Allergy to cats is a frequent cause of sensitization to indoor allergens and currently there are few alternatives to specific immunotherapy with cat native extracts. The objective is to develop and characterize a new allergoid to increase the tools available for use in clinical practice.
MethodsThe allergoid cat dander extract (ACD) was developed from a native cat dander extract (NCD) by modification with glutaraldehyde, and the optimal process control was determined by SDS-PAGE, DOT BLOT and determination of free amine groups. The ACD was characterized in protein profile by SDS-PAGE, size exclusion chromatography (SEC) and peptide footprint. The allergenic profile of ACD was determined by immunoblot, IgE CAP inhibition and IgG competition ELISA. The major allergen content in NCD was obtained by the ELISA sandwich protocol and was extrapolated to ACD.
ResultsThe control process determined the optimal development of the allergoid. The ACD obtained contains 182.28μg/mg of protein and 11.90μg/mg of Fel d 1. SDS-PAGE and SEC confirmed the presence of high molecular weight proteins in ACD, and the peptide footprint showed the presence of Fel d 1 and Fel d 7. The high degree of polymerization was evidenced with the determination of the reduction of lysine residues in the allergoid, resulting 91.96%. The ACD showed a significant loss of allergenicity respect to NCD, while the IgG-binding capacity was maintained.
ConclusionsThe ACD obtained presents a good safety profile, so would be a good alternative for treatment of cat allergy.
Domestic animals are considered risk factors for the development of allergic rhinitis and asthma in the domestic and occupational environment, constituting an important source of indoor allergens.1 Sensitization to this kind of allergens is quite ubiquitous and not dependent on geographic or climatic variables.2 The domestic animals that cause most allergies are dogs, cats and horses. Of these, cat allergy causes more complications, because their allergens can remain volatilized up to six months after the presence of the animal in a place, and is the second most frequent cause of sensitization to indoor allergens, after house dust mites in most European countries.3 Spain as the lowest percentage of prevalence of sensitization to cat allergens, not exceeding 4%,4 in contrast with Sweden where it reaches 20% of the population tested by skin prick test (SPT).3 Globally, the European population evaluated by SPT shows 25% reactivity to house dust mites, while about 10% show allergy to cat allergens.4 The percentage in the US is 15% prevalence of sensitization to cat allergens.5
Currently it is considered that specific immunotherapy (SIT) is the only treatment capable of modifying the natural history of allergic disease,6 even more so in this specific type of allergy where non-exposure to the animal does not guarantee the absence of an allergic reaction or prevent possible sensitization.7,8
The conventional strategy carried out with the treatment with SIT consists of increasing administration of allergen source doses, in order to redirect the immune response of patients, avoiding the production of mediators of immediate hypersensitivity. It has been shown that the use of allergen extracts for hyposensitization therapy is effective when the allergen dose is suitable, but augmentation of the concentration increases the risk of possible adverse reactions. Throughout the history of SIT various strategies have been adopted, such as physical or chemical modifications of the allergen which allow the administration of larger amounts, minimizing the risk of adverse reactions.9
Conformational changes of the allergen due to its chemical modification with glutaraldehyde is a well-established SIT strategy; it is possible to alter the capacity of recognition of the allergens by the IgE of the individuals sensitized, substantially reducing the ability of the allergen to induce IgE-mediated reactions.10 This allergoid, with low allergenicity, maintains its immunogenicity and can induce T cell-mediated responses that are not dependent on conformational epitopes.11 The effectiveness of allergoids in SIT is widely documented, and their ability to favor non-IgE-dependent immune responses is proven, possibly because they are not presented to T lymphocytes by IgE-dependent mechanisms.12
Based on these findings and because the current treatment with SIT for cat allergy is merely vaccines whose active principle is a native protein extract of epithelium and/or dander, the objective of this study was the development and characterization of a polymerized cat dander extract to increase the tools for use in clinical practice. An optimal characterization and standardization of allergenic extracts, is essential to ensure the quality of the extracts used for diagnosis and treatment of allergic diseases. While for the native allergen extracts there are standard methodologies described for their development, characterization and standardization, in the case of allergoids there is little information about the usefulness of these techniques and there are few reference documents available for the standardization of these products, although recently a study on the efficacy and safety of a polymerized cat dander extract has been published which shows a lower interaction with cat-specific IgE by the polymerized extract compared to the native extract, suggesting greater safety.13
The guidelines for the development, characterization and standardization of the allergoid cat dander extract were developed on the basis of what is described in both the EMA guides: Guideline on Allergen Products: Production and Quality Issues (2008),14 and the product allergens monograph European Pharmacopoeia (2010, 1063, Product Allergenic-European Pharmacopeia)15; they were also based on the ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.16
The quality of the new active principle was evaluated through the analysis of the protein, allergenic and immunogenic profiles. Since allergenic activity is essential to establish the safety of the product for use in SIT, is necessary to establish standards of both allergenic activity and immunogenic ability of the allergoid to estimate its potential quality as a hyposensitizing agent.17
MethodsAllergoid productionDevelopment of the extractThe allergoid cat dander extract (ACD) was developed from a native cat dander extract (NCD) with raw material from Allergon, manufactured under Good Manufacturing Practices (GMP). The NCD had a major allergen concentration of Fel d 1 of 8.3μg/mg quantified by Fel d 1 ELISA kit EL-FD1 (6F9/3E4) (Indoor Biotech, VA, USA) and 127.12μg/mg protein, quantified by elemental nitrogen analyzer (AEN) according to the method described in European pharmacopoeia 7.0 (2.5.33, 7B method) to determine total protein by nitrogen analysis.18 The NCD was polymerized with glutaraldehyde 10mM for 4h at room temperature. The polymerization process was stopped by addition of excess glycine, and the resulting extract was diafiltered against phosphate buffered saline (PBS) in Biomax® 100kDa membrane (Millipore, Bedford, Mass., USA), in order to remove impurities corresponding to compounds of low molecular weight, such as residues of glycine and non-polymerized protein fractions. The resulting solution was evaluated in protein concentration by AEN for dosing prior to lyophilization.
