We aimed to describe sociodemographic, comorbidities, co-medication and risk of thromboembolic events and bleeding in patients with NVAF initiating oral anticoagulants (OAC) for stroke prevention, and to estimate adherence and persistence to OAC.
SettingPrimary Health Care (PHC) in the Catalan Health Institute (ICS), Catalunya, Spain.
ParticipantsAll NVAF adult patients initiating OAC for stroke prevention in August 2013–December 2015.
MethodsPopulation-based cohort study. Persistence was measured in patients initiating OAC in August 2013–December 2014.
Data source: SIDIAP, which captures electronic health records from PHC in the (ICS), covering approximately 5.8 million people.
Results51,690 NVAF patients initiated OAC; 47,197 (91.3%) were naive to OAC and 32,404 (62.7%) initiated acenocoumarol. Mean age was 72.8 years (SD 12.3) and 49.4% were women. Platelet-aggregation inhibitors were taken by 9105 (17.6%) of the patients. Persistence and adherence were estimated up to the end of follow-up. For 22,075 patients, persistence was higher among the non-naive patients [n=258 (61.7%)] than among the naive [n=11,502 (53.1%)]. Adherence was estimated for patients initiating DOAC and it was similar in naive and non-naive patients. Among the naive to DOAC treatment, those starting rivaroxaban showed a highest proportion [(n=360 (80.1%)] of good adherence at implementation (MPR>80%) while patients starting dabigatran were less adherent [n=203 (47.8%)].
ConclusionsAcenocoumarol was the most frequently prescribed OAC as first therapy in NVAF patients. Non-naive to DOAC showed better persistence than naive. Rivaroxaban showed higher proportion of adherent patients during the implementation phase than apixaban and dabigatran the lowest.
Describir datos sociodemográficos, comorbilidades, comedicaciones y riesgo de eventos tromboembólicos y hemorrágicos de los pacientes con fibrilación auricular no valvular (FANV) que inician tratamiento anticoagulante por vía oral (TAO) para prevención del ictus. Estimar adherencia y persistencia al TAO.
EmplazamientoAtención primaria (AP) del Instituto Catalán de Salud (ICS), Cataluña, España.
ParticipantesAdultos con FANV que inician TAO para prevención de ictus entre agosto del 2013 y diciembre del 2015.
MétodosEstudio de cohortes de base poblacional. Adherencia y persistencia se midieron en pacientes que iniciaban TAO entre agosto del 2013 y diciembre del 2014.
Fuente de datos: SIDIAP, base de datos procedentes de registros electrónicos de AP del ICS, que cubre aproximadamente una población de 5,8 millones de personas.
ResultadosCincuenta y un mil seiscientos noventa pacientes con FANV iniciaron TAO, 47.197 (91,3%) eran naïve al TAO y 32.404 (62,7%) iniciaron acenocumarol. Su edad media era 72,8 años (DE 12,3) y el 49,4% eran mujeres; 90105 (17,6%) recibían tratamiento antiagregante plaquetario. Persistencia y adherencia se estimaron hasta el final del seguimiento. La persistencia a anticoagulantes orales directos (ACOD) fue mayor en no naïve que en naïve (61,7% vs. 53,1%). La adherencia a ACOD fue similar en los 2 grupos. Entre los naïve, los pacientes que iniciaban rivaroxabán (80,1%) mostraron mayor adherencia en la implementación (MPR> 80%), mientras que los que iniciaban dabigatrán fueron menos adherentes (47,8%).
ConclusionesAcenocumarol fue el anticoagulante más prescrito. Los pacientes no naïve mostraron mejor persistencia al tratamiento que los naïve. Rivaroxabán mostró mayores tasas de adherencia que apixabán y dabigatrán, las menores.
Direct oral anticoagulants (DOAC) have been authorised by the European Medicines Agency for stroke prevention and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) during the last years (in Spain the launch date was 2011 for dabigatran, 2012 for rivaroxaban, 2013 for apixaban and 2016 for edoxaban). Their efficacy and safety have been demonstrated in their respective pivotal clinical trials.1–4
The level of utilisation of the different DOAC in stroke prevention in NVAF has shown to be different among countries, and several cohort studies have shown dissimilar results on effectiveness and safety of these drugs.5–12 Adequate levels of adherence and persistence to anticoagulant treatment have shown to decrease the occurrence of embolic events,13–16 so other studies have assessed adherence and persistence to oral anticoagulants (OAC), also showing different results among them.17–23 Adherence has been defined as the extent to which the patient conforms to the medication use recommendations specified by the prescriber (frequency of administration, dosage, etc.), and it is divided in three phases: initiation, implementation and persistence.24 Initiation can be estimated by the prescriptions actually dispensed, implementation can be measured by medication possession ratio (MPR), and persistence is defined as the continuation of the treatment over time.25
Despite the introduction of DOAC for NVAF management, vitamin K antagonists (VKA) are still the first therapeutic option for stroke prevention in Spain, according to the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS).26
We have recently described apixaban patients’ characteristics according to demographics, comorbidity, risk of thromboembolic events and comedications in a paper.27
The aim of the present work was to describe the characteristics for all patients initiating any oral anticoagulant drug (DOAC and VKA) for stroke prevention in NVAF and to estimate adherence during the implementation phase and persistence to DOAC treatment.
