In recent years, the prevalence of asthma has risen in developed countries, and its extent related to a change in our indigenous microbiota. Helicobacter pylori disappearance across the population represents a fundamental change in our human microbiota and has preceded the rise in asthma prevalence.
ObjectiveTo assess the relationship between childhood asthma and Helicobacter pylori infection.
MethodsQuantitative determination of Helicobacter pylori IgG among 90 asthmatic children and 90 – age and gender – matched non-atopic, non-asthmatic healthy children was performed using ELISA in serum of all participants.
ResultsHelicobacter pylori IgG seropositivity was found in 25.6% of asthmatics compared to 44.4% of controls. Asthmatics showed lower median Helicobacter pylori IgG titre compared to healthy controls. We also detected a significant inverse relationship between Helicobacter pylori IgG titre and asthma severity.
ConclusionHelicobacter pylori seropositivity protects against childhood asthma and inversely correlates to its clinical and functional severity.
There is wide geographical variation in the prevalence of asthma and allergic conditions world-wide, with substantial differences seen between low- and high-income countries, and between urban and rural communities.1,2 Pre- and post-natal development of human immunity is remarkably continuous. The progressive immune response stabilisation at the sub-mucosal level during the first year of life arises from the interface between the host and their microflora.3 As a part of the hygiene hypothesis, a protective role of Helicobacter pylori infection in the aetiology of asthma and allergic disease has been raised. This gained support from a range of epidemiological,4–8 epigenetic,9 and animal model studies.10 Reduced risks of atopy and asthma have been reported in human studies,4,11,12 both in children4,11,13 and adults.6,12H. pylori is a bacterium which colonises the gastric mucosa of approximately half the world's population and is a main cause of peptic ulcer disease and gastric adenocarcinoma.14,15 Its infection is usually established during early childhood, persists life long, and remains asymptomatic in over 85% of cases. However, prevalence of H. pylori infection in developed countries has been declining sharply for several years.16 Additionally, the proportion of young children who got infected is extremely low nowadays, probably due to antibiotic use.17 Also, increased antibiotic use in infancy has been suggested to limit exposure to gastrointestinal microbes and to predispose to asthma in later life.18
There is ample evidence that, both, exogenous microbes and endogenous microbial communities, human microbiota, shape the developing immune system and might be involved in the prevention of pathologic pro-inflammatory trails.19 Exogenous infection and microbial substances including H. pylori infection may elicit a Th1-mediated immune response, which suppresses Th2 responses. Acquisition of H. pylori may be of importance in the induction of regulatory T cells, which could effectively reduce the possibility of allergic asthma.20 Inverse associations between H. pylori, especially cagA+ strains, with asthma and related allergic disorders were reported, especially involving younger individuals, and with early life disease onset.8,13
Therefore, we conducted this study to assess frequency of H. pylori seropositivity among asthmatic children compared to healthy controls. The relation between H. pylori IgG seropositivity in asthma clinical and functional severity was also studied.
MethodsStudy populationThis cross-sectional study was performed during the period from March 2014 till December 2015. Ninety asthmatic children (52 boys, 38 girls), aged 1–17 years (mean±SD; 7.05±3.58 years), were randomly recruited from the Paediatric Chest Clinic, Children's Hospital, Ain Shams University. Patients were selected and their clinical severity was assessed according to the global initiative for asthma “GINA, 2012”.21
Age (2–18 years, mean±SD; 7.51±4.19 years) and gender matched non-atopic, non-asthmatic healthy children (n=90) were chosen as controls. They were selected from the geographic area surrounding the place of study. There was no significant difference in school grades between children participating in the study.
This study has complied with the principles laid down in the Declaration of Helsinki, adopted by the 18th World Medical Assembly, Helsinki, Finland, in June 1964, and recently amended at the 59th World Medical Assembly, Seoul, Korea, in October 2008. The entire protocol was approved by the institutional ethical committee. All parents or care givers provided signed informed consent for participation in the study as required.
SpirometryAll participants were examined and underwent spirometry. At least eight hours before the test, short-acting bronchodilators were stopped. Dynamic spirometry (Jaeger, Germany) was performed, with measurement of forced expiratory volume in 1st sec (FEV1) % of predicted, according to standards of both European Respiratory Society and American Thoracic Academy. The highest values of FEV1 of three forced expiratory manoeuvres were used. Severity of the disease was classified as mild/intermittent or more severe. PFT results classified as normal or obstructive disease, degree of disease control on treatment was classified to controlled or uncontrolled asthma.21,22
Sample collection and processingVenous blood sample (5ml) was withdrawn from each participant under complete aseptic conditions. Blood was placed into tubes without anticoagulant and left at room temperature for 30–60min for spontaneous clotting. Serum was separated by centrifugation at 3000rpm for 10min. Serum samples were kept frozen at −80°C until used in quantitative determination of Helicobacter pylori IgG according to the manufacturer's instructions of Serion ELISA Classic Helicobacter pylori IgG kit (Virion\Serion GmbH, Würzburg, Germany) and total IgE according to the manufacturer's instructions of IgE ELISA kit (General Biologicals Corporation (GBC), Taiwan).
