The fast pyrolysis bio-oil is a promising product to be used as fuel. It is produced by means of wood particles subjected to high heat rates for very little time. However, the resulting oil has a very high oxygen content, which to be used as fuel requires upgrading processes, which makes its costs higher than mineral oil. The aim of this paper is to study the fast pyrolysis bio-oil as a precursor of carbon fibers. A heat treatment in the bio-oil was used until the optimum softening point temperature was reached for the subsequent melt spinning process. Additives such as kraft lignin and furfuryl alcohol improved the spinning process and caused the fibers to resist the carbonization step. Characterization by Fourier-transformed infrared, 13C VACP-MAS NRM and Raman revealed that the heat-treated bio-oil presented a great amount of oxygenated groups and that mainly the aromatization of the samples was verified after the carbonization process. SEM images showed that the fibers diameters were too high.
Fast pyrolysis is a thermochemical process that decomposes biomass very quickly in the absence of oxygen into charcoal, gaseous products and bio-oil. Bio-oil is the main product by fast pyrolysis process, in which residence time of vapors is short (seconds), and temperature is around 500°C. Fast pyrolysis also characterizes by its fast heating rate (up to 100°C/s) [1–3].
Bio-oil is a promising feedstock for replacement of petroleum fuels to use in heating and energy, and besides it could be used to produce value-added chemicals [4].
However, the technologies developed until now need much more improvement in order the produced bio-oil could be a drop-in biofuel. For instance, properties of fast pyrolysis bio-oil such as high viscosity, thermal instability, corrosiveness, high oxygen content are undesirable to the substitution of fossil fuels. Mostly, high oxygen content results in a fuel with low heating value [1,5]. Thereby, several upgrading process are required for bio-oil to be useful as fuel.
Applications of bio-oil as biofuel could be part of the green chemistry worldwide effort; however, it is not yet profitable besides using renewable cheap biomass. The reason for this is mainly that fuel is a commodity that must have low price in the market to keep global economy running.
Therefore, our research group is looking for the development of other products with higher value than fuel originated from fast pyrolysis process in order to make it economically viable through concepts of biorefinery. As example, we can cite the use of bio-oil as wood preservative [6], biocides from aqueous pyroligneous acid extract (ongoing research), and activated carbon and mineral fertilizers from charcoal (biochar) [7].
Herein, we describe as a proof of concept that bio-oil from fast pyrolysis can be used as precursor for carbon fiber. The main feedstock for carbon fiber are polyacrylonitrile (PAN) and mesophase pitch, which consists of common tar mixed with residual coal tar. Both of them are raw materials of fossil origin and possess high processing costs [8].
During charcoal production by slow wood pyrolysis, gases are generated which can be condensed to produce an oily liquid called wood tar. Considering the potential of carbon content contained in this liquid, researchers have developed carbon fiber from this tar [9,10].
Qin and Kadla [11] separated pyrolytic lignin-cold-water insoluble from the bio-oil – to produce carbon fiber.
Kraft lignin has been studied for some researchers as carbon fibers precursor, for being low costs and renewable resources [12–16]. Kraft lignin from hardwood and softwood blended with other polymers, such as polyethylene, polypropylene and terephthalate oxide were studied as a precursor for carbon fibers [12,13]. Maradur et al. [15] incorporated commercial hardwood lignin into PAN to produce carbon fibers.
For the best of our knowledge, there is nothing reported about the use of bio-oil from fast pyrolysis as precursor for carbon fiber. Here we show how to prepare carbon fiber using bio-oil from fast pyrolysis as the main raw material, and, furthermore, the importance of incorporating additives such as kraft lignin and furfuryl alcohol to be able to resist carbonization. Besides, we show that stabilization (oxidation) step is crucial for the mechanical resistance of the final product, although the high content of oxygen in the pristine bio-oil.
2Material and methodsBio-oil was obtained from a pilot-scale fast pyrolysis plant (Bioware, Brazil), which pyrolizes the biomass in a fluidized bed. The reaction temperature was 550°C with gas residence time of 5s. Eucalypt wood fines rejected from a chipping line were used as raw material.
Kraft lignin was provided by Suzano Pulp and Paper Industry (São Paulo, SP, Brazil) and furfuryl alcohol (98%) was purchased from Sigma-Aldrich.
2.1Elemental analysis, ash content and softening pointCarbon, hydrogen, nitrogen, sulfur and oxygen (by mass difference) contents were determined in a CHNS Elemental Analyzer from Vario Macro Cube. The bio-oil ash content was determined following the ASTM D482-13 procedure. In brief, a gram of bio-oil was weighed and subjected to burning at 800°C in a muffle. The ash content was obtained by mass difference.
The softening point values were determined using the ring-and-ball method, following the ABNT-NBR 6560 standard. Briefly, the samples were shaped in a standardized ring with a metal sphere above. The ring and sphere were positioned in an apparatus immersed in glycerin and heated. The softening temperature was recorded when the sample touches a reference plate after traveling 25.4mm. Analysis was done, in duplicate. The analysis was repeated if the measurements resulted in a difference larger than 1°C.
2.2SEM imagesThe as-spun fibers, after stabilization and after carbonization were observed in their morphology in a scanning electron microscope of the brand Shimadzu, model SS 500. The samples were prepared placing them on a carbon tape in the sample holder. Afterwards, a sample of thin layer of gold was deposited.
2.3FTIR, 13C NMR and Raman spectroscopyFourier transform infrared spectroscopy (FTIR) was performed in a Shimadzu IR Prestige 21, in the direct transmittance mode, resolution of 4cm−1 and frequency of 400–4000cm−1. FTIR spectra were recorded from potassium bromide (KBr) pellets with 1% content of samples.
Solid state 13C NMR experiments were performed on a Bruker 400MHz. Variable Amplitude Cross-Polarization Magic Angle Spinning (VACP-MAS) pulse sequence was implemented using a standard MAS probe of 4mm at room temperature. Bio-oil samples were placed on a Kel-F rotor and they were spun at 12kHz. The cross-polarization pulse sequence was accomplished value of 15.92ms, during this time a SPINAL-64 pulse sequence was performed for decoupling process between Hydrogen nuclei and Carbon nuclei. A recycle time delay was 0.5s and the mean number of scans were 64,000. The Raman spectra were measured using a SENTERRA Raman Spectrometer (Bruker) by the dispersive photon technique (Stokes mode). A laser excitation wavelength of 532nm was applied, incident power of 0.2mW. A spectral range of 40–4400cm−1 was recorded.
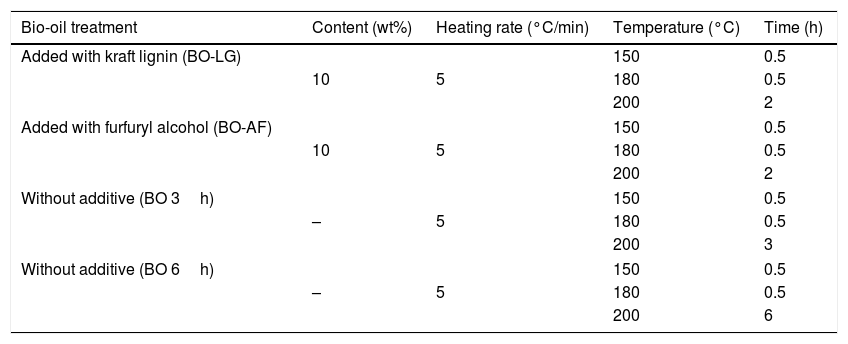
2.4Thermal treatmentsThe heat treatment of bio-oil was conducted in a stainless steel reactor electrically heated. The temperature in the bio-oil and in the wall of the reactor was controlled by thermocouples. The maximum heat treatment temperatures were 150, 180 and 200°C, and the treatment times were of 0.5, 2, 3 and 6h (Table 1). Kraft lignin or furfuryl alcohol was added to the bio-oil in a proportion of 10wt% (Table 1). The volatiles are extracted during the heat treatment. In this way, the products of higher molecular weight remain in the tar besides some oligomerization may occur. At the end of the heat treatment, the tar with or without additives was subjected to softening point analysis.
Heat treatment to remove part of the volatiles from the bio-oil with and without additives.
| Bio-oil treatment | Content (wt%) | Heating rate (°C/min) | Temperature (°C) | Time (h) |
|---|---|---|---|---|
| Added with kraft lignin (BO-LG) | 150 | 0.5 | ||
| 10 | 5 | 180 | 0.5 | |
| 200 | 2 | |||
| Added with furfuryl alcohol (BO-AF) | 150 | 0.5 | ||
| 10 | 5 | 180 | 0.5 | |
| 200 | 2 | |||
| Without additive (BO 3h) | 150 | 0.5 | ||
| – | 5 | 180 | 0.5 | |
| 200 | 3 | |||
| Without additive (BO 6h) | 150 | 0.5 | ||
| – | 5 | 180 | 0.5 | |
| 200 | 6 | |||
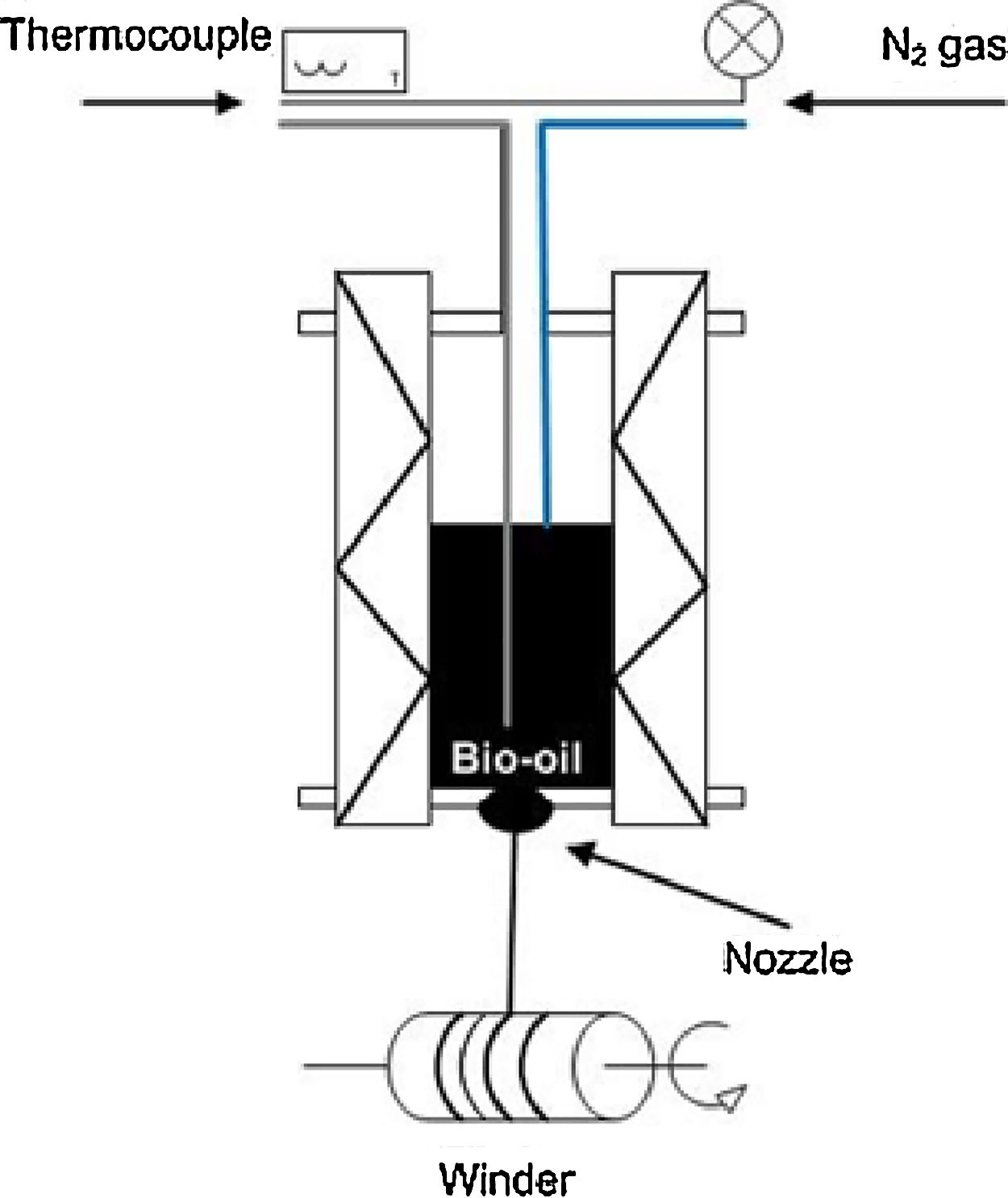
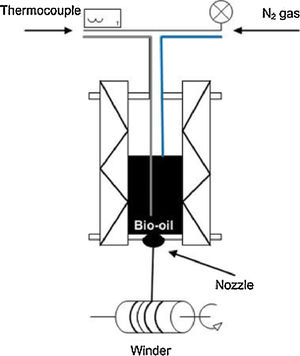
For spinning of the tar, a laboratory-scale monofilament apparatus was used (Fig. 1). The tar was extruded under nitrogen pressure ranged from 1bar to 3.5bar, through a nozzle with 0.45mm hole. The filament followed until the spin coil and rolled at different winding speeds, ranging from 200rpm to 900rpm.
2.6Stabilization and carbonizatonAfter spinning, the stabilization was conducted within a muffle of internal volume of 2100cm3. The stabilization (oxidation) process releases gases through a hole in the back of the oven, and the door remains half-open for atmospheric air entrance. The yarns were heated at a rate of 0.1°C/min to 270°C and then held at 270°C for 2h and 12h. However, BO samples without additives did not stabilized after 2h, thus they were oxidized for 12h. BO-FA samples were tested for 2h and 12h long and the oxidation on surface was visible at the first 2h. Based on this previous experience were performed for BO-LG samples just 2h of stabilization treatment. But only the BO heat treated for 3h, 6h and BO-AF were stabilized at 12h.
The thermo stabilized fibers were carbonized directly at 750°C and 1000°C and then held at 750°C and 1000°C for 1, 5, 10 and 15min. The fibers were accommodated inside a quartz glass tube and placed into the oven under nitrogen atmosphere.
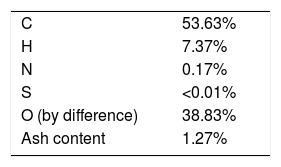
3Results and discussion3.1Characterization of the bio-oil: elemental analysis and ash contentThe fast pyrolysis bio-oil is a viscous and a dark brown liquid with relatively high oxygen content (39%), as shown in Table 2. The carbon content (54%) suggests that bio-oil from fast pyrolysis can be used as precursor of carbon fibers. The higher ash content is due to the inefficient separation process between bio char and sand – used to fast heat up the biomass – and the vapour products inside the reactor during fast pyrolysis.
3.2Heat treatment of bio-oilThe polymerization step is the transformation by heating of the liquid-consistency bio-oil into a pitch with thermoplastic characteristic. In the first hour of the heat treatment at 200°C, occurs the volatilization of the lighter compounds present in the bio-oil. In the next hour, occurs the increase of the viscosity, followed by the increment of the softening point (Table 3). Temperatures higher than 200°C cause irreversible chemical reactions in the bio-oil such as crosslinking and condensation, which transform it into a thermoset solid. In this way, the melt-spinning step is no longer possible.
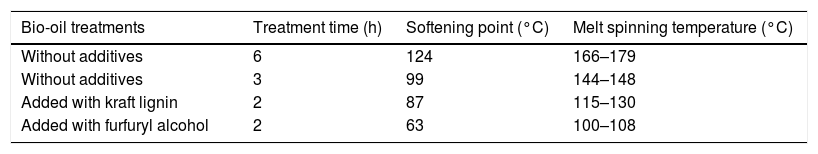
Softening point and melt-spinning temperature after heat-treatment of the samples at 200°C.
| Bio-oil treatments | Treatment time (h) | Softening point (°C) | Melt spinning temperature (°C) |
|---|---|---|---|
| Without additives | 6 | 124 | 166–179 |
| Without additives | 3 | 99 | 144–148 |
| Added with kraft lignin | 2 | 87 | 115–130 |
| Added with furfuryl alcohol | 2 | 63 | 100–108 |
The softening point increased with increasing time from 3 to 6h of heat treatment at 200°C as observed for BO without additives (Table 3). Moreover, the chemical nature of the additives also influences in the softening point being highest for lignin than for furfuryl alcohol (FA). In this case, the fact that lignin is a solid would be more influencing than the liquid FA additives despite FA is more reactive.
When softening point is too high, the bio-oil becomes more reticulated, therefore, tar does not melt again impeding spinning process. However, highest the softening point, the temperature that will be used in the stabilization step may be higher as well. This could decrease process time and costs. Therefore, an optimization should be persecuted.
The 2h of heat treatment for the bio-oil with additives (lignin and furfuryl alcohol) improved the winding of the fused wires wound correctly on spin coil with homogeneous diameter. In contrast, longer time of treatment for samples with additives cause too much crosslinking chemical reactions impeding a proper winding during melt-spinning process.
3.3Melt spinningThe spinning temperature of the yarns was 45°C to 50°C above the softening point of the tar.
A pressure of around 1bar of nitrogen was insufficient to extrude the melted pitches through the hole. At a pressure of 3.5bar, the pitches was expelled very quickly and do not wind on the coil. The pressure of 2bar was ideal for the continuous flow through the hole and resulting in correctly spun fibers.
The bio-oil treated for 6h had formed inhomogeneous filaments. In addition, they generated yarns of small malleability, quite fragile and brittle. The same had been observed for the bio-oil heat-treated for 3h.
The addition of kraft lignin and furfuryl alcohol in the bio-oil allowed the formation of filaments easier than bio-oil without additives.
The diameters of spun fibers with addition of furfuryl alcohol (BO-FA) decreases from 131μm to 107μm with the increase in winding speed from 500rpm to 700rpm. Probably because the filament is more stretched at higher torque [17]. This result suggests that the furfuryl alcohol confers plasticity character to the fiber even after heat treatment and during melt spinning.
Unexpected opposite behavior was observed for the lignin added fibers (BO-LG). The as-spun fibers BO-LG at 400rpm resulted in a fiber diameter with 70μm, increasing to 89μm with increasing fiber winding at 500rpm.
3.4Oxidative stabilizationThe stabilization treatment was necessary to prevent fiber fusing during carbonization. This process introduces oxygen into the fiber structure at temperature of 270°C in atmospheric air. Even though, there was weight loss. Prauchner et al. [10] attributed this weight loss to the release of volatile compounds and the degradation of the lateral aliphatic chains present in the chemical structure of tar.
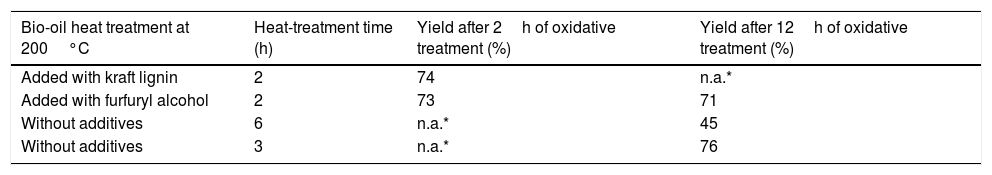
The yield after oxidative stabilization is shown in Table 4.
Yield in the oxidative stabilization at 270°C at a heating rate of 0.1°C/min (the bio-oil was polymerized at 200°C before these oxidative stabilization).
| Bio-oil heat treatment at 200°C | Heat-treatment time (h) | Yield after 2h of oxidative treatment (%) | Yield after 12h of oxidative treatment (%) |
|---|---|---|---|
| Added with kraft lignin | 2 | 74 | n.a.* |
| Added with furfuryl alcohol | 2 | 73 | 71 |
| Without additives | 6 | n.a.* | 45 |
| