Evaluate whether an intervention applied to general practitioners to prevent clinical inertia had an impact on pain, functionality, and health-related quality of life (HRQoL) of patients with hip and/or knee osteoarthritis.
DesignThis was a cluster-based, multicentre, prospective, randomized, parallel-group study. Clusters of physicians working were assigned to one of two study groups. Physicians in Group 1 received a training session while those in Group 2 did not.
SettingPrimary Care Health centers representative of the entire Spanish territory.
Participants329 general practitioners of primary healthcare centre.
InterventionsThe intervention consists of a motivational session to propose a proactive care, based on current recommendations.
MeasurementsVisual analogue scale (VAS); functionality (WOMAC scale) and global perception of health by SF-12. Effects were measured in two visits six months apart.
ResultsA total of 1361 physicians, and 4076 patients participated in the study. No significant differences were observed in the clinical benefit obtained between patients assigned to Group 1 and Group 2. Nevertheless, a significant improvement was observed in the combined population (Groups 1 + 2) in the VAS (p<0.001), WOMAC (p<0.0001) and SF-12v2 (p<0.001) questionnaires in Visit 2 compared to Visit 1.
ConclusionsThe results indicate that, although this specific intervention carried out on physicians did not provide an additional clinical benefit to patients with knee and/or hip osteoarthritis, an increased awareness of the patient's disease through the use of functionality indexes, as well as the mere fact of being observed, seem to improve patient-reported pain, functionality and HRQoL.
Evaluar si una intervención aplicada a médicos de familia para evitar la inercia clínica tuvo un impacto en el dolor, funcionalidad y calidad de vida relacionada con la salud (CVRS) de los pacientes con artritis de cadera y/o rodilla.
DiseñoEstudio de grupos paralelos de cluster, multicéntrico, prospectivo, aleatorizado. Los médicos fueron asignados a 2 grupos, el grupo 1 recibieó una sesión de entrenamiento, el grupo 2, no.
EmplazamientoCentros de salud representativos del territorio español.
ParticipantesMédicos de familia de 329 centros de salud.
IntervencionesConsistieron en una sesión motivadora para proponer una atención proactiva, basada en recomendaciones actualizadas.
Mediciones principalesEscala analógica visual (EVA); funcionalidad (escala WOMAC) y percepción global de salud mediante SF-12 en 2 visitas separadas por 6 meses.
ResultadosParticiparon 1.361 médicos y 4.076 pacientes. No se observaron diferencias significativas en el beneficio clínico obtenido entre los pacientes asignados al grupo 1 y grupo 2. Sin embargo, se observó una mejora significativa en la población total (grupos 1 + 2) en la EVA (p<0,001), WOMAC (p<0,0001) y el SF-12V2 (p<0,001) en la visita 2 en comparación con la visita 1.
ConclusionesEsta intervención sobre médicos de familia no proporcionó un beneficio clínico adicional a los pacientes. Se observó en ambos grupos una mayor conciencia de la enfermedad del paciente por el uso novedoso de índices de funcionalidad y CVRS, que parece mejorar el dolor percibido, la funcionalidad y la calidad de vida relacionada con la salud.
Osteoarthritis is the most common type of arthritis in Western populations, and is a major cause of chronic musculoskeletal pain and mobility disability in elderly populations worldwide.1 The prevalence of osteoarthritis varies depending upon the diagnostic method used (clinical or radiological), the joint(s) studied, and the characteristics of the study population; nonetheless, it is considered one of the ten most disabling diseases in developed countries, affecting 10% of men and 18% of women over the age of 60.2 As with other chronic conditions, treatment of osteoarthritis is complex and involves a combination of pharmacological and non-pharmacological measures for its optimal management.3,4
Failure to implement recommendations contained in the available guidelines of any chronic disease is one of several factors contributing to clinical inertia. Clinical inertia is a leading cause of potentially preventable adverse events. According to Phillips et al.5 factors that could contribute to clinical inertia include the overestimation of the quality of the care delivered by the physician or the underestimation of the number of patients who need an intensification of pharmacotherapy. Additionally, other factors that could contribute are that some physicians lack the appropriate knowledge, tools and clinic facilities to deliver adequate care to patients with chronic diseases.5 As has been demonstrated in several randomised clinical trials and cohort studies, the deleterious consequences of clinical inertia for the patient can be significant, especially in certain chronic diseases such as diabetes mellitus or arterial hypertension.6–8 Unfortunately, information on the impact of clinical inertia on other diseases with low mortality but high morbidity, such as osteoarthritis, is limited or non-existent.
