrating that total pancreatectomy induced diabetes in the dog6. One year later, Minkowski (fig. 3) administered the first dried fresh pancreas (which he called pancreatine); after observing its failure, he injected pancreatic extracts subcutaneously, also without an effective response. In 1909, Jean de Meyer introduced the word insulin to describe the product of the islets of Langerhans7.
The discovery of insulinThe discovery of the antidiabetic hormone was a multi-step process shared by many investigators. Frederick C. Banting and John J.R. Macleod were awarded the Nobel Prize for Physiology and Medicine in 1923 for the discovery of insulin; nevertheless, a great deal of controversy has been focused upon this issue.
In 1892, Caparelli used an extract obtained by trituration of fresh pancreas in saline solution, and injected it into the abdominal cavity of a pancreatectomised dog. A substantial reduction of glycosuria was observed8.
Between 1902 and 1904, J. Rennie and T. Fraser, from Aberdeen Royal Infirmary, administered orally and hypodermically, fish islet extracts to 5 diabetic subjects, without significant changes in urine sugar content9.
Georg L. Zuelzer in Berlin, investigated from 1906, and reported the reduction of urine glucose excretion of depancreatised dogs, and also in diabetic subjects, after the administration of pancreatic extracts10. A peculiar case was a 6 year old child who successfully recovered from a severe episode of heavy glycosuria and ketosis on July 1907 after the intravenous administration of an emulsion containing 1 gram of the pancreatic extract. Zuelzer registered the extract, and named it acomatol (a USA patent would be awarded in 1912). The actions of Zuelzer's extract were confirmed by Forschbach, who carried out clinical experiments at the Minkowski Clinic in Breslau11; nevertheless, human treatments were stopped after the appearance of serious side effects (fever, attacks of shivering, sweating and vomiting). Zuelzer improved the potency of the extracts; the animals tested, mainly dogs, experienced severe convulsions, never seen before. Zuelzer could not recognise the induction of hypoglycaemic shock (nor did he estimate blood sugar content in those experiments). Thus, Zuelzer was the first to use an effective pancreatic extract in both animal experiments and in the treatment of clinical diabetes12.
Israel Kleiner and co-workers from the Department of Physiology and Pharmacology at the Rockefeller Institute for Medical Research observed, in normal animals, that 90 minutes after the infusion of dextrose, the concentration of glucose in blood reached nearly the same level as that prior to the infusion. In non-treated depancreatised dogs, the glucose levels was at least twice, whereas in the animals treated with the pancreatic emulsion, the disappearance of the administered glucose load was similar to that observed in normal animals13. Kleiner did not observe much toxicity of the pancreatic extracts, probably because the extract was highly diluted and very slowly injected14.
Nicolae C. Paulescu, Professor of Physiology at Bucharest initiated investigations on the pancreatic extract and its effects on carbohydrate, fat, and protein metabolism, as early as December 1916, although the publication had to until 192015. Four communications, covering nine experiences (fig. 4), were published by Paulescu in July 192116–19, as well as in a most comprehensive article regarding the actions of the antidiabetogenic hormone of the pancreas published on August 31, 1921 by Archives Internationales de Physiologie20. The Romanian investigator made important efforts to purify the extract to make it suitable for human therapy21. Both intravenous and subcutaneous administrations of the extract was shown to be effective; this was not the case for oral or intestinal cannula22. Paulescu carried out limited clinical experiments with diabetic subjects, due to the development of acute fever and other side effects. He gave the name pancreine to the active ingredient of the pancreatic extract and a license was provided by the Romanian Government on April 10, 192223.
Nicolae C. Paulescu published in 1921 most comprehensive evidence regarding the actions of the pancreatic antidiabetic hormone (pancreine). Adapted by C. Ionescu-Tirgoviste18.
On November 7th, 1920, the surgeon Frederick C. Banting visited John J.R. Macleod, Professor of Physiology, University of Toronto, asking for his cooperation to develop an experimental program to administer pancreatic extracts to diabetic dogs. Banting became impressed after reading an article by Moses Barron24, showing that the ligature of the pancreatic duct was followed by degeneration of the cells of the pancreas; since the acinous, but not the islet tissue, degenerates after this operation, the hypothetic pancreatic hormone could be extracted from the intact islets. The hypothesis of Barron, accepted by Macleod and Banting, ignored that Heidenhein as early as 1875 had shown that extracts of the fresh pancreas have no proteolytic activity except a zymogen which under certain conditions formed an active ferment25. Bayliss and Starling, following Langley, showed that the proteolytic ferment was in the fresh pancreatic gland in the form of the precursor trypsinogen26. On May 1921, Macleod, Banting, and Charles H. Best, a fourth year student of Physiology and Biochemistry, initiated the experimental research project. As Banting had never before performed a pancreatectomy, Macleod showed the surgical procedure with the first dog, leaving behind a small remnant, following the two-step protocol described by Hédon in 190927. The technique would allow the pancreas to function while the animal recovered. About a week later, the completion of total pancreatectomy would induce diabetes in the experimental animal. Later on, an injected emulsion of the «degenerated gland» would overcome the hyperglycaemia and glycosuria of depancreatised dogs. Best used the Myers-Bailey technique for estimating blood sugar, a modification of the Lewis-Benedict procedure, and the Benedict's method for the determination of glycosuria. He also measured the glucose to nitrogen (G:N) ratio, an another parameter of the disease. Macleod then left for a summer vacation in Scotland.