Statistical analysisCollected data were reviewed, coded, entered personal computer then analysed statistically by SPSS software version 15 (SPSS Inc., Chicago, IL, USA). Obtained data were presented as count and percentage for categorical variables. Data are presented as mean±SD. The Mann Whitney U test was used to analyse differences between two groups. Comparison of three groups was performed using analysis of variance (ANOVA) and Fisher's protected least significant difference test or Chi-squared test. Kruskal–Wallis (χ2) test was used for comparison of more than two groups in non-parametric variables. Correlations between data were analysed using Spearman's rank correlation test. Statistical significance was set at a value of p<0.05.
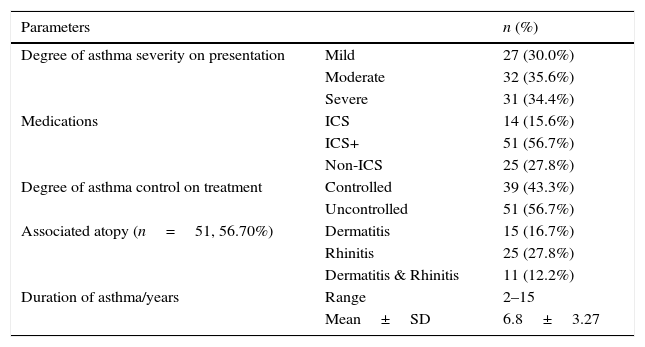
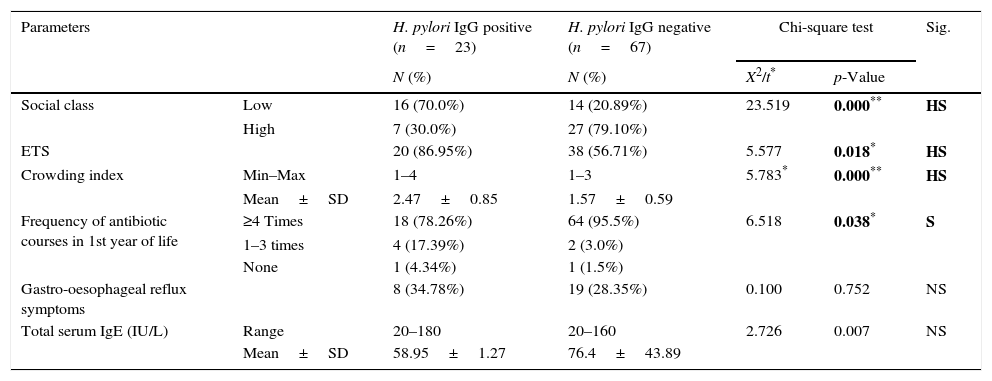
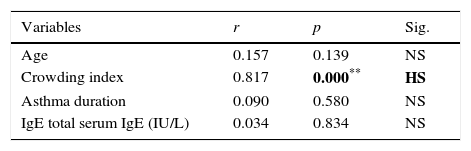
ResultsClinical data of the asthmatic group are shown in Table 1. Symptoms suggestive of gastro-oesophageal reflux were significantly more frequent among asthmatics (25.55%) when compared to healthy control (8.90%) (p=0.006). Our data reported higher median level of serum IgE in asthmatics (69.74IU/l) compared to healthy controls (30.70IU/l, p<0.001). H. pylori IgG seropositivity were found in 23/90 (25.6%) of asthmatic cases compared to 40/90 (44.4%) of controls with a high significant difference between the two groups (p=0.008). Medians (IQ) of H. pylori IgG were 19.6U/ml (15–57.8) and 34U/ml (20.8–89.6, p<0.001) in asthmatics and healthy controls, respectively. By comparing asthma subgroups subdivided according to asthma severity,21 we found statistical decrease in median H. pylori IgG titre with increase asthma severity (p=0.042), Table 2. Environmental Tobacco Smoking Exposure (ETSE) was higher among asthmatics (64.40%) compared to healthy controls (35.55%, p=0.001). Our study found that, in the first year of life, there was a statistically significant higher antibiotic intake among asthmatics in comparison to healthy controls (p=0.001), with 90% of asthmatic children exposed to more than four courses of antibiotics. Moreover, when we compared between asthmatics regarding H. pylori IgG seropositivity, we detected higher titres among low social class children (p<0.001), high environmental tobacco smoking exposure (p=0.018) and lower IgE levels (p=0.007) as well as less antibiotic intake among H. pylori IgG seropositive asthmatics (78.26%) in comparison with seronegative ones (95.5%, p=0.038), Table 3. However, the study did not report significant statistical differences between H. pylori IgG seropositive and seronegative asthmatics as regards age, sex, breastfeeding duration and timing of weaning (p>0.005). We found a significant positive association between H. pylori IgG levels and crowding index among asthmatics, Table 4.