Material and methodsThis is an observational population-based cohort study of adult patients receiving DOAC and VKA for stroke prevention in NVAF, identified in the primary healthcare (PHC) database SIDIAP (Information System for Research in Primary Care)28 in Catalonia, Spain. The study cohort included all individuals diagnosed with NVAF who had a new prescription for apixaban, dabigatran, rivaroxaban or VKA (acenocoumarol or warfarin) from August 2013 until December 2015. We excluded from the study those patients with a registered diagnosis of valvular heart disease, including patients with mitral prosthetic valves. All patients enrolled were subdivided in four groups according to the treatment initiated: apixaban, dabigatran, rivaroxaban and VKA. Patients were considered naive if they did not have prior prescription of any OAC during 12 months before index date, or non-naive if they had been previously treated during the previous 12 months with a different OAC from the OAC that caused the entrance to the study cohort. Patients were followed-up until the discontinuation of anticoagulant treatment.
The data source was SIDIAP,28 which currently collects anonymized information from 279 primary health care centres managed by the Catalan health institute (ICS), which covers more than 5.8 million patients (approximately 80% of the Catalonia population, or more than 10% of the Spanish population). The information in SIDIAP is generated from different data sources: (1) ECAP™ (electronic health records in PHC); which includes information since 2006 on sociodemographic characteristics, health conditions registered as ICD-10 codes,29 General Practitioners’ prescriptions, clinical parameters and toxic habits. (2) Laboratory data. (3) Pharmacy invoice data available since 2005: information on all pharmaceutical products dispensed by community pharmacies with ICS prescriptions, by ATC codes.30
We first described demographics, comorbidity, risk of thromboembolic events and comedications of all patients with a new prescription of OAC, and secondly, we described medication adherence during implementation and persistence to anticoagulant treatment for those patients whose dispensing data were available and had at least one year of follow-up in SIDIAP database, during the period between August 2013 and December 2014 in order to analyse data of at least one year after initiation.
To calculate adherence during implementation and persistence phases we only took into account those patients who were adherent during initiation, meaning those who had OAC prescribed and dispensed. Persistence was defined as no discontinuation of treatment. Discontinuation rates of OAC were defined by lack of subsequent dispensing of the index drugs within 2 months after last supply day of the last dispensing and were analysed by calculating the cumulative percentage of discontinuation (treatment withdrawal or switch) rate.
Therapeutic adherence during the implementation phase was estimated by MPR, which is the ratio between the days of treatment covered by the medication dispensed and the total number of days between the index date and the last dispensing, and it was measured in persistent patients (those with at least one year of no discontinuation). Values of MPR above 80% were considered as good adherence during implementation. The package size has been used to estimate days of supply for DOACs (rivaroxaban once daily, dabigatran and apixaban twice daily) as the WHO method estimation using defined daily dose does not separate between standard and low dose.30 Adherence of VKA treatment cannot be properly estimated with the same method, as doses are not the same for every day of the week, they change after INR alterations and, moreover, warfarin and acenocoumarol pharmaceutical products in Spain are available at different doses and contain different numbers of tablets. Thus, adherence data in this paper are only described for DOAC.
Statistical analysisSociodemographic characteristics for the four groups at the start date are provided: (1) for all variables, number and percentage of missing data; (2) for categorical variables (sex, toxic habits and MEDEA index31), number and percentage for each category; (3) for continuous variables (age, body mass index), mean and standard deviation (SD). We report the total number of patients with at least one disease and the number and percentage of patients with each specific condition for all groups (apixaban, dabigatran, rivaroxaban and VKA). Concurrent use of medications at the start date was quantified by the number and percentage of users. Risk of stroke and major haemorrhage event were assessed at the start date with CHA2DS2VASc32 and HAS-BLED.33 For each score, number and percentage of each category for the five drugs groups are described.
Monthly discontinuation rates for all anticoagulant during the first year of treatment were also calculated in order to estimate the persistence. We conducted survival analyses of the time from the index date to the discontinuation date using survival curves drawn using the Kaplan–Meier method. Censoring was considered at end of follow-up, death or date of treatment discontinuation. For each DOAC group, number and percentage of users with good adherence and summary statistics for MPR were estimated.
Analysis was conducted using SAS software, version 9.4 (SAS Institute). Detailed methodology for summary and statistical analyses of data collected in this study are documented in the Statistical Analysis Plan, which is dated, filed and maintained by the sponsor and can be accessed at request to the corresponding author.
Ethics approvalThe study protocol was approved by the Ethics Committee of the ‘IDIAPJGol’ and classified by the AEMPS.