The lack of experience by Banting explained most animal deaths (fourteen out of nineteen between the end of May and July 10, 1921), either from the anaesthesia or the surgery, although the surgical performance eventually improved. In most animals the «degenerated pancreas» showed a normal appearance, and it looked almost impossible to induce experimental diabetes in depancreatised dogs. On August 3, Banting and Best decided to abandon the unsuccessful and laborious Hédon protocol, and to proceed with total pancreatectomy in a single time. The administration of the extract of «degenerated» pancreas was able to reduce the hyperglycaemia of pancreatectomised dogs. They also developed more active extracts from foetal and adult ox pancreas, without the need for duct ligature. The antidiabetogenic substance was named iletin at first. The original article, describing the research activities performed up to November 10, 1921, was submitted for publication with the title of «The internal secretion of the pancreas». It was accepted by the Journal of Laboratory and Clinical Medicine, and published in the issue of February 5, 192228.
The results of this work were essentially the same as those previously reported by Zuelzer, Kleiner and Paulescu.
Treating diabetic patients with pancreatic extract was a main drive for Frederick G Banting, but Professor Duncan Graham denied the request by Banting to administer the extract to treat diabetic patients at Toronto General Hospital (TGH). Graham thought that a surgeon not currently practising medicine, and without specific qualifications for conducting clinical research, could not be allowed to assume such a task, for the benefit of patients. Nevertheless, Banting would persuade Macleod and Graham to allow the use, for the first treatment, of the extract made by him and Best. Dr. Walter R Campbell, on duty at the Medical Word at TGH, ordered Dr. Ed Jeffrey to inject the first dose of pancreatic extract to the patient Leonard Thompson (LT), a 14 year old boy, admitted to the hospital on December 2, 1921. LT had a diagnosis of overt diabetes since December 1919. On admission, he appeared malnourished, weigh 65 pounds (30 Kg), acetone smell in his breath, hair falling out; urine test for sugar and ketones strongly positive; blood glucose, 580mg/dL. A diet with 450 calories was prescribed, but careful dietetic regulation failed to improve the course of the disease. On January 11, 1922, the first dose of Banting and Best pancreatic extract, a «murky, light-brown liquid», was administered. A total of 15mL (7.5mL into each buttock) was injected. A sterile abscess developed at one of the sites of injections, without clinical benefit other than a 25% fall in blood glucose and slight lowering of glucose excretion in the urine. Therefore, the pancreatic extract prepared by Banting and Best failed when clinically tested.
The incorporation of James B. Collip to the team of Macleod in Toronto led to the successful purification of the extract (fig. 5) after intensive work during December 1921 and January 192229. On the night of January 19, Collip discovered a limit; the active ingredient itself precipitated in over 90% alcohol. Using this cut-off point, he was able to remove most of protein contaminants as a precipitate, below 90% ethanol. Then, moving to a higher concentration of alcohol, the active product could be precipitated and isolated. The preparation of pancreatic extract under these conditions was far purer than before.
The first diabetic patient treated with the Collip's pancreatic extract at the Toronto General Hospital was also Leonard Thompson. Daily subcutaneous injections of the extract from January 23 to February 4 to Leonard Thompson resulted in an immediate improvement. BG dropped from 520mg/dL to 120. Excretion of sugar became much less; during treatment it varied from 71g to 9g; ketone bodies disappeared from urine; the patient looked brighter, felt better, and became more active. By February 1922, six more patients were treated, all with favourable results. W.R. Campbell and A.A. Fletcher were the clinicians assigned by Prof. Duncan Graham to work out the many problems in using this new therapy on the wards of the Medical Service of the Toronto General Hospital. The final manuscript was sent to the Canadian Medical Association Journal, a publication with little circulation outside Canada, to ensure quick publication. Banting (and Best) had little to do with the writing of this paper or the clinical work. Finally, the article was published in March 192230.
On May 30, 1922, a collaboration was established between the University of Toronto and Eli Lilly Co. On January 23, 1923, an American patent of both insulin and Toronto's method of making it would be awarded to Banting, Collip and Best. The three discoverers assigned their patent rights to the Board of Governors of the University. Lilly agreed that the word «insulin» would be the generic name, and «iletin» only to refer to the specific Lilly product. Lilly accepted a new and non-exclusive licensing contract. Lilly's first chemist, George Walden, led to the most important next step in insulin production and purification. Precipitation at the isoelectric point yielded the best preparation, with a stability and purity up to 100 times greater than any other obtained before. Early in 1923, the supply of insulin was adequate to meet the requirements of various institutions selected for its clinical use. The impact of insulin treatment was impressive worldwide. By the 1940's, for those patients with onset of diabetes at the age of 10, life expectancy gained 34 years; for those first diagnosed at the age of 30, life span was increased by insulin to additional 26 years; finally, for those with onset at the age of 50, the life expectancy increased by 8 additional years31.
Self Monitoring Blood Glucose (SMBG) versus Continuous Glucose Monitoring (CGM)Monitoring metabolic control in diabetes: historical reviewThe spill over of sugar into urine was probably first noted by Chinese (Zhen Li-yan, ca. 600AD) and Indian physicians (Sushrut and Chakrata (ca. 400-600AD). It took approximately 20 centuries until the sweetness of diabetes-associated urine was actually documented.
Theophratus Bombastus von Hohenheim (Paracelsus) (1493-1541) advocated the testing of the urine, but failed to taste the evaporated urine, mistaking it for saltpetre. In 1674, Thomas Willis (1621-1675) re-emphasised the presence of sweet like sugar or honey urine in persons suffering from «Diabetes, or the Pissing Evil».
Mathew Dobson (1745-1784) documented in 1776 that both urine and, for the first time, serum from a patient with diabetes had a taste indistinguishable from sugar, which was later demonstrated by the French chemist Chevreul (1786-1889).