Clinical data of asthmatic group (n=90).
| Parameters | n (%) | |
|---|---|---|
| Degree of asthma severity on presentation | Mild | 27 (30.0%) |
| Moderate | 32 (35.6%) | |
| Severe | 31 (34.4%) | |
| Medications | ICS | 14 (15.6%) |
| ICS+ | 51 (56.7%) | |
| Non-ICS | 25 (27.8%) | |
| Degree of asthma control on treatment | Controlled | 39 (43.3%) |
| Uncontrolled | 51 (56.7%) | |
| Associated atopy (n=51, 56.70%) | Dermatitis | 15 (16.7%) |
| Rhinitis | 25 (27.8%) | |
| Dermatitis & Rhinitis | 11 (12.2%) | |
| Duration of asthma/years | Range | 2–15 |
| Mean±SD | 6.8±3.27 |
ICS: inhaled corticosteroids; ICS+: inhaled corticosteroids and leukotriene receptor antagonist; non-ICS: only on demand bronchodilator (mild intermittent asthma).
Comparison between asthmatics sub-groups according to asthma severity regarding H. pylori IgG titre.
| Parameter | Mild (n=25) | Moderate (n=14) | Severe (n=51) | Kruskall Wallis | Sig. | ||||
|---|---|---|---|---|---|---|---|---|---|
| H. pylori IgG titre (U/ml) | Median | IQR | Median | IQR | Median | IQR | K | p-Value | |
| 21.9 | 15.9–70 | 22.1 | 15.4–63.8 | 15.5 | 13.3–20.9 | 6.326 | 0.042* | S | |
Comparison between asthmatics according to H. pylori IgG seropositivity.
| Parameters | H. pylori IgG positive (n=23) | H. pylori IgG negative (n=67) | Chi-square test | Sig. | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | X2/t* | p-Value | |||
| Social class | Low | 16 (70.0%) | 14 (20.89%) | 23.519 | 0.000** | HS |
| High | 7 (30.0%) | 27 (79.10%) | ||||
| ETS | 20 (86.95%) | 38 (56.71%) | 5.577 | 0.018* | HS | |
| Crowding index | Min–Max | 1–4 | 1–3 | 5.783* | 0.000** | HS |
| Mean±SD | 2.47±0.85 | 1.57±0.59 | ||||
| Frequency of antibiotic courses in 1st year of life | ≥4 Times | 18 (78.26%) | 64 (95.5%) | 6.518 | 0.038* | S |
| 1–3 times | 4 (17.39%) | 2 (3.0%) | ||||
| None | 1 (4.34%) | 1 (1.5%) | ||||
| Gastro-oesophageal reflux symptoms | 8 (34.78%) | 19 (28.35%) | 0.100 | 0.752 | NS | |
| Total serum IgE (IU/L) | Range | 20–180 | 20–160 | 2.726 | 0.007 | NS |
| Mean±SD | 58.95±1.27 | 76.4±43.89 | ||||
Statistical correlations between H. pylori IgG titre level versus other variables of asthmatic group (n=90).
| Variables | r | p | Sig. |
|---|---|---|---|
| Age | 0.157 | 0.139 | NS |
| Crowding index | 0.817 | 0.000** | HS |
| Asthma duration | 0.090 | 0.580 | NS |
| IgE total serum IgE (IU/L) | 0.034 | 0.834 | NS |
H. pylori IgG seropositivity was found in 25.6% of asthmatic cases compared to 44.4% of controls with high significant difference (p=0.008) in this cross-sectional study. These results demonstrated a significant negative association between H. pylori infection and asthma. It has been specifically noticed that H. pylori IgG seropositivity was higher among low social class children, high environmental tobacco smoking exposure, those with lower total serum IgE levels and less antibiotic intake. Furthermore, we detected a significant positive association between H. pylori IgG levels and crowding index among asthmatics. Thus, these findings represent further evidence for the role of infection with H. pylori in the pathogenesis of asthma.
Previous studies about the association between H. pylori status and asthma risk generate conflicting findings.23 These studies were conducted in adults, mostly small-scale, and additionally did not address the modifying roles of age. Similarly, Zevit et al.,24 found an inverse association of H. pylori with asthma, especially early-onset asthma.