We carried out a cluster-based, multicentre, prospective, randomised, parallel-group study to evaluate whether patients with hip and/or knee osteoarthritis would benefit from a specific type of intervention received by their general practitioners, which was designed to reduce clinical inertia. Secondary objectives were to learn about the clinical characteristics of osteoarthritis patients attending primary care clinics, and to identify those factors related to clinical inertia that, when modified, may result in clinical benefit for the patient.
Materials and methodsStudy designA multicentre, prospective, randomised, parallel-group study of physician clusters (general practitioners) was performed to compare the effectiveness of two different healthcare approaches. The study protocol was approved by the Ethics Committee of the Hospital Universitario 12 de Octubre (Madrid, Spain) and by the Scientific Committee of the Spanish Family and Community Medicine Society (SemFYC).
Study populationsGeneral practitioners (GP) working in the same primary healthcare centre for a period of at least six months after the start of the study were selected to participate in the study. Physicians involved in other studies related to healthcare improvement in osteoarthritis or similar were excluded. GP included in the study enrolled patients who attended their clinics with a diagnosis of hip and/or knee osteoarthritis according to the criteria of the American College of Rheumatology.9 Patients who had a prosthetic treatment of osteoarthritis, a prosthetic implantation planned within the next six months, osteoarthritis involving exclusively other joints besides the hip or knee, concomitant diagnosis of other rheumatic disorders, and/or inability to participate in the study were excluded.
With an anticipated effectiveness of 50% in group 2 and an estimated clinically relevant difference of at least 10%, a type I error of 5% and a study power of 90%, the estimated sample size for this study was 350 primary care centres with 5 general practitioners per site (1,750 GP). Assuming that 10% of physicians would probably drop out, the final sample size estimated for this study was 1,925 GP from the 350 primary care centres.
Study proceduresClusters of physicians working at the same healthcare centre for more than six months were randomly assigned with a ratio 1:3 to one of two study groups. Group 1 (proactive intervention group) physicians received a specific scientific training session on the current management guidelines of osteoarthritis disease to avoid clinical inertia. Group 2 (control group) physicians did not receive any specific intervention and, hence, delivered usual healthcare to patients with hip and/or knee osteoarthritis.
Each GP included the first three patients with hip and/or knee osteoarthritis who fulfilled selection criteria and agreed to participate in the study. Consequently, a total of 5,775 patients were expected to be included in the study.
The intervention delivered to Group 1 was a single clinical session of 45-60minutes, in which a family physician, who had been previously trained, explained the assessment methods which would be used during the study: visual analogue scale (VAS) (range 0-100mm),10 Western Ontario and McMaster Universities (WOMAC)11 and the Short Form-12 version 2 (SF-12v2) Health Survey12 questionnaires. Motivational techniques were used and the latest EULAR evidence-based recommendations for the management of osteoarthritis disease were delivered.3,4 The session was addressed to family physicians with daily clinical practice, who are supposed to have enough knowledge level to make decisions on the patients; thus, the aim of meeting was to motivate, break the weight that inertia supposes and generate proactive behaviour to lead physicians to search and update their knowledge of osteoarthritic disease and its treatment.
Additionally, GP from both study groups received a brief summary of the study protocol, the case report forms, and the questionnaires which would be used (VAS, WOMAC and SF-12v2). Scores were estimated for four of the health concepts (physical functioning, physical role, emotional role and mental health) using two items each, while the remaining four (pain, general health, vitality and social functioning) were represented by a single item. All 12 items are used to calculate the Physical and Mental Component Summary scores, which yield a mean of 50 and a standard deviation (SD) of 10.13 Thus, patients are classified as above or below average. If a patient's physical health difference score was negative but close to zero, he or she was considered in ‘average’ health. However, if a patient's score was around -20, he or she was considered ‘below average’, or in poor health.14
Patients selected by GP were scheduled for at least two visits with a six months interval. During Visit 1, age, weight, height, medical history, time elapsed since diagnosis, presence of concomitant diseases, and pharmacological treatment(s) received were recorded. In addition, patient's global health perception, general health status, pain and functionality were assessed using the SF-12v2, WOMAC and VAS questionnaires. During Visit 2, patients underwent a physical examination, information about current pharmacological and non-pharmacological treatments and the presence of additional relevant events was recorded, and SF-12v2, WOMAC and VAS tests were administered.