Process controlThe suitability of the production process was assessed at various points by different techniques:
SDS-PAGE: Samples were collected at different times during the course of the polymerization process. These samples were loaded under reducing conditions in 4–20% acrylamide-bisacrylamide gradient gels, with a glycine running buffer (25mM Tris, 192mM Glycine and 0.1% SDS). The silver staining was performed using ProteoSilver™ (Sigma-Aldrich).
DOT-BLOT: Samples were collected over the course of the polymerization process, stopping the process with glycine. Subsequently the samples were loaded in duplicate on a nitrocellulose membrane, 0.2μm (Bio-Rad, CA, USA), which was incubated overnight with a pool of sera from patients sensitive to Felis domesticus with a specific IgE of 7.1 KUA/L, analysis performed in ImmunoCAP system (Thermo Fisher Scientific, MA, USA). This pool of sera (hereafter p56) was provided by Dr. Benito Rodríguez Domínguez from area of Allergology of the Hospital Virgen de Altagracia de Manzanares (Ciudad Real, Spain), where the patients gave their informed consent for this publication, operating in full compliance with the principles embodied in the Declaration of Helsinki of 1964. It was used for all other immunochemical assays described in this article. As secondary antibodies, anti-Human IgE (¿-chain specific) – peroxidase antibody produced in goat (A9667, Sigma-Aldrich) and anti-Human IgG (whole molecule) – peroxidase antibody produced in goat (A8667, Sigma-Aldrich) were used.
Quantification of free amino groups: Prior to adding glycine, a sample of polymerized solution was collected to determine the amount of free amino groups. This data can be directly related to the degree of polymerization of the extract. The sample was analyzed and compared with NCD reconstituted under identical conditions. Serial dilutions of the samples in borate buffer 0.1M were performed and incubated for 30min with picrylsulfonic acid solution TNBS (Sigma-Aldrich) 1%; the reading was performed at 420nm in a double-beam spectrophotometer, Cary 100 (Agilent Technologies, CA, USA). Consequently, a regression line was obtained which correlated the concentration of protein in the sample with the absorbance. When A is the slope of the line obtained from the NCD and B from the ACD, the degree of modification obtained is ((A−B)/A)×100.19
Quantification of amino acid glycine: The percentage of glycine removed during the diafiltration process was evaluated in a sample collected at the beginning of the process and another at the end. The analysis was performed in accordance with the method for amino acid analysis described in US Pharmacopeia USP-29.20
Allergoid characterizationProtein profileAEN: The protein quantification of new ACD was performed by means of an elemental nitrogen analyzer.18 Combustion of the sample was performed in oxygen at 950°C which produced nitric oxide from the nitrogen present in the sample, and then it was detected by a chemiluminescence detector.
SDS-PAGE: Samples of NCD and ACD were loaded under reducing conditions with a total amount of 50μg of protein in gels 15% acrylamide-bisacrylamide, with a tricine running buffer. Staining was performed using Coomassie with Brilliant Blue R (Sigma-Aldrich, St. Louis, MO, USA) and silver staining ProteoSilver™ (Sigma-Aldrich).
Size exclusion chromatography (SEC): Recommended by the Guideline on Allergen Products: Production and Quality Issues.14 Analysis was performed by gel permeation chromatography with the isocratic method; the mobile phase was a saline buffer at pH 7 (130mm NaCl; 20mM KCl and 50mM Na2HPO4) and a wavelength of 280nm. The equipment used was a HPLC LC2010 CHT (Shimadzu Corporation, Kyoto, Japan) and a size exclusion column Zorbax GF-250 (4.6×250mm Analytical 4-Micron Silica, Agilent). The NCD and ACD with injections at a protein concentration of 4.5mg/ml were analyzed.
Peptide footprint: NCD and ACD were proteolytically digested with trypsin which decomposed them into peptides which were chromatographed by RP-HPLC (Waters XBridge BEH C18 HPLC column (5μm, 150×1mm, Waters Corporation, USA)) which was coupled online with an Agilent XCT Plus Ion Trap mass spectrometer using an electrospray interface. Data processing was performed with a data analysis program for LC/MSD Trap Version 3.3 (Bruker Daltonik, GmbH, Germany) and Spectrum Mill MS Proteomics Workbench (Rev A.03.02.060B, Agilent Technologies). The Spectrum Mill score was based on a points system; points are added for each peak assigned to an ion fragment type allowing for a candidate peptide sequence, and different types of fragment ions were worth different points. This scheme was composed of scoring three main components; the protein score, the score and the peptide Scored Peak Intensity (SPI). A peptide score above 5 and SPI above 70% were considered as valid for guarantee of the identity of the protein.