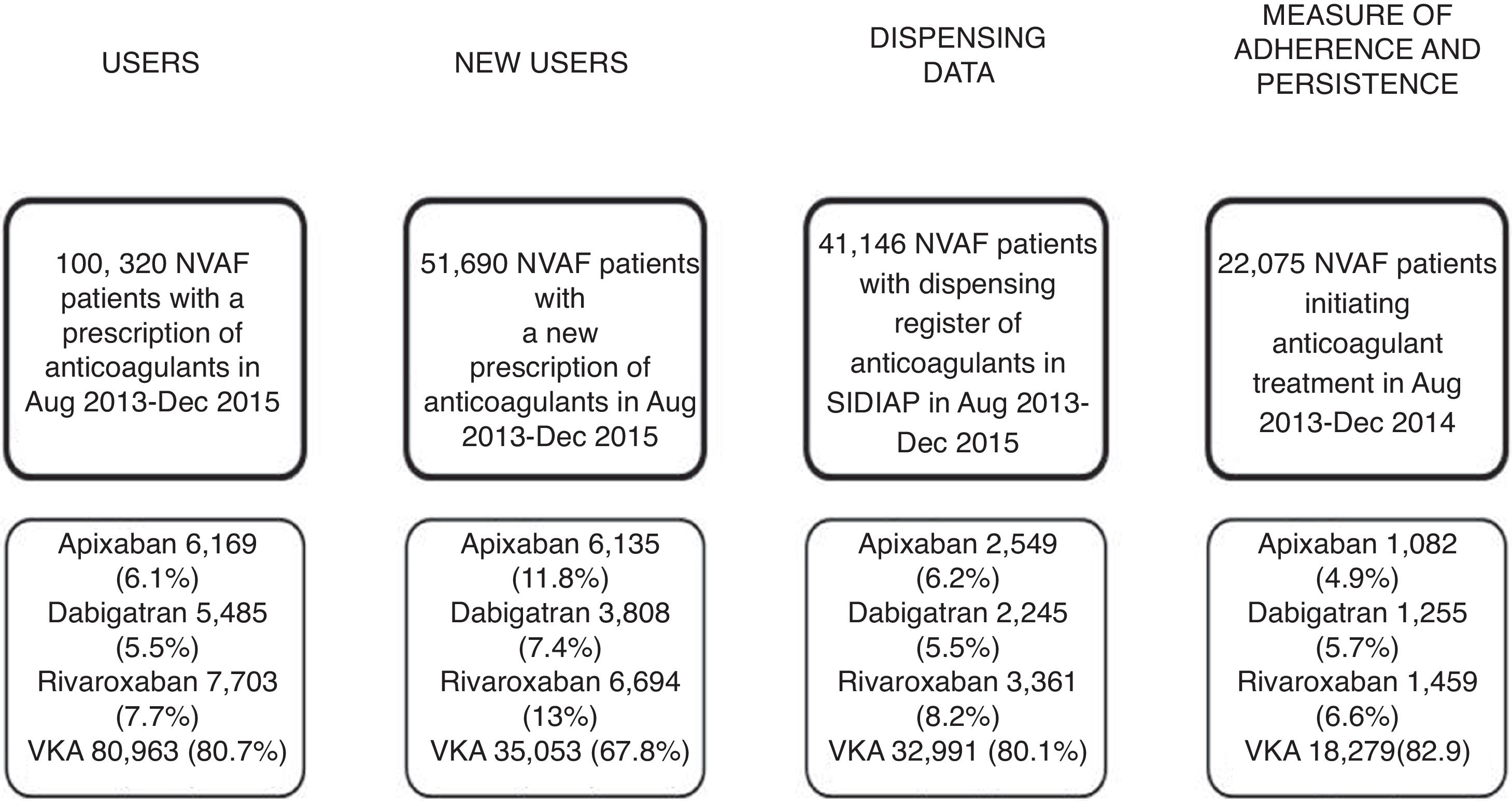
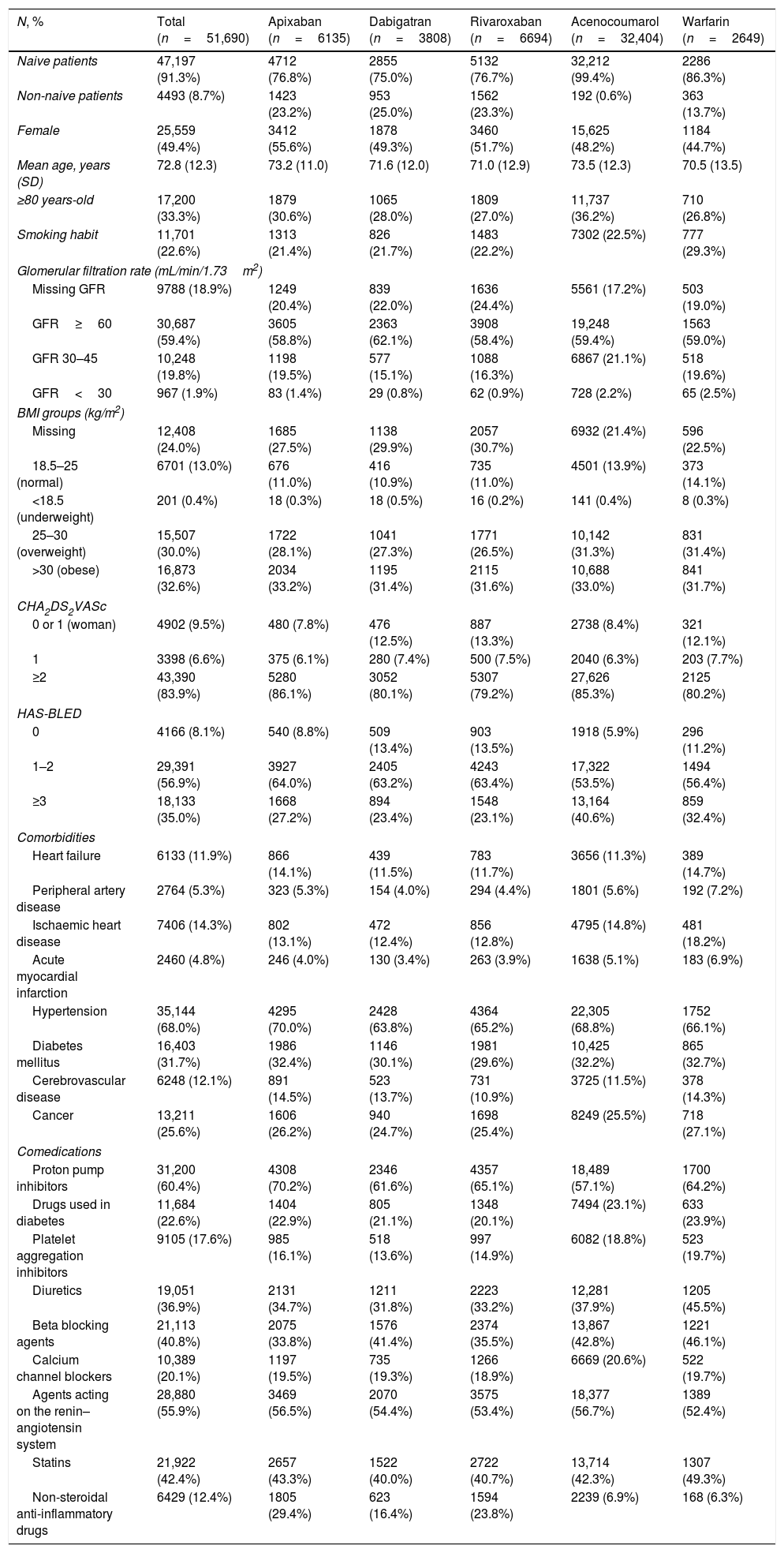
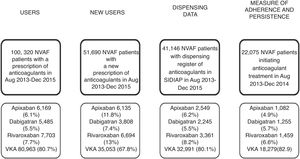
ResultsWe analysed 51,690 NVAF patients with a new OAC prescription during the study period; 47,197 (91.3%) were OAC naive patients and 4493 (8.7%) were non-naive, and out of the overall number of patients, 16,637 (32.2%) received a DOAC prescription and 35,053 (67.8%), VKA (Fig. 1, Table 1). Demographics, risk of stroke and haemorrhage, comorbidities and comedications are described in Table 1. Patients initiating anticoagulants had a mean age of 72.8 years (SD 12.3), 49.4%% of them were women and 83.9% of patients had a CHA2DS2VASc score ≥2. The youngest patients were those initiating warfarin (70.5 years) and the oldest, those with acenocoumarol (73.5). There was a higher proportion of patients prescribed with apixaban with a CHA2DS2VASc score ≥2 than for the rest of the drugs (86.1%) and the highest haemorrhagic risk was for acenocoumarol-treated patients (40.6% with HAS-BLED ≥3 as compared to 23.1–35.0% patients with HAS-BLED ≥3 in patients receiving other OAC). The most frequent comorbidities were hypertension (68%), diabetes (31.7%) and cancer (25.6%), and the most frequent comedications were proton pump inhibitors (60.4%), agents acting on the renin–angiotensin system (55.9%) and statins (42.4%). Dispensing data were available for 41,146 patients (79.6%) of those patients; 8155 (19.9%) patients initiated DOAC treatment and 32,991 (80.1%) initiated VKA (Fig. 1).
Baseline characteristics of patients with new prescriptions of anticoagulants.
| N, % | Total (n=51,690) | Apixaban (n=6135) | Dabigatran (n=3808) | Rivaroxaban (n=6694) | Acenocoumarol (n=32,404) | Warfarin (n=2649) |
|---|---|---|---|---|---|---|
| Naive patients | 47,197 (91.3%) | 4712 (76.8%) | 2855 (75.0%) | 5132 (76.7%) | 32,212 (99.4%) | 2286 (86.3%) |
| Non-naive patients | 4493 (8.7%) | 1423 (23.2%) | 953 (25.0%) | 1562 (23.3%) | 192 (0.6%) | 363 (13.7%) |
| Female | 25,559 (49.4%) | 3412 (55.6%) | 1878 (49.3%) | 3460 (51.7%) | 15,625 (48.2%) | 1184 (44.7%) |