Statistical analysisQualitative variables were expressed as percentages. Continuous variables were described using mean, median, SD, range, and 95% confidence intervals (CI). Frequency distributions of qualitative variables were compared by Pearson X2 or Fisher exact tests. Health centre and physician were considered random effects, and perception of patient changes was considered as fixed effect. All analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC).
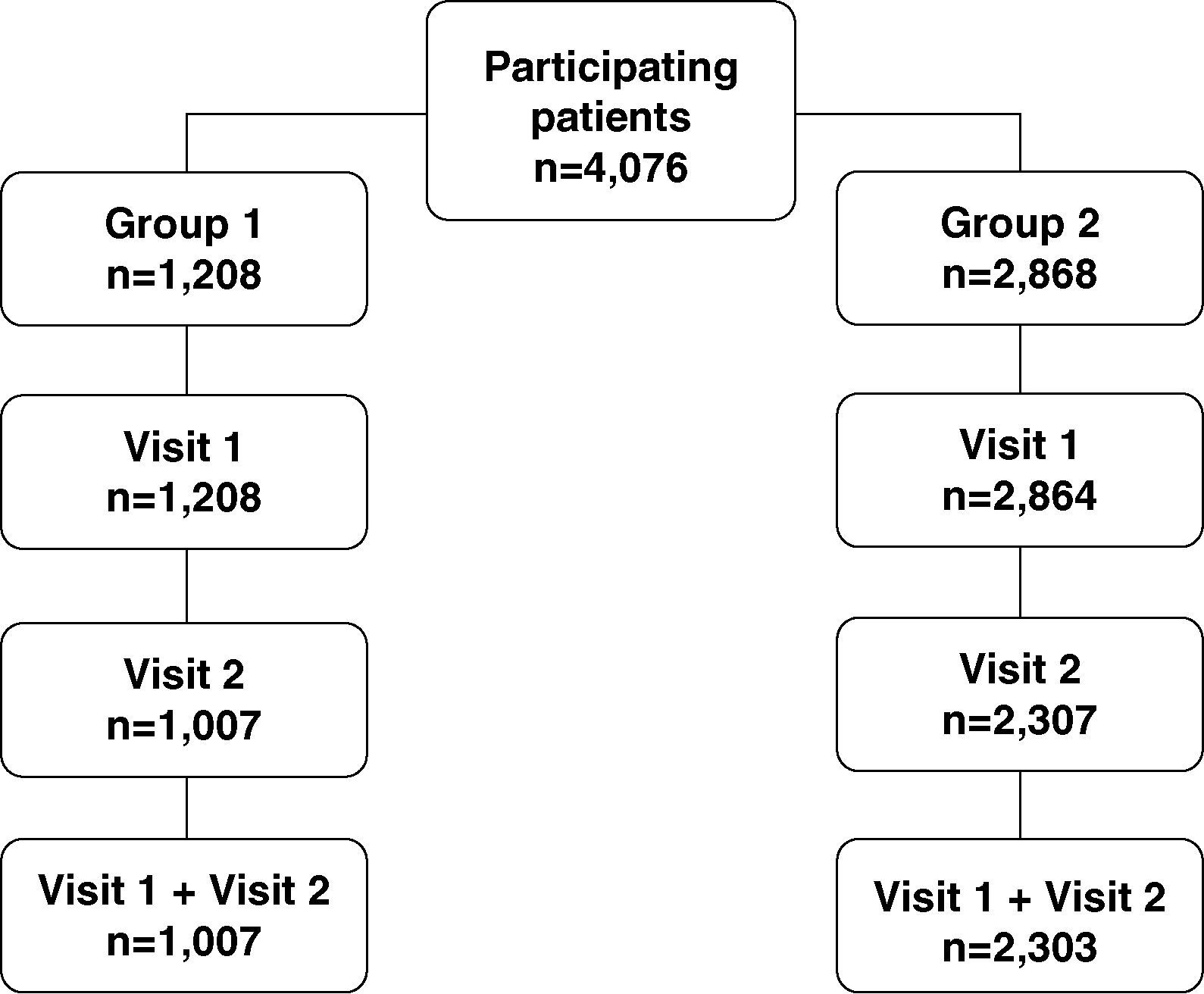
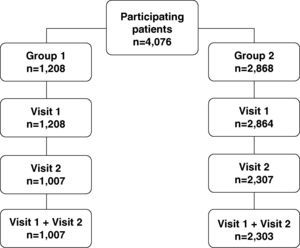
ResultsBetween September and October 2007, a total of 1361 GP were selected: 403 (30%) were assigned to Group 1 and 958 (70%) to Group 2. No significant differences were observed between groups regarding the number of years practicing, scientific and academic activity, age and gender.