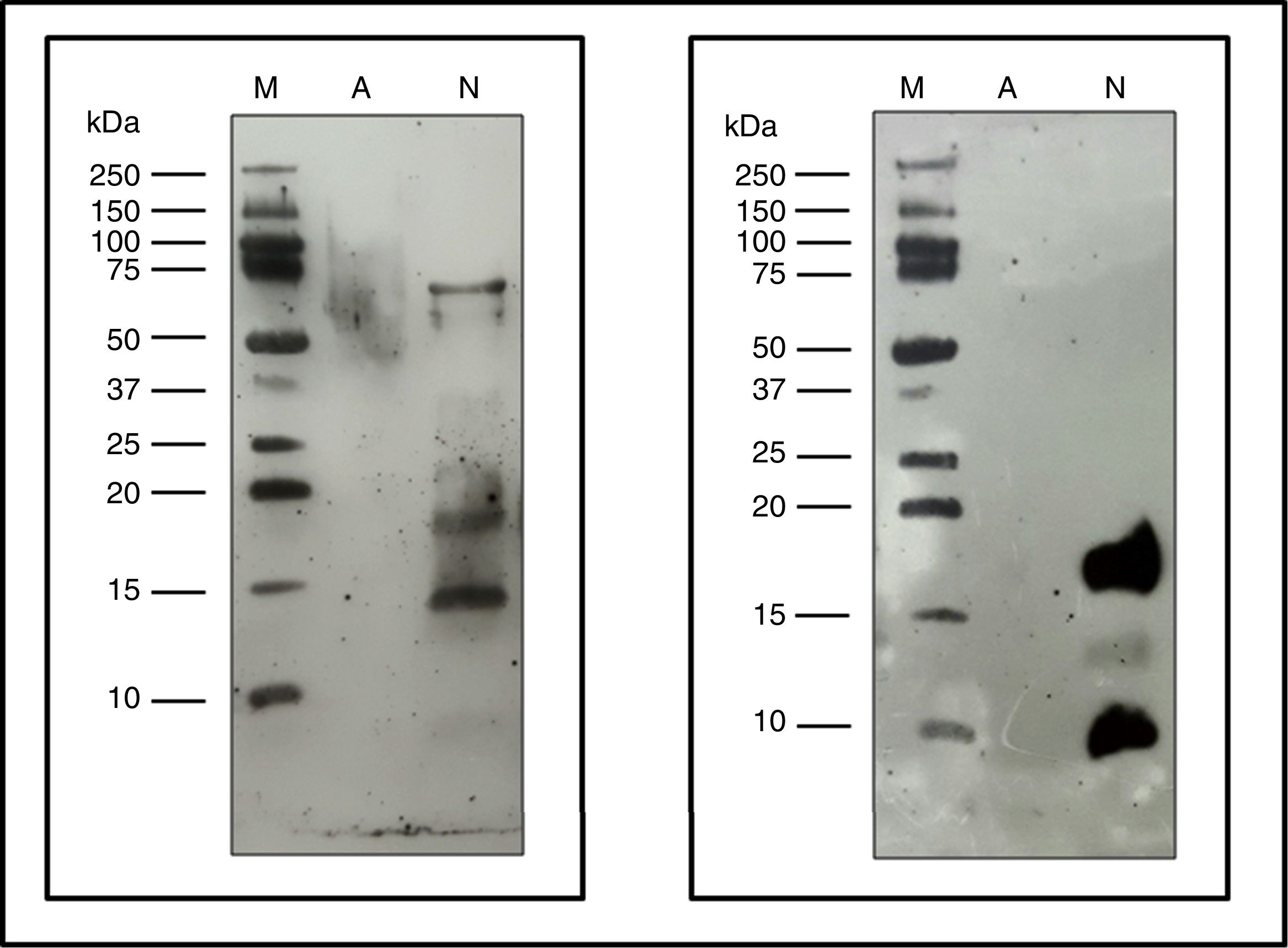
Allergenic profileImmunoblot: Proteins from NCD, ACD and a native Fel d 1 protein (Indoor Biotech) were separated by SDS-PAGE as described above and these proteins were transferred in duplicate to 0.2μm nitrocellulose membranes (Bio-Rad).
One membrane was incubated with p56 as the primary antibody and using the anti-human IgE (A9667, Sigma-Aldrich) as a secondary antibody. The other membrane was incubated using as primary antibody α-Fel d 1 6F9/3E4 (Indoor biotech), and as a secondary antibody anti-mouse IgG (whole molecule)-Peroxidase antibody produced in rabbits (A9044, Sigma-Aldrich). Both membranes were developed using the Clarity™ Western ECL Substrate (Bio-Rad) following the manufacturer's instructions.
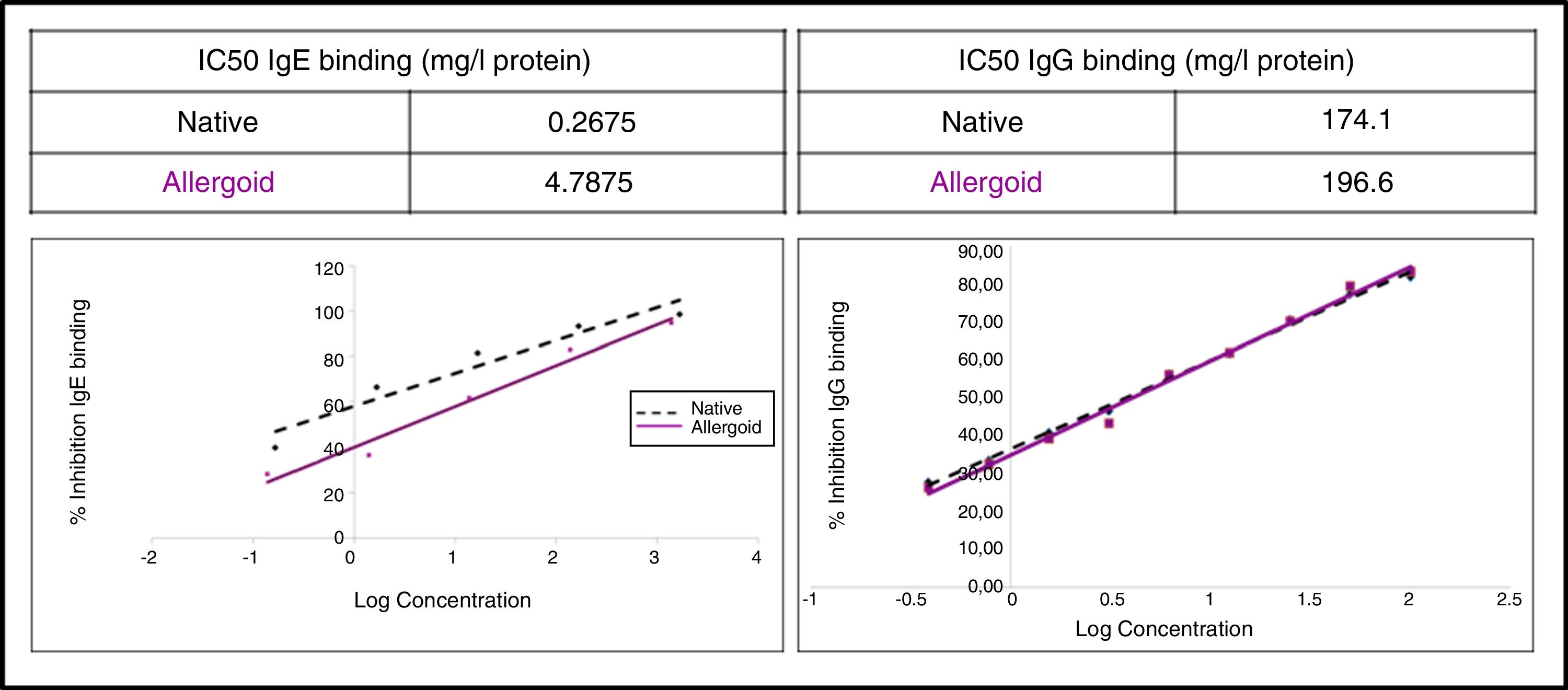
IgG competition ELISA: Performed in multiwell Maxisorp™ immunoplate (Thermo Fisher Scientific), using as antigen an in-house reference native extract and the ACD and NCD as inhibitors. As primary antibody p56 was used, and as a secondary antibody anti-human IgG (A8667, Sigma-Aldrich). For reading at 450nm in spectrophotometer Multiskan™ (Thermo Fisher Scientific), SIGMAFAST™ OPD peroxidase substrate (Sigma-Aldrich) was used, in accordance with the manufacturer's instructions. The half maximal inhibitory concentration (IC50) was calculated, and indicated the ability to inhibit 50% of IgG-binding between the antigen and specific IgG from p56.