| Mean age, years (SD) | 72.8 (12.3) | 73.2 (11.0) | 71.6 (12.0) | 71.0 (12.9) | 73.5 (12.3) | 70.5 (13.5) |
| ≥80 years-old | 17,200 (33.3%) | 1879 (30.6%) | 1065 (28.0%) | 1809 (27.0%) | 11,737 (36.2%) | 710 (26.8%) |
| Smoking habit | 11,701 (22.6%) | 1313 (21.4%) | 826 (21.7%) | 1483 (22.2%) | 7302 (22.5%) | 777 (29.3%) |
| Glomerular filtration rate (mL/min/1.73m2) | ||||||
| Missing GFR | 9788 (18.9%) | 1249 (20.4%) | 839 (22.0%) | 1636 (24.4%) | 5561 (17.2%) | 503 (19.0%) |
| GFR≥60 | 30,687 (59.4%) | 3605 (58.8%) | 2363 (62.1%) | 3908 (58.4%) | 19,248 (59.4%) | 1563 (59.0%) |
| GFR 30–45 | 10,248 (19.8%) | 1198 (19.5%) | 577 (15.1%) | 1088 (16.3%) | 6867 (21.1%) | 518 (19.6%) |
| GFR<30 | 967 (1.9%) | 83 (1.4%) | 29 (0.8%) | 62 (0.9%) | 728 (2.2%) | 65 (2.5%) |
| BMI groups (kg/m2) | ||||||
| Missing | 12,408 (24.0%) | 1685 (27.5%) | 1138 (29.9%) | 2057 (30.7%) | 6932 (21.4%) | 596 (22.5%) |
| 18.5–25 (normal) | 6701 (13.0%) | 676 (11.0%) | 416 (10.9%) | 735 (11.0%) | 4501 (13.9%) | 373 (14.1%) |
| <18.5 (underweight) | 201 (0.4%) | 18 (0.3%) | 18 (0.5%) | 16 (0.2%) | 141 (0.4%) | 8 (0.3%) |
| 25–30 (overweight) | 15,507 (30.0%) | 1722 (28.1%) | 1041 (27.3%) | 1771 (26.5%) | 10,142 (31.3%) | 831 (31.4%) |
| >30 (obese) | 16,873 (32.6%) | 2034 (33.2%) | 1195 (31.4%) | 2115 (31.6%) | 10,688 (33.0%) | 841 (31.7%) |
| CHA2DS2VASc | ||||||
| 0 or 1 (woman) | 4902 (9.5%) | 480 (7.8%) | 476 (12.5%) | 887 (13.3%) | 2738 (8.4%) | 321 (12.1%) |
| 1 | 3398 (6.6%) | 375 (6.1%) | 280 (7.4%) | 500 (7.5%) | 2040 (6.3%) | 203 (7.7%) |
| ≥2 | 43,390 (83.9%) | 5280 (86.1%) | 3052 (80.1%) | 5307 (79.2%) | 27,626 (85.3%) | 2125 (80.2%) |
| HAS-BLED | ||||||
| 0 | 4166 (8.1%) | 540 (8.8%) | 509 (13.4%) | 903 (13.5%) | 1918 (5.9%) | 296 (11.2%) |
| 1–2 | 29,391 (56.9%) | 3927 (64.0%) | 2405 (63.2%) | 4243 (63.4%) | 17,322 (53.5%) | 1494 (56.4%) |
| ≥3 | 18,133 (35.0%) | 1668 (27.2%) | 894 (23.4%) | 1548 (23.1%) | 13,164 (40.6%) | 859 (32.4%) |
| Comorbidities | ||||||