A total of 4076 patients with hip and/or knee osteoarthritis were included in the study, 1208 (30%) attended by GP assigned to Group 1 and 2868 (70%) attended by GP belonging to Group 2. Overall, 1007 (83%) group 1 patients and 2303 (80%) group 2 patients attended both study visits. Discontinuation rate was a slightly higher in the control group (20% vs 17%).
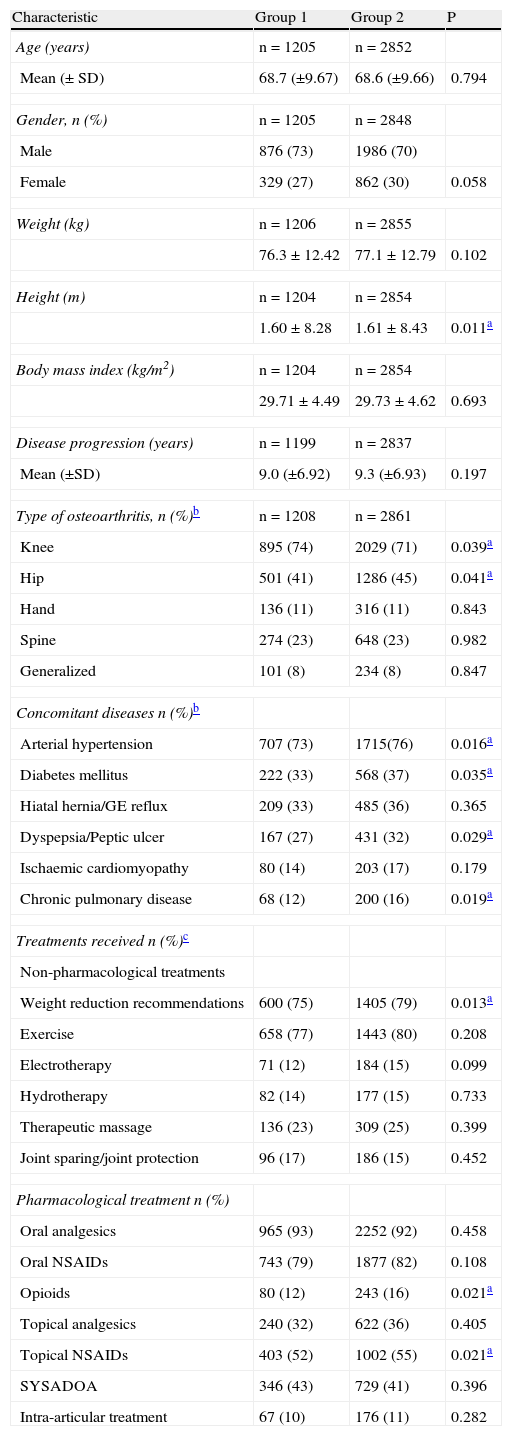
Baseline characteristics of the study groups are described in Table 1. There were small significant differences regarding the type of osteoarthritis (in Group 1, knee osteoarthritis was more frequently observed: 74% vs. 71%, P=.039; and hip osteoarthritis less frequently observed: 41% vs 45%, P=.041). There were small differences in some concomitant diseases: arterial hypertension, diabetes mellitus, hiatal hernia or gastroesophageal reflux, and dyspepsia or peptic ulcer. A large proportion of patients were being treated with non-pharmacological measures. Most of those who were treated with pharmacotherapy took: analgesics (>90%), oral and topical non-steroidal anti-inflammatory drugs (NSAIDs) (>70% and >50%, respectively), and SYSADOAS (Symptomatic Slow Acting Drugs for Osteoarthritis) (>40%). The only differences observed were patients receiving topical NSAIDs (P=.021) and opioids (P=.021).