IgE CAP inhibition: Performed in Phadia® 100 UNICAP (Thermo Fisher Scientific), where the IgE-binding capacity of the ACD and NCD was assessed.
ELISA sandwich: The quantification of the major allergen Fel d 1 in the NCD using the commercial ELISA kit Fel d 1 (6F9/3E4, Indoor biotech) was performed. The Fel d 1 concentration in the ACD was extrapolated based on NCD determination.
Degree of polymerizationQuantification of amino acid lysine: The amount of lysine, involved in the polymerization process, was calculated in ACD to compare it with NCD. The proteins of both extracts were hydrolyzed with 6M HCl (150°C; 4h). Then the hydrolyzed proteins were derivatized with dabsyl chloride and chromatographed by RP-HPLC in accordance with what is described in amino acids analysis (method 6, United States Pharmacopeia USP-29).20
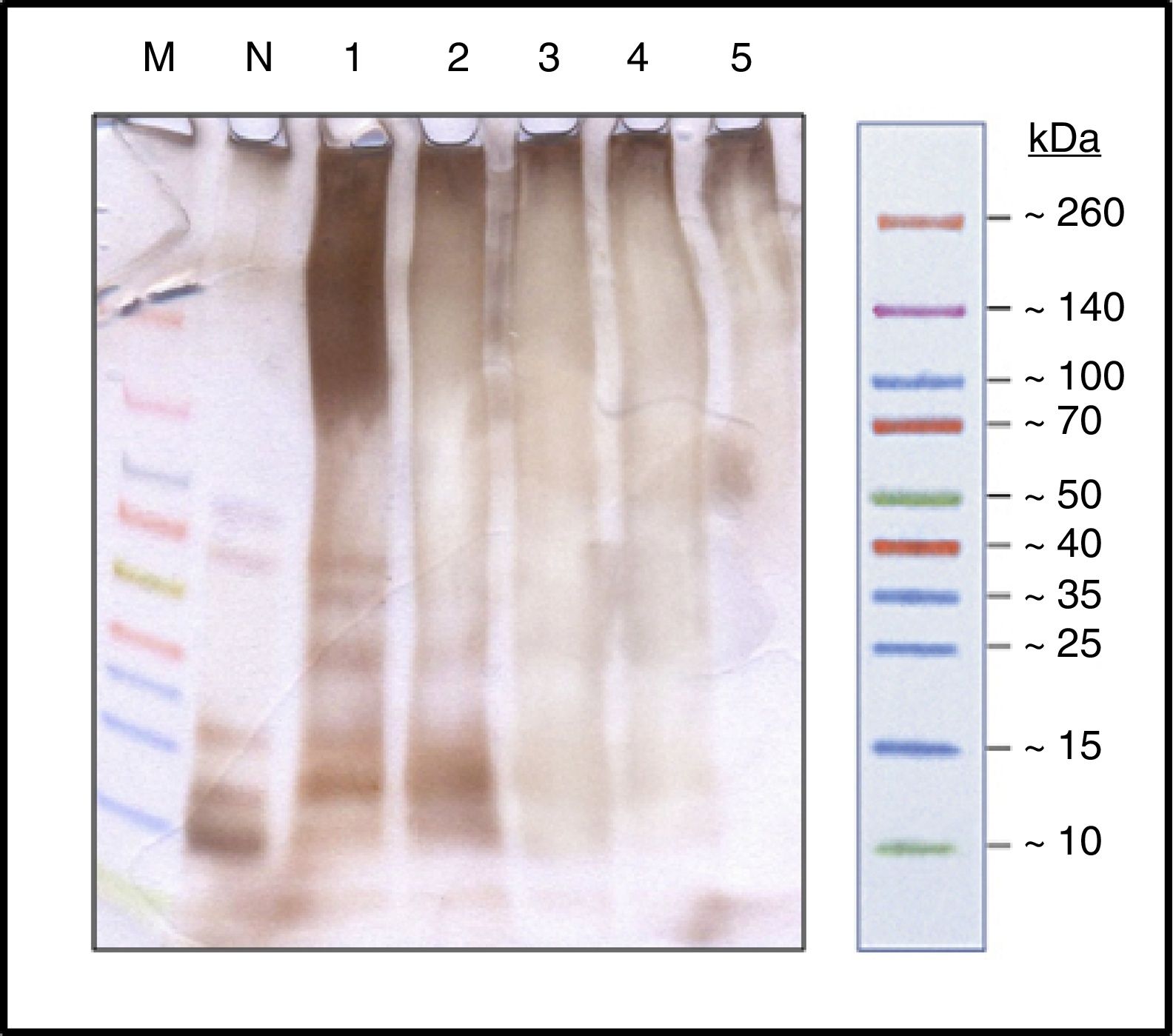
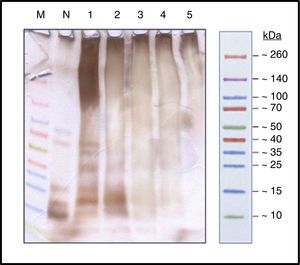
ResultsProcess controlProtein profileBy SDS-PAGE in the samples at different times (Fig. 1) it has shown the disappearance of bands, during the polymerization process, corresponding to proteins of low molecular weight, belonging to the different allergens of NCD that resulted in a single upper band of high molecular weight (>100kDa) which constituted the new ACD. It is also noted that the process of protein aggregate formation of high molecular weight was stabilized after the four hours.
SDS-PAGE silver staining (running buffer tricine 100mM). Spectra™ Multicolor Broad Range Protein Ladder (M); Protein solution with the native cat dander extract (N); Polymerized protein solution of cat dander: T-0h, lane 1; T-1h, lane 2; T-2h, lane 3; T-3h, lane 4 and T-4h, lane 5.
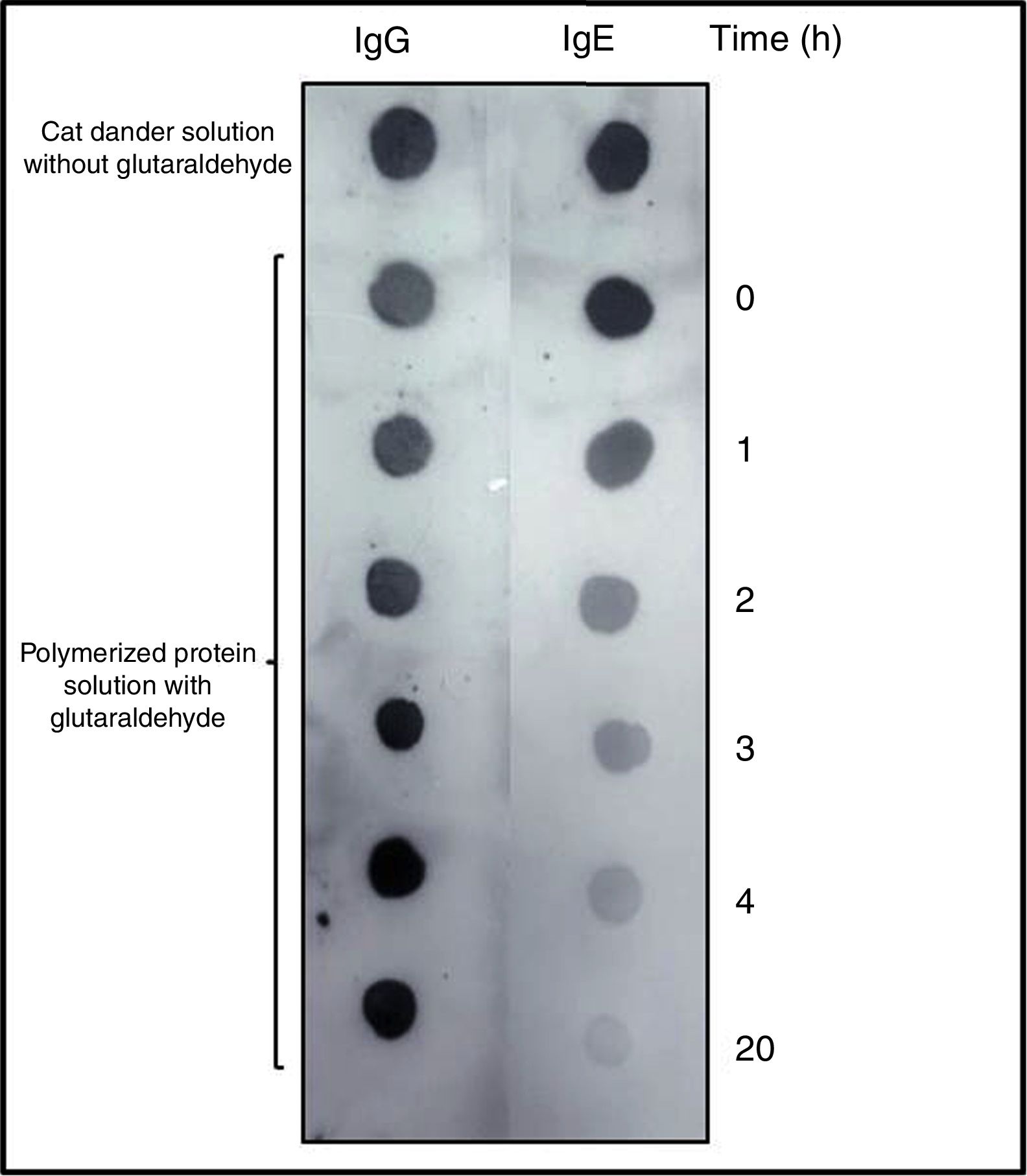
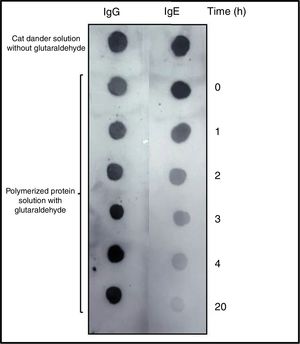
DOT-BLOT confirmed that the process of formation of a protein aggregate was proportional to the loss of the IgE-binding capacity of p56 by this new allergoid in development. It was observed that the IgG-binding capacity was maintained throughout the process (Fig. 2). The allergenicity decreased during the modification process with glutaraldehyde, maintaining the IgG-binding capacity.
DOT-BLOT with samples of the protein solution at different times of the polymerization process on nitrocellulose membrane performed with a pool of sera from patients sensitized to Felis domesticus (p56) as primary antibody. As secondary antibody Left: anti-Human IgG. Right: anti-Human IgE.