A chemical test for glucose then emerged, initially with the introduction of the polarimetric measurement of glucose in 1833 by Baptise (1774-1862), followed by Trommer (1806-1879) in 1841 with his copper sulphate test, later modified in 1848 by Fehling (1812-1885), and commonly referred to as the Fehling's test (fig. 6). The first semi-quantitative urine glucose test was devised in 1911 by Benedict. For many years, and still in developing countries, urine testing has been the only way to monitor sugar levels in diabetic subjects, in spite of its important limitations. The test is done by dipping into a small collection of urine, a test strip, the end of which is coated with an enzyme that changes colour in the presence of sugar. Initially, testing for glucose in blood required large volumes. Micromethods became available in 1913 by Bang (1869-1918), quickly followed by Lewis and Benedict in 1915 and in 1918 by Hagedorn (1888-1971) and Jensen (1889-1946)32.
The first self-sampling at home for blood sugar determination was introduced by Harry Keen, in a publication in the Lancet in 1962, who developed a method whereby the patient at home applied a drop of blood onto a small filter paper pad, which was then allowed to dry before been dispatched by mail to the hospital laboratory for analysis. The method of glucose estimation was colorimetric33. In 1964, Rennie described a rapid (1min) enzyme test strip-based semiquantitative method for estimating capillary blood glucose (Dextrostix, Ames)34.
Clinic-based devices to help physicians manage their patients’ blood glucose levels were not available until the late 1960s. Like the earlier urine test strips, the strip changed colour based upon the amount of sugar in a drop of blood. The darker the colour, the higher the level of blood glucose. Nevertheless, visually comparing the colour on the strip with the colour on a reference scale of glucose values did not provide a precise value. Attempts were made to eliminate observer errors made by visually judging blood colour changes to reflect glucose concentrations. A lightweight, portable battery-operated reflectance meter (Eyetone, Ames) in conjunction with the strip (Dextrostix) was introduced and evaluated against the Autoanalyzer35. Not until the late 1970s did people with diabetes have wide access to devices that could help them manage their own blood glucose levels. The introduction of portable reflectance-meters, reliable quantitative devices to measure light reflected from the test strip, allowed the self-blood glucose monitoring (SBGM) of capillary blood by the diabetic patient on an ambulatory basis, and in realtime (fig. 7). This technology, coupled with diabetes education, has facilitated the empowerment of people with diabetes, allowing them to make adjustments in daily therapy, in close collaboration with the healthcare team.
In the late 1970s, two simultaneous articles appeared in the Lancet demonstrating that rapid methods of SMBG were a means of improving diabetes control, helping to adjust insulin doses with less frequent episodes of hypoglycaemia36-37.
The benefits of controlling blood glucose in pregnancy were already evident38-39. The importance of using SMBG in pregnancy was illustrated by Lowy in 1975. One of her patients insisted on monitoring at home instead of being hospitalised. This was the first pregnant patient successfully managed at home for the third trimester, instead of being admitted to the hospital from 26 weeks onwards. The experience laid the foundations for adopting SMBG in routine care by the time the same patient became pregnant 2 years later40. SMBG has made it easier to estimate the insulin requirements and their changes throughout pregnancy, and together with intensive insulin therapy have made it possible to optimise glycaemic control in diabetic pregnancies, as well as maternal and foetal outcomes41. Additional innovative solutions for improving SMBG have included: the developing of meters able to measure the electric current generated when blood sugar reacts with the enzyme of the strips; «talking» meters for visually-impaired patients; finger stick devices for minimising pain; data management capabilities for keeping electronic records that can be downloaded into computers for storage and statistics; systems requiring almost imperceptible amounts of blood.
The Diabetes Control and Complications Trial (DCCT) conducted in North America, convincingly demonstrated that improvement in glycaemic control delayed the onset and progression of microvascular (retinopathy, nephropathy, and neuropathy) complications in patients with type 1 diabetes42. Five years later, comparable evidence emerged from the United Kingdom Prospective Diabetes Study (UKPDS), exalting the benefit of improving blood glucose control and also blood pressure control in people with type 2 diabetes43,44. The successful implementation of SMBG relies on both education and motivation. Training is essential to provide accurate and reliable results.
Continuous Glucose Monitoring(CGM) : History and perspectiveThe use of SMBG only intermittently assesses glucose, thus precluding precise and accurate assessment of the impact of various interventions —medications, exercise, stress, other lifestyle factors— on blood glucose fluctuations and rate of change45.
In 1967, Updike and Hicks were the first to develop a miniature electrical transducer of glucose that could be implanted in an animal to monitor glucose continuously. They reported the first successful effort to use the enzyme glucose oxidase to monitor glucose on a continuous basis. They placed a layer of glucose oxidase in close approximation to an electrode, and showed that a continuous electrical current could be reproduced when the glucose was oxidized by the enzyme46. Their concept was based on the oxygen electrode described by Clark in 195647.
In the early 1970s, various groups reported progress with glucose electrodes48–50. In the following two decades, important advances were made in the development of potentially viable continuous glucose sensing, although none of the systems emerged into commercial sensors. The Food and Drug Administration (FDA) approved the first commercial CGM system (MiniMed CGMS) in 1999; although it did not provide real-time glucose measurements, the results could be down-loaded in a physician's office and used to improve glycaemic control51.