Baseline patient characteristics.
| Characteristic | Group 1 | Group 2 | P |
| Age (years) | n=1205 | n=2852 | |
| Mean (± SD) | 68.7 (±9.67) | 68.6 (±9.66) | 0.794 |
| Gender, n (%) | n=1205 | n=2848 | |
| Male | 876 (73) | 1986 (70) | |
| Female | 329 (27) | 862 (30) | 0.058 |
| Weight (kg) | n=1206 | n=2855 | |
| 76.3±12.42 | 77.1±12.79 | 0.102 | |
| Height (m) | n=1204 | n=2854 | |
| 1.60±8.28 | 1.61±8.43 | 0.011a | |
| Body mass index (kg/m2) | n=1204 | n=2854 | |
| 29.71±4.49 | 29.73±4.62 | 0.693 | |
| Disease progression (years) | n=1199 | n=2837 | |
| Mean (±SD) | 9.0 (±6.92) | 9.3 (±6.93) | 0.197 |
| Type of osteoarthritis, n (%)b | n=1208 | n=2861 | |
| Knee | 895 (74) | 2029 (71) | 0.039a |
| Hip | 501 (41) | 1286 (45) | 0.041a |
| Hand | 136 (11) | 316 (11) | 0.843 |
| Spine | 274 (23) | 648 (23) | 0.982 |
| Generalized | 101 (8) | 234 (8) | 0.847 |
| Concomitant diseases n (%)b | |||
| Arterial hypertension | 707 (73) | 1715(76) | 0.016a |
| Diabetes mellitus | 222 (33) | 568 (37) | 0.035a |
| Hiatal hernia/GE reflux | 209 (33) | 485 (36) | 0.365 |
| Dyspepsia/Peptic ulcer | 167 (27) | 431 (32) | 0.029a |
| Ischaemic cardiomyopathy | 80 (14) | 203 (17) | 0.179 |
| Chronic pulmonary disease | 68 (12) | 200 (16) | 0.019a |
| Treatments received n (%)c | |||
| Non-pharmacological treatments | |||
| Weight reduction recommendations | 600 (75) | 1405 (79) | 0.013a |
| Exercise | 658 (77) | 1443 (80) | 0.208 |
| Electrotherapy | 71 (12) | 184 (15) | 0.099 |
| Hydrotherapy | 82 (14) | 177 (15) | 0.733 |
| Therapeutic massage | 136 (23) | 309 (25) | 0.399 |
| Joint sparing/joint protection | 96 (17) | 186 (15) | 0.452 |
| Pharmacological treatment n (%) | |||
| Oral analgesics | 965 (93) | 2252 (92) | 0.458 |
| Oral NSAIDs | 743 (79) | 1877 (82) | 0.108 |
| Opioids | 80 (12) | 243 (16) | 0.021a |
| Topical analgesics | 240 (32) | 622 (36) | 0.405 |
| Topical NSAIDs | 403 (52) | 1002 (55) | 0.021a |
| SYSADOA | 346 (43) | 729 (41) | 0.396 |
| Intra-articular treatment | 67 (10) | 176 (11) | 0.282 |
GE: gastroesophageal reflux; NSAIDs: non-steroidal anti-inflammatory drugs; SD: standard deviation; SYSADOA: symptomatic slow acting drugs for osteoarthritis.
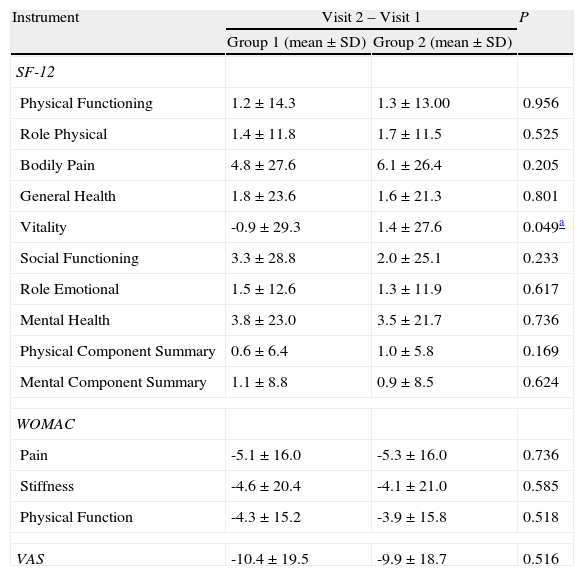
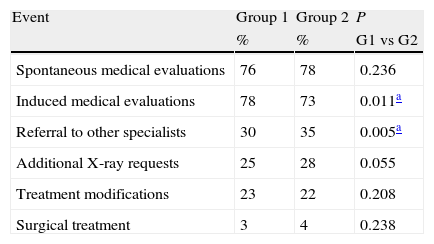
No significant differences were observed in any of the items evaluated by the SF-12v2, the WOMAC or the VAS questionnaires in visits 1 and 2 (Table 2), except the vitality evaluated by SF-12v2 (P=.049). In the comparison of the incidence of relevant events occurring during the six-month study period (Table 3), Group 1 showed significantly more induced medical evaluations (P=.011) and less referrals to other specialties.