| Heart failure | 6133 (11.9%) | 866 (14.1%) | 439 (11.5%) | 783 (11.7%) | 3656 (11.3%) | 389 (14.7%) |
| Peripheral artery disease | 2764 (5.3%) | 323 (5.3%) | 154 (4.0%) | 294 (4.4%) | 1801 (5.6%) | 192 (7.2%) |
| Ischaemic heart disease | 7406 (14.3%) | 802 (13.1%) | 472 (12.4%) | 856 (12.8%) | 4795 (14.8%) | 481 (18.2%) |
| Acute myocardial infarction | 2460 (4.8%) | 246 (4.0%) | 130 (3.4%) | 263 (3.9%) | 1638 (5.1%) | 183 (6.9%) |
| Hypertension | 35,144 (68.0%) | 4295 (70.0%) | 2428 (63.8%) | 4364 (65.2%) | 22,305 (68.8%) | 1752 (66.1%) |
| Diabetes mellitus | 16,403 (31.7%) | 1986 (32.4%) | 1146 (30.1%) | 1981 (29.6%) | 10,425 (32.2%) | 865 (32.7%) |
| Cerebrovascular disease | 6248 (12.1%) | 891 (14.5%) | 523 (13.7%) | 731 (10.9%) | 3725 (11.5%) | 378 (14.3%) |
| Cancer | 13,211 (25.6%) | 1606 (26.2%) | 940 (24.7%) | 1698 (25.4%) | 8249 (25.5%) | 718 (27.1%) |
| Comedications | ||||||
| Proton pump inhibitors | 31,200 (60.4%) | 4308 (70.2%) | 2346 (61.6%) | 4357 (65.1%) | 18,489 (57.1%) | 1700 (64.2%) |
| Drugs used in diabetes | 11,684 (22.6%) | 1404 (22.9%) | 805 (21.1%) | 1348 (20.1%) | 7494 (23.1%) | 633 (23.9%) |
| Platelet aggregation inhibitors | 9105 (17.6%) | 985 (16.1%) | 518 (13.6%) | 997 (14.9%) | 6082 (18.8%) | 523 (19.7%) |
| Diuretics | 19,051 (36.9%) | 2131 (34.7%) | 1211 (31.8%) | 2223 (33.2%) | 12,281 (37.9%) | 1205 (45.5%) |
| Beta blocking agents | 21,113 (40.8%) | 2075 (33.8%) | 1576 (41.4%) | 2374 (35.5%) | 13,867 (42.8%) | 1221 (46.1%) |
| Calcium channel blockers | 10,389 (20.1%) | 1197 (19.5%) | 735 (19.3%) | 1266 (18.9%) | 6669 (20.6%) | 522 (19.7%) |
| Agents acting on the renin–angiotensin system | 28,880 (55.9%) | 3469 (56.5%) | 2070 (54.4%) | 3575 (53.4%) | 18,377 (56.7%) | 1389 (52.4%) |
| Statins | 21,922 (42.4%) | 2657 (43.3%) | 1522 (40.0%) | 2722 (40.7%) | 13,714 (42.3%) | 1307 (49.3%) |
| Non-steroidal anti-inflammatory drugs | 6429 (12.4%) | 1805 (29.4%) | 623 (16.4%) | 1594 (23.8%) | 2239 (6.9%) | 168 (6.3%) |
SD: standard deviation, GFR: glomerular filtration rate, BMI: body mass index.