The slopes of the regression lines obtained with NCD and the polymerized solution were compared. With this technique the degree of polymerization obtained was 76%.
To assess the purity of the polymerized extract a quantification of glycine by RP-HPLC contained in the polymerized solution before and after the diafiltration process was carried out, showing a removal of 83.77% in this process, from an initial concentration solution of 35.72mM to a final 5.91mM.
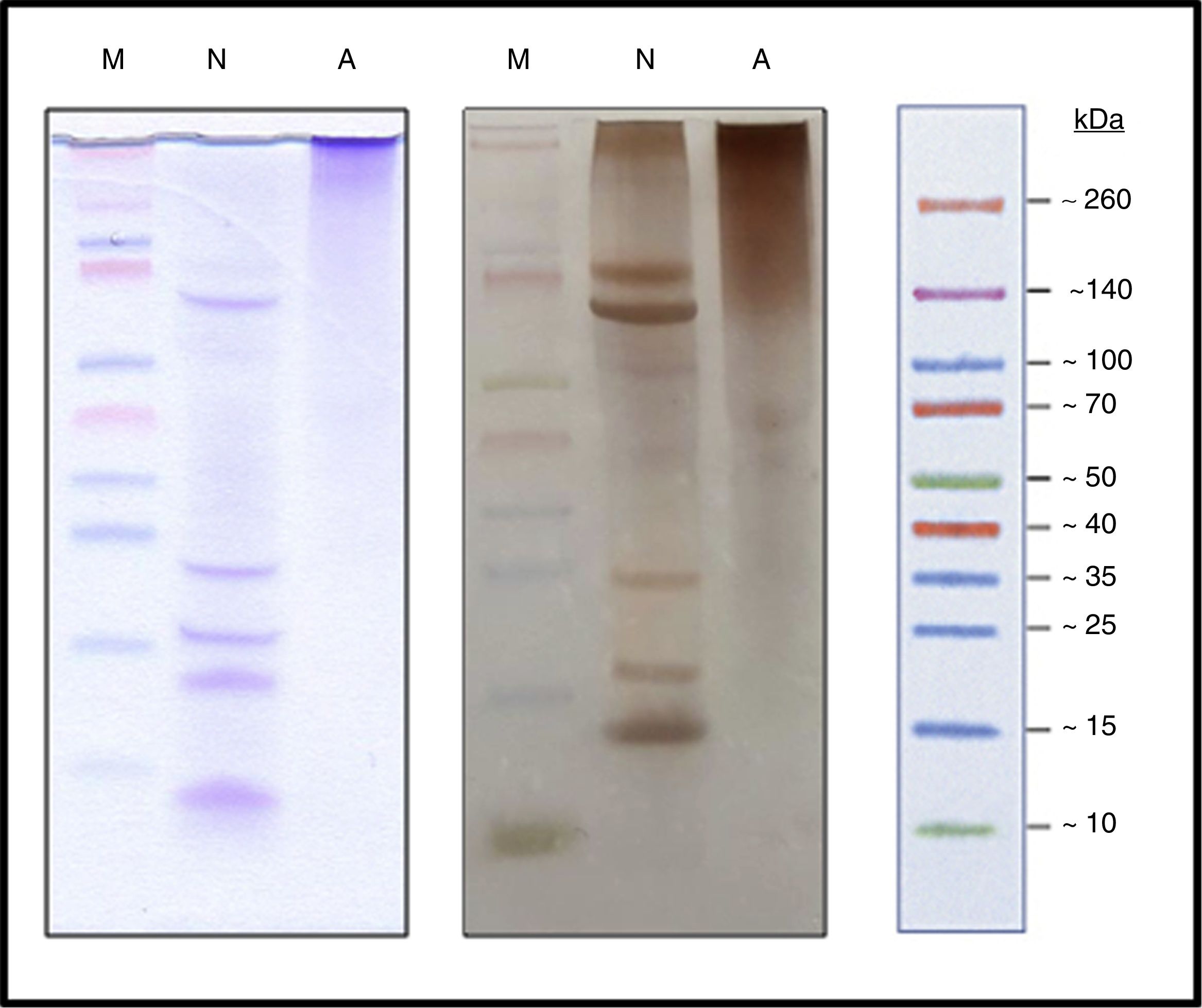
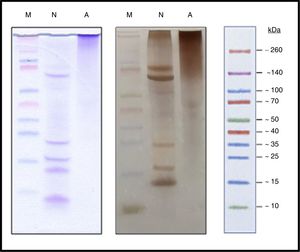
Characterization of the new allergoidProtein profileThe lyophilized ACD showed a protein concentration of 182.28μg/mg determined by AEN. It confirmed the presence of high molecular weight proteins. Using SDS-PAGE a protein profile showed a single protein band with a molecular weight above 100kDa was obtained. The NCD showed several bands of lower molecular weights such as those belonging to the two chains of Fel d 1, which is a heterodimer consisting of two non-covalent bonds to form a tetramer with apparent molecular weight of between 35 and 39kDa under native conditions, but under reduced conditions it shows each dimer separately, in turn divided into two polypeptide chains, chain 1 (4kDa) and chain 2 (14kDa). Also it showed a set of bands whose size may correspond to different allergens from cat (Fig. 3), such as Fel d 2 (69kDa), Fel d 3 (11kDa), Fel d 4 (22kDa), Fel d 7 (17.5kDa) and Fel d 8 (24kDa).