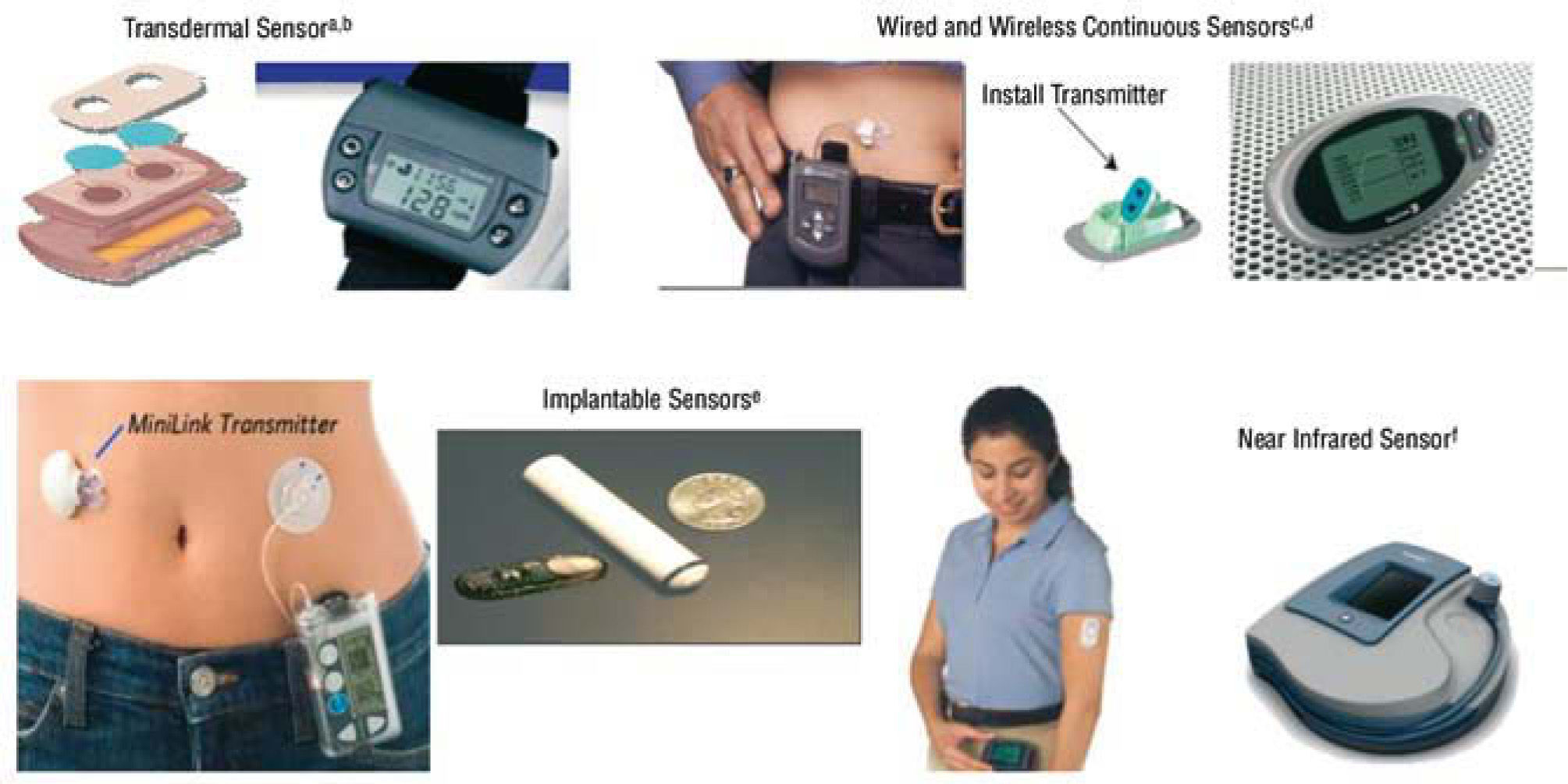
Continuous Glucose Monitoring Systems (CGMS) consist of three major components : a) a sensor, which is inserted under the skin; b) a transmitter, which attaches to the sensor and sends data to c) a receiver, which displays data. In CGMS, glucose readings are stored and/ or displayed every few minutes. Most applied technology is an «amperometric enzyme electrode», and a needle or fine flexible probe, implanted in the tissue. As the active part of the probe, the enzyme glucose-oxidase is immobilized at a charged electrode, such as platinum. The production of hydrogen peroxide is monitored by a current flow. The electrical current is proportional to glucose levels. Readings are stored every few minutes. The monitor is down-loaded to a computer; graphs display the continuous glucose data, highlighting meals, exercise, and other events.
In 2004, MiniMed obtained FDA approval for the Guardian system in 2004, which was able to alert patients to potentially dangerous high and low blood glucose values by sounding an alarm. In 2005, FDA approved also MiniMed Guardian - RT, a system that displays updated real-time glucose values every five minutes. In 2006, FDA approved MiniMed Paradigm Real-Time system, prepared to display CGM on an insulin pump. The system has been clinically validated52.
DexCom Inc (San Diego, CA) has developed several systems based on the technology by Updike. The STS glucose monitor was approved for 3 days of use per sensor (2006). The SEVEN, approved for seven days (2007), and more recently, SEVEN PLUS, the last version, displaying updated real-time glucose values every 5 minutes, alarms, rate of change arrows, and the ability to track meals, insulin, and activity. DexCom sensors have been extensively investigated in clinical trials53,54. The FDA approved the FreeStyle Navigator CGM from Abbott in March 2008. This system is based on wired enzyme technology using an osmium-based mediator molecule. The system displays glucose values every 5 minutes for 5 days55,56 (fig. 8).
Glucose sensor. aTamada et al. JAMA. 1999;282:1839-44. bGarg and Jovanovic54. cFeldman et al. Diabetes Technol Ther. 2003;5:769-79. dBoland et al. Diabetes Care. 2001;24:1858-62. eGarg et al. Diabetes Care. 2004;27:734-8. fMonfre et al. European Association for the Study of Diabetes, 2004.
In Europe, Menarini Diagnostics marketed the Gluco-Day, a semi-invasive CGM, based on a «microdialysis technique», evaluated in clinical trials57,58.
The advantages of CGM over SMBG includes, among other benefits, all of the following:
- –
Sensors provide information about direction of change; meters do not.
- –
Sensors provide information about rate of changes; meters do not.
- –
Sensors provide information about rate of acceleration (slowing-down and speeding-up); meters do not.