Adherence at implementation and persistence were assessed through dispensing data for patients who initiated anticoagulant treatment with OAC between August 2013 and December 2014 (n=22,075), being only 1.9% of them (n=418) non-naive patients. Of the 22,075 patients, 3796 (17.2%) received DOAC; 3425 (n=15.8%) of them were naive and 371 (88.7%) were anticoagulant-experienced patients (Table 2).
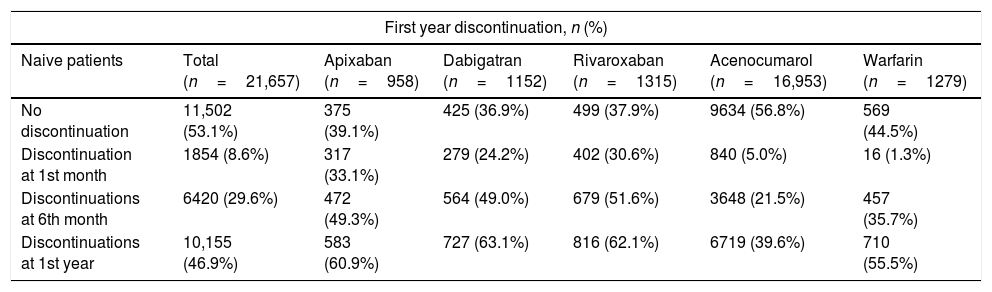
Descriptive of adherence and discontinuation rates of anticoagulants in naive and non-naive patients.
| First year discontinuation, n (%) | ||||||
|---|---|---|---|---|---|---|
| Naive patients | Total (n=21,657) | Apixaban (n=958) | Dabigatran (n=1152) | Rivaroxaban (n=1315) | Acenocumarol (n=16,953) | Warfarin (n=1279) |
| No discontinuation | 11,502 (53.1%) | 375 (39.1%) | 425 (36.9%) | 499 (37.9%) | 9634 (56.8%) | 569 (44.5%) |
| Discontinuation at 1st month | 1854 (8.6%) | 317 (33.1%) | 279 (24.2%) | 402 (30.6%) | 840 (5.0%) | 16 (1.3%) |
| Discontinuations at 6th month | 6420 (29.6%) | 472 (49.3%) | 564 (49.0%) | 679 (51.6%) | 3648 (21.5%) | 457 (35.7%) |
| Discontinuations at 1st year | 10,155 (46.9%) | 583 (60.9%) | 727 (63.1%) | 816 (62.1%) | 6719 (39.6%) | 710 (55.5%) |
| Non-naive patients | Total (n=418) | Apixaban (n=124) | Dabigatran (n=103) | Rivaroxaban (n=144) | Acenocumarol (n=12) | Warfarin (n=35) |
|---|---|---|---|---|---|---|
| No discontinuation | 258 (61.7%) | 82 (66.1%) | 50 (48.5%) | 93 (64.6%) | 6 (50.0%) | 27 (77.1%) |
| Discontinuation at 1st month | 23 (5.5%) | 2 (1.6%) | 13 (12.6%) | 7 (4.9%) | 1 (8.3%) | 0 (0.0%) |
| Discontinuations at 6th month | 99 (23.7%) | 19 (15.3%) | 39 (37.9%) | 33 (22.9%) | 5 (41.7%) | 3 (8.6%) |
| Discontinuations at 1st year | 160 (38.3%) | 42 (33.9%) | 53 (51.5%) | 51 (35.4%) | 6 (50.0%) | 8 (22.9%) |
During the first month of treatment, all DOAC-naive patients presented high discontinuation rates, being the discontinuation rates higher for apixaban (33.1%) than for rivaroxaban (30.6%) and for dabigatran (24.2%). Discontinuation rates in naive patients after one year of treatment were similar between the three DOAC, with approximately 60% of patients stopping treatment. In all the naive patients the persistence at one year was less than 50% except for the acenocoumarol naive patients in whom it was of 56.8%. Persistence was higher in non-naive patients with an observed persistence of more than 50% at one year, being higher for warfarin non-naive patients (77.1%) than for DOAC, where the maximum persistence was observed for apixaban patients (66.1%) and the minimum for the dabigatran (48.5%) patients.