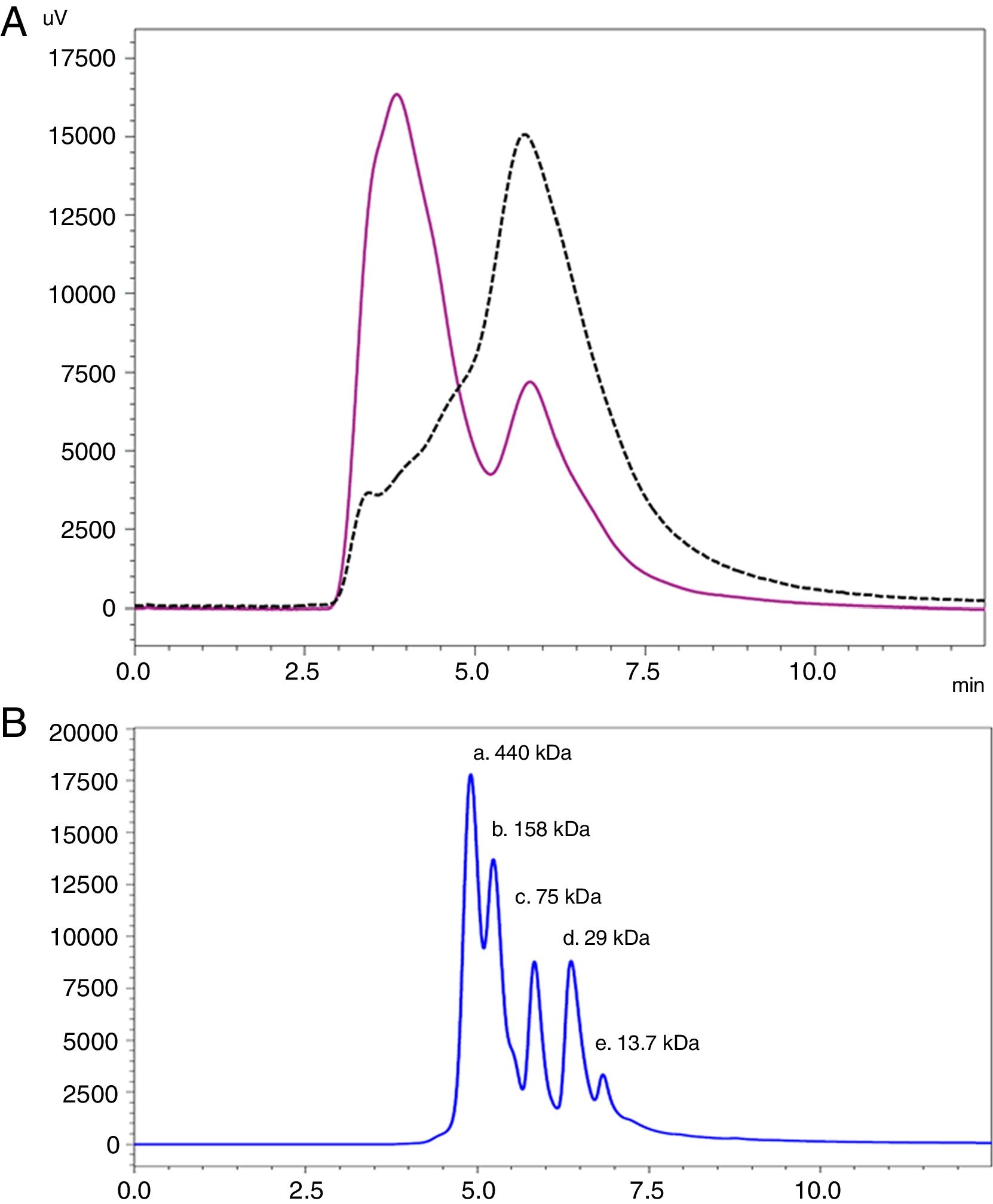
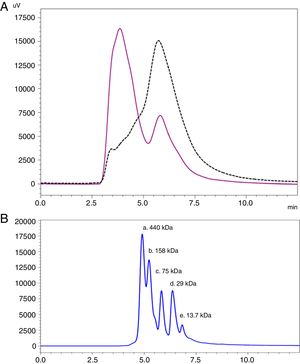
Analysis by SEC showed a shift in the proteinogram in ACD compared to NCD, due to the shorter retention time of higher molecular weight proteins. Furthermore, the percentage area under the curve of the proteins above 100kDa in ACD was 78.72% (Fig. 4).
SEC proteinogram detected at 280nm and elution time in minutes. (A) Data comparison with native cat dander extract (black discontinuous line) versus allergoid cat dander extract (purple continuous line). (B) Calibration Kit proteins profile (GE Healthcare) (a: Ferritin 440kDa; b: Aldolase 158kDa; c: Conalbumin 75kDa; d: Carbonic Anhydrase 29kDa; e: Ribonuclease A 13.7kDa).
Analysis by peptide footprint of the ACD showed the presence of the major allergen Fel d 1 (GI 38492847) with a coverage sequence of 37%, and Fel d 7 (GI 325652162) with a coverage sequence of 16%. The analysis performed in the NCD showed the presence of four allergens of Felis domesticus, where coverage sequence was 45% to Fel d 1 (GI 38492847), 18% to Fel d 2 (GI 1351908), 27% to Fel d 4 (GI 45775300) and 10% to Fel d 7 (GI 325652162).
Degree of polymerizationThe analysis by RP-HPLC showed a reduction of the concentration of the amino acid lysine in the ACD when compared to the NCD, resulting in 91.69%. The considerable reduction of this amino acid was due to the modification of lysine residues involved in the polymerization process using glutaraldehyde.
Allergenic profileThe content of the major allergen Fel d 1 in ACD was 11.90μg/mg. This data was obtained by extrapolation, relating the quantification of Fel d 1 in the NCD by ELISA sandwich (8.30μg/mg) and the amount of protein contained in ACD and NCD by AEN.
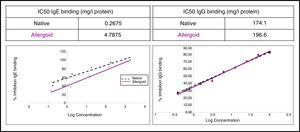
The study of the allergenic profile showed a loss of allergenicity of ACD compared to NCD, while its IgG-binding capacity was maintained. The assays developed by IgE CAP inhibition showed IC50 displacement by ACD (4.79mg/l protein) in comparison with the NCD (0.27mg/l protein) (Fig. 5). The ACD needed a higher concentration (almost 18 times) than NCD to generate an inhibition of 50% of IgE-binding using p56. With the competitive ELISA assays of IgG, no significant shift of the IC50 by the ACD was observed compared with the NCD (Fig. 5).