- –
Sensors can predict an area of possibilities that extends into the future; they are predictive, and meters are not.
- –
In the future, it is likely that sensors will be routinely integrated with meters and perhaps with tools like an insulin pen, as well as pumps. Also, future integration efforts will facilitate communication means, with mobile phones and computer screens, providing automatic reports about the metabolic state of the patient to health care centres and emergency services59.
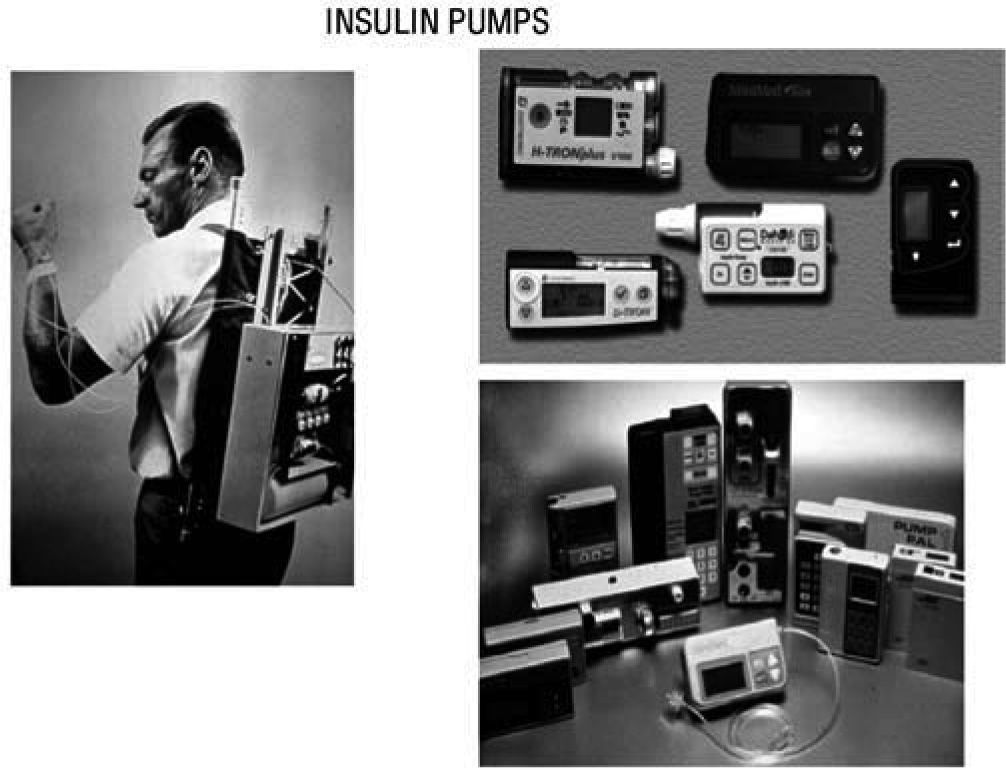
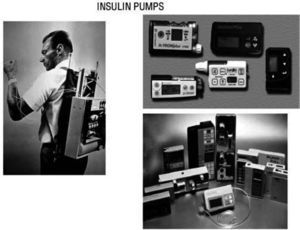
The Juvenile Diabetes Research Foundation launched in 2006 a multi-year initiative to accelerate the availability of an artificial pancreas to people with diabetes. One key step has been The Continuous Glucose Monitoring Study published in 200860, demonstrating that CGM improved glycaemic control in adult type 1 diabetic subjects, if patient's compliance is satisfactory. In 1974 Gérard Slama designed the first prototype for an intravenous insulin pump. First CSII system was used by John Pickup and Harry Keen, in 1978. Pumps have subsequently been reduced in size and programs developed for multiple basal rates of insulin infusion and a pre-prandial bolus (fig. 9).
In 1974 Gérard Slama designed the first prototype for an intravenous insulin pump (left). First CSII system was used by John Pickup and Harry Keen, in 1978. Later on, pumps have been reduced in size and developed programs for multiple basal rates of insulin infusion and premeal bolus (right).
Sensor-augmented pump therapy integrates a continuous glucose-monitoring device and a precise insulin pump into one system; it allows patients and physicians to monitor the glycaemic control and treatment with the aid of Internet-based adapted software. A one year multicentre, randomised, controlled trial compared the efficacy of sensor-augmented pump therapy with that of a regimen of multiple daily injections (MDI) in 329 adults and 156 children. After one year of follow up, the baseline mean glycated haemoglobin level (8.3% in both groups) decreased in the experimental group to 7.5%, whereas almost did not change in the MDI group (8.1%; P<.001). The rate of severe hypoglycaemia did not differ between the two experimental groups61. This integrated system uses a technologic platform in which the sensor will allow the insulin dose to be modified in closed-loop modalities62.
Closed loop therapy: Telemedical Artificial Pancreas (TAP)Automating insulin delivery is an active field of research. There have been multiple algorithms proposed for closedloop control of blood glucose63.
One of these algorithms has evolved from within the medical community, based on clinical experiences: a run-to-run (R2R) control strategy to adjust the insulin -to-carbohydrate ratio based on post-prandial blood glucose measurements. In order to optimise the basal rates, the patient wears a CGM system for a period of 3 days, recording the blood glucose concentration every 5 minutes, thus providing a full history of the glucose changes. During this time, a meal is missed each day, with the purpose of obviating the effects of the prandial insulin bolus and the glucose appearing from the corresponding meal. Basal infusion rates are adjusted to target afasting blood glucose of 90mg/dL). The circadian variation in insulin sensitivity and changes in physical activity allows the basal insulin delivery to be set at different rates for distinct segments of the day, due to the sleep-awake cycle and activity levels; a range of one to seven segments are common. In the clinical testing, an important challenge will be the meal-related insulin dose. The core of the model of Hovorka consists of two compartments for glucose (plasma and tissue) and one compartment for plasma insulin. The model divides the action of insulin on glucose kinetics into 3 additional components (glucose transport; glucose disposal; endogenous glucose production). A set of cases is created from actual subject pump infusion profiles64.