Global differences between Group 1 and Group 2 in Visit 2 compared with Visit 1.
| Instrument | Visit 2 – Visit 1 | P | |
| Group 1 (mean±SD) | Group 2 (mean±SD) | ||
| SF-12 | |||
| Physical Functioning | 1.2±14.3 | 1.3±13.00 | 0.956 |
| Role Physical | 1.4±11.8 | 1.7±11.5 | 0.525 |
| Bodily Pain | 4.8±27.6 | 6.1±26.4 | 0.205 |
| General Health | 1.8±23.6 | 1.6±21.3 | 0.801 |
| Vitality | -0.9±29.3 | 1.4±27.6 | 0.049a |
| Social Functioning | 3.3±28.8 | 2.0±25.1 | 0.233 |
| Role Emotional | 1.5±12.6 | 1.3±11.9 | 0.617 |
| Mental Health | 3.8±23.0 | 3.5±21.7 | 0.736 |
| Physical Component Summary | 0.6±6.4 | 1.0±5.8 | 0.169 |
| Mental Component Summary | 1.1±8.8 | 0.9±8.5 | 0.624 |
| WOMAC | |||
| Pain | -5.1±16.0 | -5.3±16.0 | 0.736 |
| Stiffness | -4.6±20.4 | -4.1±21.0 | 0.585 |
| Physical Function | -4.3±15.2 | -3.9±15.8 | 0.518 |
| VAS | -10.4±19.5 | -9.9±18.7 | 0.516 |
SF-12v2: Short Form 12-item version 2 Health Survey; VAS: Visual Analogue Scale; WOMAC: Western Ontario and McMaster Universities Index.
Incidence of relevant events in patients assigned to Group 1 and Group 2.
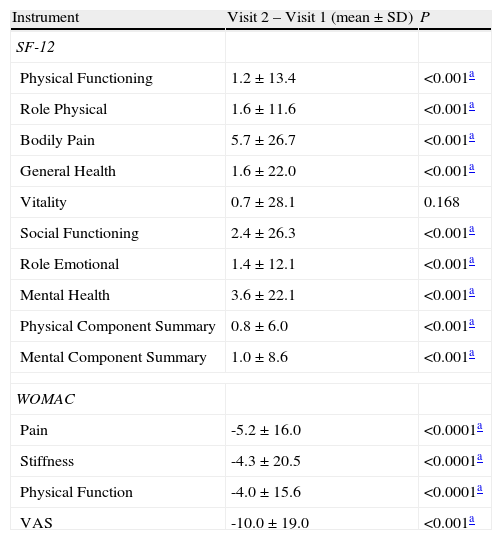
Comparing the results of the two visits for the whole study population, significant differences were observed in most of the items evaluated (Table 4).
Global differences between Visit 2 and Visit 1.
| Instrument | Visit 2 – Visit 1 (mean±SD) | P |
| SF-12 | ||
| Physical Functioning | 1.2±13.4 | <0.001a |
| Role Physical | 1.6±11.6 | <0.001a |
| Bodily Pain | 5.7±26.7 | <0.001a |
| General Health | 1.6±22.0 | <0.001a |
| Vitality | 0.7±28.1 | 0.168 |
| Social Functioning | 2.4±26.3 | <0.001a |
| Role Emotional | 1.4±12.1 | <0.001a |
| Mental Health | 3.6±22.1 | <0.001a |
| Physical Component Summary | 0.8±6.0 | <0.001a |
| Mental Component Summary | 1.0±8.6 | <0.001a |
| WOMAC | ||
| Pain | -5.2±16.0 | <0.0001a |
| Stiffness | -4.3±20.5 | <0.0001a |
| Physical Function | -4.0±15.6 | <0.0001a |
| VAS | -10.0±19.0 | <0.001a |
SF-12v: Short Form 12-item version 2 Health Survey; VAS: Visual Analogue Scale; WOMAC: Western Ontario and McMaster Universities Index.