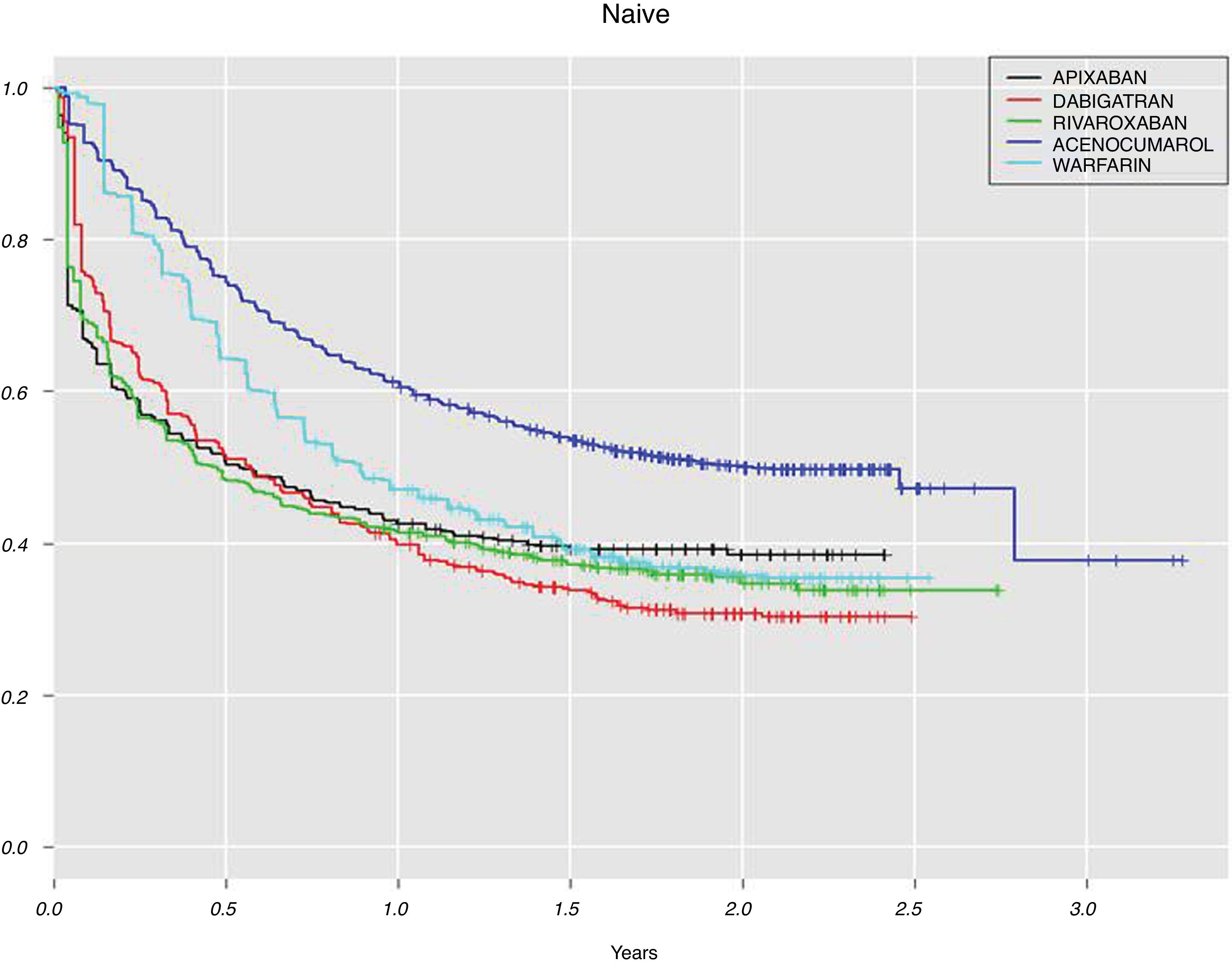
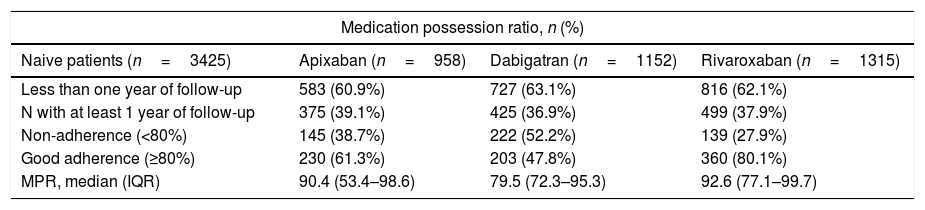
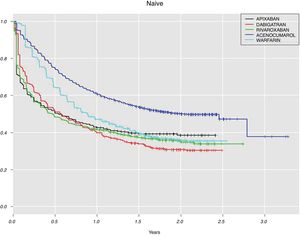
For the DOAC naive patients with at least one year of follow-up (n=1299, 37.9%, Table 3), the highest adherence at implementation was for rivaroxaban (80.1%), followed by apixaban (61.3%) and dabigatran (47.8%). For DOAC non-naive, 225 (60.6%) patients did not discontinue DOAC treatment during the first year and among them, the largest proportion of patients with good adherence was for rivaroxaban (81.7%) and the lowest for dabigatran (34.0%). Although the measure of persistence for VKA should be different to the measure for DOAC, in Fig. 2 we show data for both groups of anticoagulants in order to compare them. This figure shows Kaplan–Meier curves for treatment discontinuation of DOAC and VKA in naive patients; there were high DOAC discontinuation rates at treatment start and, over time apixaban showed lower discontinuation rates.
Descriptive statistics of adherence rates of direct oral anticoagulants in naive and non-naive patients.
| Medication possession ratio, n (%) | |||
|---|---|---|---|
| Naive patients (n=3425) | Apixaban (n=958) | Dabigatran (n=1152) | Rivaroxaban (n=1315) |
| Less than one year of follow-up | 583 (60.9%) | 727 (63.1%) | 816 (62.1%) |
| N with at least 1 year of follow-up | 375 (39.1%) | 425 (36.9%) | 499 (37.9%) |
| Non-adherence (<80%) | 145 (38.7%) | 222 (52.2%) | 139 (27.9%) |
| Good adherence (≥80%) | 230 (61.3%) | 203 (47.8%) | 360 (80.1%) |
| MPR, median (IQR) | 90.4 (53.4–98.6) | 79.5 (72.3–95.3) | 92.6 (77.1–99.7) |
| Non-naive patients (n=371) | Apixaban (n=124) | Dabigatran (n=103) | Rivaroxaban (n=144) |
|---|---|---|---|
| Less than one year of follow-up | 42 (33.9%) | 53 (51.5%) | 51 (35.4%) |
| N with at least 1 year of follow-up | 82 (66.1%) | 50 (48.5%) | 93 (64.6%) |
| Non-adherence (< 80%) | 27 (32.9%) | 33 (66.0%) | 17 (18.3%) |
| Good adherence (≥80%) | 55 (67.1%) | 17 (34.0%) | 76 (81.7%) |
| MPR, median (IQR) | 94.5 (53.4–98.6) | 75.1 (71.6–94.7) | 99.7 (84.4–99.7) |
MPR: medication possession ratio, IQR: interquartile range.