An artificial neural network model (NNM) has also been implemented. The inputs of the NNM are the values provided by the CGM sensor during the preceding 20 minutes, while the output is the prediction of glucose concentration at the chosen prediction horizon (PH) time. The method performance is assessed using data sets from 2 different CGMS: Three different PHs are used : 15, 30, and 45 minutes. The NNM accuracy was estimated by using the root mean square error and prediction delay65.
An inverse controller (IC) obtained by the conversion of an existing mathematical model and validated with synthetic patients simulated with a different model has been developed and compared with a proportional-integralderivative controller. The results have shown the viability of using an IC for closed loop diabetes control66.
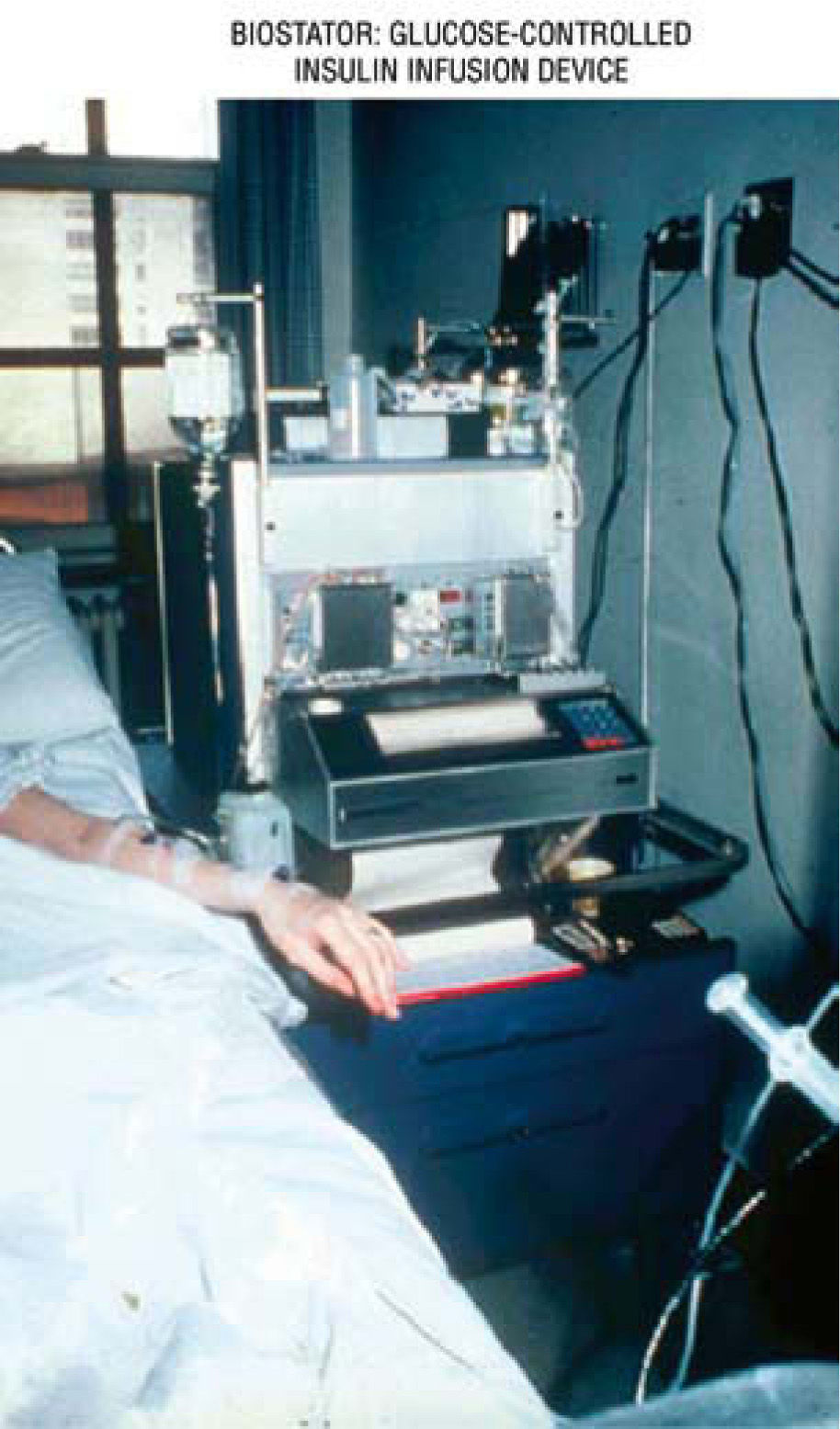
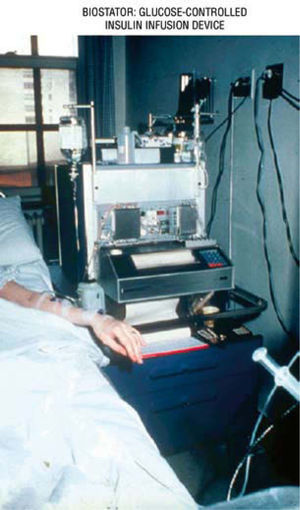
The artificial beta cell system contains three primary components: a) continuous insulin infusion (insulin pump); b) continuous glucose sensing (CGM); c) close-loop mathematical algorithm to regulate the insulin infusion rate based upon the glucose sensor reading. An early prototype of the artificial beta cell system was the Biostator (fig. 10), a bedside system using the intravenous route for insulin and glucose delivery, and continuous-flow blood glucose sampling; the goal was to maintain BG levels at a predefined value67. Although the intravenous route is ideal for practical results, in real life and ambulatory environment, the subcutaneous delivery is the right choice, in spite of the inconveniences of the delayed absorption of insulin, delay in glucose measurement, and the need to translate interstitial fluid (IF) glucose readings into blood glucose concentrations.