The results of our study can be grouped into three major observations: (1) patients with osteoarthritis have a high degree of comorbidity and a low health-related quality of life (HRQoL), as reflected by the SF-12v2, WOMAC and VAS scores; (2) patients did not seem to obtain a clinical benefit from the intervention studied, as there were no significant differences between visit 2 and visit 1; and (3) patient-reported pain, functionality and HRQoL significantly improved in the overall study population.
Study patients seem to have a higher degree of impairment and a worse health status in relation to the general Spanish population. This was tested by Vilagut et al.,12 who observed that the mean SF-12v2 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores of a representative sample of the Spanish population were 45.05 (SD: 10.17) and 47.81 (SD: 9.20), respectively, indicating a somewhat better quality of life than our study group.
One limitation of the study is the discontinuation rate, around 20%, higher than the 10% used to calculate the sample size, despite the motivation strategy, follow-up, phone and e-mail reminders. It was not possible to do a systematic analysis of discontinued patients, as many were due to job changes of GP, but were similar in number in both groups. The high prevalence of some diseases, like diabetes mellitus (33% and 37% in the control and intervention group) are consistent with the average age of the sample (68 years) and the existence of high rates of comorbidity.
As regards the clinical benefit obtained by patients treated by the GP who had received the specific intervention, our findings call for careful interpretation, and several aspects must be taken into consideration. Therefore, despite the efforts to modify clinical inertia in GP, it is likely that these were insufficient, probably, among other reasons, because they focus on a single factor.
The observation that HRQoL perception improved significantly in overall population from Visit 1 to Visit 2, regardless of the study group, is important. Since the majority of patients had one or more concomitant diseases, osteoarthritis may have been unimportant until enrolment in this study. Thus, a small increase in GP attention may have represented a significant change to the patient in the quality of medical care received, which in turn, had an effect on the HRQoL. We are aware that the mere fact of having been included in a study, which produces a psychological effect of being observed, may have generated a positive response in the physicians, which in turn affected the quality of healthcare delivered to patients, irrespective of the group assigned to. This Hawthorne effect is expected in all types of studies in which the investigator or subject are aware of their participation.15 Therefore, the long-term effects on HRQoL will need further evaluation.
The results of this study should be interpreted in the light of potential limitations. First, considering the chronic nature of osteoarthritis, the six months follow-up period might not have been long enough. Secondly, as discussed above, the type of intervention we designed in order to modify clinical inertia may have fallen short, or the factors we intended to modify were not the most appropriate. Nevertheless, since the actual recruitment was 1361 physicians and 4076 patients, we consider that the power of the study is enough to support the main conclusions. Also, although the fact that approximately 20% of patient data was lost on follow-up when an estimation of 10% was initially planned, does not invalidate our results. Moreover, it could be an additional factor contributing to clinical inertia due to a lack of an adequate follow-up of the disease.16 The search for factors that might contribute to clinical inertia through interventions on primary care physicians have been used in other cluster randomised trials,17,18 yielding positive results regarding the implementation of interventions carried out on physicians to reduce clinical inertia.18 Future educational interventions on practitioners could use the recent Clinical Practice Guidelines for Osteoarthritis of the Royal College of Physicians as a reference.19
In conclusion, the results suggest that minor interventions, such as an increased awareness of the patient's disease through the use of functionality indexes, as well as the mere fact of being observed, may be improving patient-reported pain, functionality and HRQoL. Nevertheless, because this specific intervention carried out on physicians to prevent clinical inertia did not provide an additional clinical benefit to our sample of patients with knee and/or hip osteoarthritis, efforts should be made to identify potentially modifiable factors that lead to clinical inertia other than physician-related factors.
- •
Osteoarthritis (OA) is the most common type of arthritis in Western populations, and is a major cause of chronic musculoskeletal pain, mobility disability and health services use in populations worldwide.
- •
Clinical inertia, do not initiate or intensify therapy when clinically indicated is described in many chronic conditions and it also applies to OA.
- •
Patients with osteoarthritis have a high degree of comorbidity and a low HRQoL, as reflected by the scores obtained in the SF-12v2, WOMAC and VAS questionnaires;
- •
Patients did not seem to obtain a clinical benefit from the intervention we made on their treating general practitioners, given that inertia was not modified.