We included 51,690 new users of OAC in this population-based cohort study, 41,146 of them had dispensing data available in SIDIAP (79.6%). Approximately 80% of the patients initiated VKA rather than DOAC, pointing out that VKA are still the first therapeutic option for anticoagulation in NVAF in our setting, as recommended by AEMPS.26 Most patients (83.9%) had CHA2DS2VASc score ≥2, which is the criterion to anticoagulate in NVAF patients according to guidelines.34,35 Patients with highest risks of stroke were those in the groups of acenocoumarol and apixaban, as shown in previous studies for the other VKA, warfarin.14,22,36
Therapeutic adherences at implementation and persistence to OAC were assessed in those patients who were adherent at initiation and started anticoagulation treatment before 2015. Only one third of naive patients received DOAC treatment during at least one year of follow-up, for VKA the proportion was higher. Between them, rivaroxaban group showed the highest percentage of patients with good adherence at implementation (MPR ≥80) and dabigatran the lowest. Similar results were also found by Forslund et al.22 or by Beyer-Westendorf et al.23 although this last study only analysed rivaroxaban and dabigatran. On the opposite, other studies found higher MPR in apixaban-treated patients.14,37
Regarding persistence to DOAC in naive patients, apixaban showed higher discontinuation rates during the first month of treatment but at one year, all DOAC showed similar rates. Several studies analysed discontinuation rates at different times during follow-up. After one year, apixaban users were more persistent than other DOAC and VKA users in the studies conducted by Forslund et al.22 and Johnson et al.21
Other studies which analysed persistence in naive patients only included dabigatran and rivaroxaban. Rivaroxaban presented better persistence than dabigatran and VKA.23,38
Manzoor et al.37 or Martínez et al.39 studied all DOAC together as a group. The first one showed higher levels of persistence to DOAC in anticoagulant-experienced patients over time. Martínez et al. found higher levels of persistence in DOAC versus VKA users, which slightly decreased during the first year of follow-up (from 94.7% of persistents to DOAC at 3 months to 72.9% at 12 months).
For anticoagulant-experienced patients with more than one year of follow-up in our study, again rivaroxaban showed the largest proportion of patients with good adherence during implementation. Only Manzoor et al.37 analysed MPR in non-naive patients and apixaban showed higher MPR in apixaban at 6 months and dabigatran at 9 months. Discontinuation rates in our non-naive patients were much lower than for the naive ones; during the first month since treatment initiation 1.6% apixaban, 4.9% rivaroxaban and 12.6% dabigatran patients discontinued the treatment, and after one year rivaroxaban users showed lower persistence rates (48.5%) than apixaban and dabigatran (66.1% and 64.6%, respectively). Manzoor et al.37 compared persistence between naive and non-naive patients receiving DOAC and the last ones showed higher levels of persistence. Johnson et al.21 described similar discontinuation rates than for naive patients and at the end-of follow-up patients prescribed apixaban showed improved persistence over dabigatran, rivaroxaban and VKA.
The differences in treatment persistence between naive and anticoagulant-experienced patients in our study could be motivated by a better knowledge of the anticoagulation importance of these patients who previously received mainly VKA, and they were used to attend monthly to PHC centres for INR determination and had optimal levels of drug adherence.
Suboptimal adherence to anticoagulant therapy places patients with AF at risk for stroke or bleeding complications. Our study concludes as most observational studies, that the guidelines recommendations regarding anticoagulant therapy are not routinely followed in clinical practice, and adherence is substantially lower than in clinical trials.3,40
Some specific limitations in our database are the lack of association between GP's prescriptions and dispensing associated with these prescriptions. This study has missing data from pharmacy claims and for some variables as it is common in observational studies using electronic databases (information bias). The strengths of our study are representativeness for the general population, with a database that covers almost the 80% of the Catalonian population, with complete sociodemographic and health records, long follow-up, and real clinical practice data.
- •
The level of utilisation of the different DOAC in stroke prevention in NVAF has shown to be different among countries, and several cohort studies have shown dissimilar results on adherence, effectiveness and safety of these drugs.
- •
Adequate levels of adherence and persistence to anticoagulant treatment have shown to decrease the occurrence of embolic events.
- •
Vitamin K antagonists are still the first option to anticoagulate NVAF patients in our setting.
- •
We measured adherence and persistence for DOAC patients. Adherence was higher for rivaroxaban, followed by apixaban and dabigatran.
- •
Persistence to DOAC was low in our setting, which may result in higher risk of thromboembolic events. Discontinuation rates were higher in naive than in non-naive patients. This may be caused by better knowledge of the importance of treatment adherence in patients who have previously received VKA, which need a more strict management.
The authors declare that they have no conflict of interest.
The authors thank María Aragón and Darío García from SIDIAP for data management.