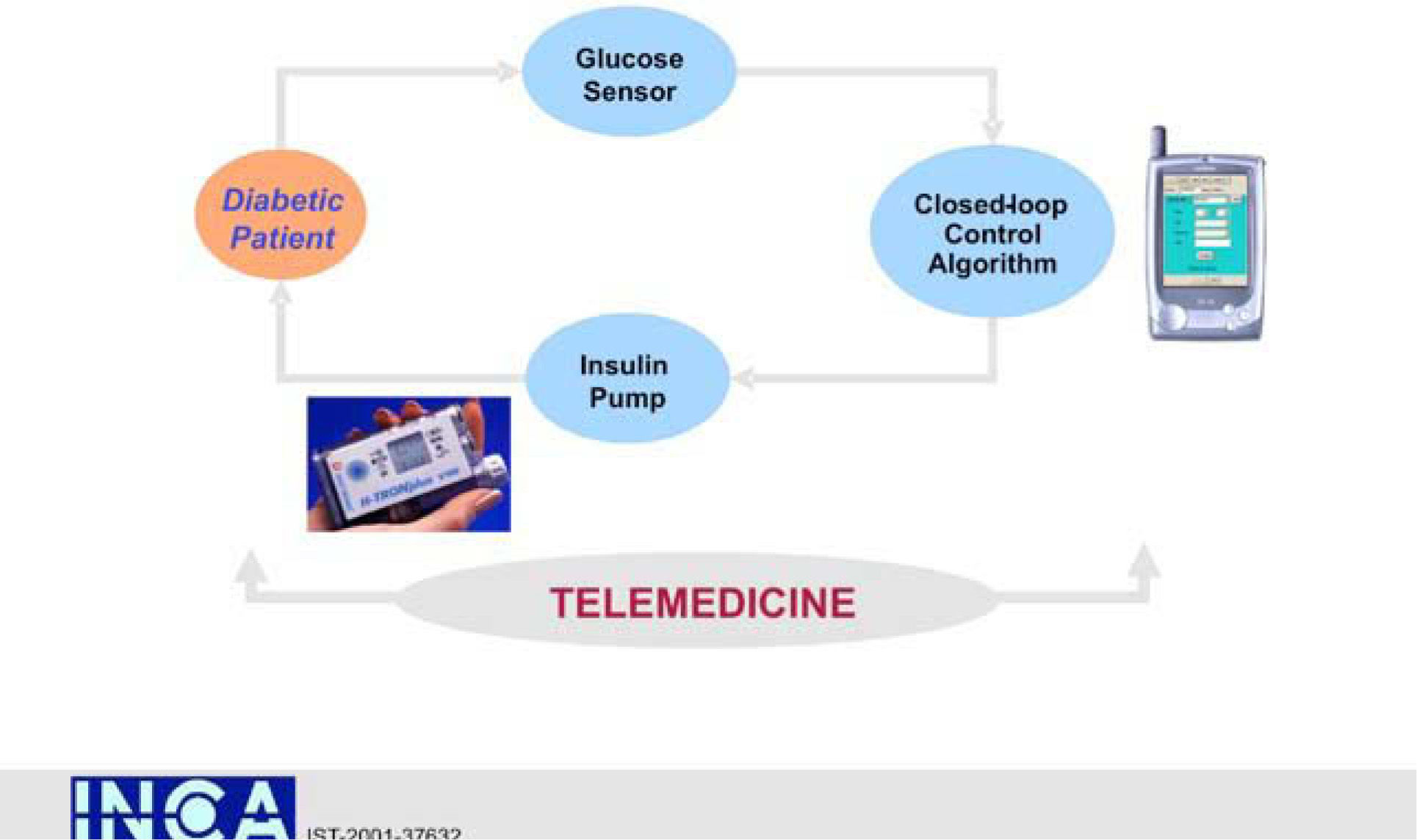
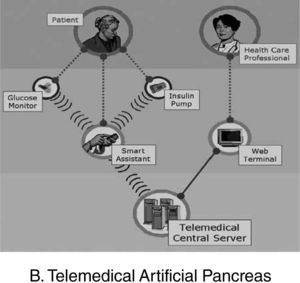
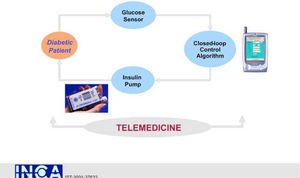
An interdisciplinary team of academic bioengineers and clinical investigators from the Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autónoma, Barcelona (EDUAB-HSP), and Polytechnic University of Madrid (GBT-UPM), have designed and validated a telemedical artificial pancreas system (TAP), incorporating these new technologies with the intention of optimising the glycaemic control of diabetic subjects. The technological platform integrates a multi-access telemedicine system, a real-time continuous glucose sensor (CGM-RT), continuous subcutaneous insulin infusion (CSII, insulin pump), a smart assistant (SA), and closed loop control algorithms. SA is a personal intelligent assistant based on a handheld digital device to provide both personal and remote loop strategies, using CGM, CSII, and mobile general packet radio service (GPRS) communication with a telemedicine central server (TMCS)68.
TAP is built in two interlinked loops. The «personal loop» allows wireless communication between the SA, the insulin pump, and the CGM device. The «remote loop» connects the diabetic subject to the health care professional via the SA and the wireless connection to the TMCS. The model predictive control module calculates the modification of the continuous insulin infusion rate provided by the pump, following the physiological model of Hovorka. This implies two control modes: 1) the patient decides changes in the insulin pump program by using the information coming from the glucose sensor (free-mode), and 2) the patient gets advice, through the SA, on the insulin bolus before each meal (advisory mode)69.