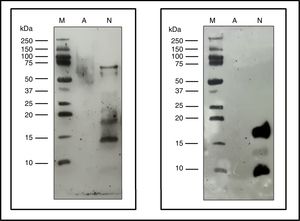
The immunoblot assay with p56 showed the absence of IgE-binding by the proteins from ACD, while the NCD showed bands corresponding to allergens that react with the IgE of p56 (Fig. 6). In the case of the membrane incubated with α-Fel d 1, no reaction was observed by the ACD while the NCD showed a very intense band corresponding to chain 1 of Fel d 1 (4kDa), a lower intensity band corresponding to chain 2 of Fel d 1 (14kDa) and another very intense band of 19kDa corresponding to the dimer formed by the union of both chains of Fel d 1 (Fig. 6).
Immunoblotting assays. Precision Plus Protein™ Western C™ (M), Native cat dander extract (N), Allergoid cat dander extract (A). 50μg extract/lane. Left: pool of sera from patients sensitized to Felis domesticus (p56) as a primary antibody. Right: monoclonal antibody α- Fel d 1 6F9 as a primary antibody.
In the search for alternative treatment with immunotherapy for Felis domesticus allergy with safer strategies, this new ACD was developed. This type of allergy, whose main associated symptoms are asthma, rhinoconjunctivitis and atopic dermatitis,21 shows a good prognosis with standard treatment with native allergen extract vaccine, inoculated in increasing concentration for at least three years. However, immunological analyzes in a study evaluating patients five years after stopping treatment with SIT indicate a return of the disease.22 This disease return indicates that a sufficient amount of allergen extract to ensure its effectiveness is needed,23 but the increase of the concentration of allergens in the extract can cause a high significant percentage of adverse reactions.24 Working in this line, it was considered that the development of a polymerized extract by modification with glutaraldehyde, in widespread use for other allergens such as pollens and mites, might be an adequate tool. In the case of allergenic animal sources such an extract does not yet exist. For cat allergy, in addition to conventional treatment, experimental treatments with another alternatives such as treatments with peptides from the major allergen Fel d 1 have been tested with good results but accompanied by adverse reactions.25
The safety indicators used to analyze the new ACD showed a high degree of safety. The protein profile by SDS-PAGE showed the formation of large molecular aggregates; moreover, the absence of low molecular weight proteins within the sizes where most allergenic proteins are described was observed. The protein profile obtained by SEC shows significant differences between the ACD and NCD, due to the higher molecular weight proteins of the former. A chromatographic assay by SEC with a broad range of molecular weights would more accurately define the molecular size of the protein aggregate. The conformational change produced led to reduced allergenicity with a decreased capacity of allergoid to bind specific IgE of Felis domesticus. The capacity to bind specific IgG of Felis domesticus remained intact as it was demonstrated in the process control analysis by DOT-BLOT and then by ELISA inhibition, where the IgG-binding capacity obtained similar results for the native extract and polymerized extract. These data show the optimum development of the ACD regarding the size of its molecular aggregates, as it appears that the molecular size is an important parameter for the maintenance of immunogenicity.26
When the biological potency was compared, the ACD showed a lower ability to inhibit specific IgE of Felis domesticus compared to NCD, indicating an optimal polymerization process, and the accuracy thereof was analyzed by controlling the high degree of polymerization in accordance with the indications of the guidelines of the European Medicines Agency (EMEA/CHMP/BWP/304831/2007) by assessing the percentage reduction of the amino acid lysine after the process of chemical modification.
The analysis by peptide footprint of ACD evidenced only the presence of Fel d 1 and Fel d 7 allergens, although this does not necessarily imply nonexistence of the other relevant allergens in the ACD because these modified extracts have less proteolytic cleavage than natives, due to the polymerization process itself. Proteolytic cleavage is performed by C-terminal ends of several basic amino acids, including the amino acid lysine, but this amino acid is involved in the polymerization process; lysine is responsible for the glutaraldehyde union in allergoids, which means that these chemical modifications of allergoids prevents them from being digested. In the peptide footprint analysis with a less restrictive score and SPI, all cat allergens described in allergen database (WHO/IUIS Allergen Nomenclature Sub-committee) in both ACD and NCD were detected.
Considering these results as preliminary study, carrying out further in vivo studies is suggested, in which both safety and efficacy will be evaluated. A good starting point would be the development of a safety test in vivo to study skin response to the allergoid, as the first step determined by the level of reaction produced by the ACD against NCD, using wheal diameter reduction as indicative. Subsequently, in a second step the security of the SIT with this allergoid would be established by evaluating the clinical tolerance.
The results obtained with the characterization of the new polymerized extract of cat dander have demonstrated a good safety profile of the product, which added to the extensive literature concerning the optimal use of these chemically modified allergen extracts as hyposensitization agents in SIT, show this allergoid to be a potentially good alternative treatment for a cat allergy without ignoring the minor allergens and minimizing adverse reactions.
FundingThe assays described in this article funded by Probelte Pharma S.L.U., were developed in its laboratories.
Authors’ contributionsMP coordinated and supervised all the assays developed in the study. JPS developed the allergoid. JPS and AC made biochemical and immunological characterizations, YP made the footprint and quantification of major allergen. JPS and YP wrote the manuscript. JPS, YP, AC and MP approved and agreed the manuscript to publish.
Conflict of interestAll authors are employees of Probelte Pharma S.L.U. JPS is a student of the doctoral school of the National University of Distance Education (UNED) of Spain in the department of pharmaceutical chemistry. All authors declare no competing financial interests.
The authors would like to thank Dr. Benito Rodríguez Dominguez from the area of Allergology of the Hospital Virgen de Altagracia de Manzanares (Ciudad Real, Spain) for his collaboration in the study. We thank Dr. José Luis Martínez Guitarte from the Faculty of Chemistry of the National Distance Education University (UNED) of Spain for his teaching support.
This document has been revised by Ann Hannigan-Breen, BA (UCD); HDipEd; Member of CIOL (Chartered Institute of Linguists) UK.