Environmental tobacco smoke (ETS) exposure among children carries a lot of hazards; it affects the immune system and predisposes to asthma25 and the gut microbiota,26 and increases the risk of H. pylori infection.27 We found a high frequency of ETS among asthmatics compared to healthy controls and among H. pylori IgG seropositive asthmatics compared to seronegative ones. Interestingly, the association of smoking exposure with active H. pylori infection may result from various mechanisms including the role of smoking in increasing acid and pepsin secretion and the changes in gastric motility, prostaglandin synthesis, gastric mucosal blood flow, and mucus secretion.28 In addition, smoking increases mucus production (as much as seven-fold), decrease ciliary movement, beat frequency, and transport, increase white blood cells production mainly eosinophils, and increase mucosal permeability to allergens.29
As socioeconomic status plays an important role in H. pylori infection30,31; current data documented higher frequency of seropositive H. pylori IgG asthmatics among low social class compared to high social class. This was in agreement with Jafri et al.,32 data which reported more distribution of asthmatics among high social class (66.70%) and more distribution of H. pylori IgG seropositive asthmatics among low social class (86.95%). Similarly, among Egyptian children, H. pylori prevalence was highest in children attending school in socially-deprived areas.31
Two studies23,31 explained the observations that developed countries show higher prevalence of asthma than developing countries according to the “hygiene hypothesis”, which postulates that childhood development in an overly hygienic environment reduces microbial exposure and promotes atopic immune responses and the risk of asthma. Moreover, Okada et al.,33 demonstrated that the lack of microbial burden in developed countries in early childhood, which normally favours a strong Th1-biased immunity, redirects the immune response towards a Th2 phenotype and therefore predisposes the host to allergic disorders. In addition, another explanation for “hygiene hypothesis” is the “Antigenic competition”, which states that the development of strong immune responses against antigens from microbial agents could inhibit responses to ‘weak’ antigens such as auto-antigens and allergens.34
Owing to the role of household crowding in H. pylori infection23,31,32; the current results found that crowding index was higher among H. pylori IgG seropositive asthmatics compared to seronegative ones. Moreover, a significant positive correlation was found between H. pylori IgG titre in asthmatics and crowding index. This comes in agreement with the “hygiene hypothesis” which would have a protective effect against asthma.23,33 Moreover, H. pylori-infection status and childhood living conditions are associated with signs of allergic diseases in an occupational population.34
In agreement with Voort et al.,35 who found that short duration of breastfeeding and non-exclusivity are associated with increased risks of the asthma-related symptoms during the first four years of life, with the strongest effect estimated during the first two years. Newburg36 explained the underlying mechanisms for that association by the presence of immunoglobulin A, cytokines, especially transforming growth factor-β1, and long-chain fatty acids in breast milk that stimulate the infant's immune system. Studies about the role and duration of breastfeeding in H. pylori infection were variable, Pearce et al.,37 reported that increased duration of exclusive breastfeeding in infancy may have a long-term protective effect against chronic H. pylori infection; this could be due to maternal antibodies, the lactoferrin and other protective factors in the human milk. However, Chak et al.,38 argued that this protection lasts less than one year and does not seem to influence the prevalence of the infection later in life. Rodrigues et al.39 suggested that breastfeeding does not protect against acquisition of H. pylori; conversely, an infected mother may have an important role in transmission of the disease to the child, this may be explained by placing the breastfed children in close contact with the infected mother.
Our data confirmed that early antibiotic exposure is considered one of the most important predictors of childhood asthma. It affects the human gut microbiota – including H. pylori – with disturbance of the immune system.40 Several studies included the number of courses of antibiotics in their analysis; the highest risk for asthma/wheezing was found in children exposed to more than four courses of antibiotics in the first year of life.26,30
Interestingly, the relation between H. pylori serum IgG titre and asthma severity revealed a statistical significant inverse relationship between H. pylori serum IgG titre and asthma severity. This could be explained by the fact that respiratory allergy is less frequent in people heavily exposed to orofecal and food borne microbes which prevent allergic diseases by switching T-helper cells towards the Th1 direction.24,26 Additionally, infection with CagA+ H. pylori strains is reported to result in even greater Th1 responses and reduced Th2 responses.6–8 The H. pylori neutrophil-activating protein (HP-NAP) is an important Th1-promoting virulence factor, which has been shown to modulate Th2 responses in humans and mice. H. pylori infection also influences the Th1/Th2 balance via effects on gastric hormones. When levels of somatostatin are reduced and gastrin production is increased which inhibits Th2 cytokine release and promotes Th1 responses. Moreover, regulatory cytokine IL-10 and CD103+CD11b− dendritic cells are necessary to successfully protect against asthma in H. pylori-infected mice.40
In conclusion, H. pylori seropositivity protects against childhood asthma and inversely correlates to its clinical and functional severity.
Financial supportNone.
Conflict of interestNone.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.