TAP has been evaluated by a research consortium funded by the European Commission: The Intelligent Control Assistant for Diabetes (INCA Project) (fig. 11). A feasibility pilot was carried out on a group of type 1 pump treated diabetic patients investigated at the Institut für Diabetesforschung (Munich, Germany) with satisfactory outcomes in ambulatory scenarios70.
Two randomised crossover ambulatory clinical studies have been carried out to investigate the clinical impact of TAP in «real life». The Clinical Study 1 focused on evaluating the operation of the original web-based telemedicine system71. The Clinical Study 2 evaluated the results of CGM-RT in cooperation with a telemedicine system on HbA1c and glucose variability in insulin pump treated type 1 diabetic patients72. These two clinical investigations demonstrated that real-time CGM in conjunction with TM (TAP) improved glucose control (HbA1c) and reduced glucose variability in adult type 1 diabetic subjects treated with insulin pumps. Further clinical evaluation has also shown the validation with TAP of the runto-run bolus calculator, and prediction tools to avoid situations of hypoglycaemia and hyperglycaemia73.
The development of a closed-loop artificial pancreas has been a long-time objective that could transform diabetes management. Recent progress in the field helps to predict the short-term delivery of a semi-automated system to people with diabetes74.
The introduction of TAP will be gradual and progressive, in parallel with the advance of knowledge and clinical experience, accuracy of the system, and, in particular, patient safety.
Chinese and Indian Physicians noticed the spillover of sugar into the urine (600AD).
Thomas Willis re-emphasized «sweet like sugar» urine in human diabetes (1674).
Johann Conrad Brunner successfully performed eight pancreatectomies on dogs, and precisely described polyphagia, polyuria and polydipsia (1683).
Chemical test for glucose emerged with the introduction of the polarimetric measurement by Baptise (1833), followed by the copper sulphate test (Trommer, 1841), the Fehling's test (1848), and the first semiquantitative urine glucose test (Benedict, 1911).
Initial tests for blood glucose required large volumes. Micromethods became available in 1913 (Bang), quickly followed by Lewis and Benedict (1915), Hagedorn and Jensen (1918).
The first self-sampling at home for blood glucose was introduced by Harry King (1962); two years later, a rapid enzyme test-strip-based semiquantitative method for capillary blood glucose became available (Rennie; Dextrostix). In 1970, battery-operated reflectance-meter allowed quantitative estimations for SBGM (Eyetone, Dextrostix).
O. Minkowski demonstrated that total pancreatectomy induced diabetes (1889).
The discovery of insulin was a multi-step process shared by many investigators (G. Zuelzer, N.C. Paulescu, J.J.R. Macleod, F.G. Banting, J.B. Collip, 1908-1922).
SBGM, coupled with diabetes education, has facilitated the empowerment of people with diabetes, allowing them to adjust of daily insulin therapy. Increased frecuency of SMBG is associated with better glycemic control.
In 1967, a miniature electrical transducer of glucose was implanted, for the first time, in an animal to monitor glucose continuously (Updike and Hicks). In 1999, the FDA approved the first commercial CGM system.
CGM does not replace SMBG; CGM requires a blood glucose meter for calibration. CGM readings should be used as an adjunct to decision making; BG concentration should be verified prior to making treatment decisions.
The evidence for the benefit of SMBG in DM-1, DM-2, and GDM (independent from the type of pharmacological treatment) is unquestionable. SMBG is particularly beneficial if baseline HbA1C is above 8%, and patients are properly educated and take actions based on results.
Glucosensors provide information about direction and rate of change of blood glucose; they are predictive, allowing rapid adjustments of blood glucose, particularly when reading in real time.
CGM collects significant information about the dynamics of BG fluctuations, and facilitates the development of calculations to analyze the risks associated with temporal BG variability and exposure to hyperglycemia.
CGM identifies areas (time periods) were glucose goals are not being met or detected by SMBG; it is of special relevance to detect biochemical hypoglycemia or hypoglycemia unawareness.
CGM-RT (real time), achieves a greater reduction of HbA1C in adult type 1 diabetic subjects, and allows quick adjustments in therapy. Patients on CGM-RT spend more time in euglycemia and less time in hypoor hyperglycemia.
Patient compliance is a key factor for these achievements.
It has been shown that achievement of glycemic control, using automated insulin delivery (closed-loop system, based on subcutaneous CGM and subcutaneous insulin) is feasible.
A critical concern in the development of closed-loop therapy/artificial pancreas, is sensor performance.
Mean glucose levels during closed-loop control and CGM is similar to that achieved under standard CSII therapy, but the variance about the mean is reduced.
A critical concern in the development of closed-loop therapy/artificial pancreas, is sensor performance.
Mean glucose levels during closed-loop control and CGM are similar to those achieved under standard CSII therapy, but the variance about the mean is reduced.
Occurrences of biochemical hypoglycaemia, detected by CGM, leads to suspension of insulin delivery in all instances by closed-loop therapy.
Although closed-loop insulin delivery results in tighter glucose control than that achieved by standard CSII, the control in not as good as in non-diabetic subjects on a similar diet.
Telemedicine can be integrated with CGM, CSII and SA (TAP, INCA, Project, EC).
Glycaemic control using automated insulin delivery system is an achievable goal. Closed-loop system, based on subcutaneous CGM and insulin delivery, is feasible.
The authors thank Dr. Lois Jovanovic, Chief Officer, Sansum Research Diabetes Institute (Santa Barbara, CA, USA), for her kind help, facilitating reports and written documents, as well as pictures from her own research files.