- •
Minor, in clinic, interventions, such as an increased awareness of the patient's disease through the use of functionality indexes, as well as the mere fact of being observed, may improve patient-reported pain, functionality and HRQoL.
AGC, ATV and FLV developed the original idea for the paper.
AGC, ATV and FLV contributed to the design of the study.
AGC, ATV, FLV, DLP, APM, FVN and JHH participated in the execution of the study.
The Clinical Epidemiology Unit of the Hospital 12 de Octubre performed the analysis and interpretation of data.
AGC, ATV, and FLV wrote the article, helped by Dr. Ximena Alvira from HealthCo SL (Madrid, Spain).
AGC, ATV, and FLV made the decision on submitting the manuscript for publication.
All authors approved the final version of the submitted article.
Researchers were independent from funders and sponsors, and they had access to all the study data.
Competing interestsThe authors declare that they have no competing interests.
The authors thank Dr. Ximena Alvira from HealthCo SL (Madrid, Spain) for assistance in the preparation of the manuscript, and SERMES CRO who performed data collection. This project was financially supported by the Spanish Family and Community Medicine Society (SemFYC), granted mainly by Merck Sharp and Dohme Spain. Merck Sharp and Dohme Spain was not involved in the study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Juana Redondo Sánchez, Centro de Salud Lucano (Córdoba), Carlos Alberto Cabrera Rodríguez (Granada), Francisca Leiva/Hospital (Málaga), Antonio Hormigo Pozo, Centro de Salud Puerta Blanca (Málaga), José María Fernández Rodríguez-Lacín (Asturias), Enrique Nieto Pol, Centro de Salud Concepción Arenal (A Coruña), José Fernando Espinosa Díaz, Centro de Salud Villanueva de La Serena (Badajoz), Ramón Orueta Sánchez, Centro de Salud Sillería de Toledo (Toledo), Carlos Cerezo Goyeneche, ABS Montilivi (Girona), María del Mar Rodríguez Álvarez (Barcelona), Juan José Antón Álvarez, CAP Manso (Barcelona), Ángel Donado Mazarrón Romero, (Tarragona), Alejandro Tejedor Varillas, Centro de Salud Las Ciudades-Getafe (Madrid), Juan Carlos Hermosa Hernán, Centro de Salud Las Ciudades- Getafe (Madrid), Fernando León Vázquez, Centro de Salud San Juan de la Cruz-Pozuelo (Madrid), Juan de Dios González Caballero, Consultorio Aljorra (Murcia), Antonio Fuertes Fortea, Centro de Salud Alginet (Valencia), Carlos Fluixá Carrascosa, Centro de Salud Benimaclet (Valencia), Carlos Vilaplana Bernabeu, Centro de Salud Castalla (Alicante), Carmen Fernández Fernández, Centro de Salud Arquitecto Benassar (Mallorca), Adolfo Hervás Angulo, Centro de Salud Tafalla (Tafalla), José Luis Torres Baile, Centro de Salud Rodríguez Paterna (La Rioja), Francisca González Rubio, Centro de Salud Delicias Sur (Zaragoza), Julia Echevarría Portell, Centro de Salud Zuazi-Baracaldo (País Vasco) Agustín Gómez de la Cámara, Hospital Universitario 12 de Octubre (Madrid), Miguel García López, (León), Miguel Torrecilla García, (Salamanca), Esperanza Delgado Vicente,CS. Ávila Norte, Miguel Ángel Castilla Fernández, (Valladolid), Álvaro Pérez Martín,CS. Los Valles (Santander), Martín Astorga Romón, (Burgos), Enrique Alcaráz Vera, Centro de Salud La Laguna (Cádiz), Vicente Rodríguez Pappalardo, Centro de Salud De Camas (Sevilla), Gustavo Moreno Valentín, Centro de Salud Alcaravaneras (Las Palmas), Francisco Vargas Negrín, Centro de Salud Dr. Guigou (Tenerife), Antonio Pérez Márquez, Centro de Salud La Orden (Huelva), Vicente Reyes Adrian, Centro de Salud La Vileta (Palma de Mallorca), Emilio Aberasturi CS. Abechucho Vitoria Gasteiz
The list of members of the ArtroPro Study Group is shown in Appendix 1.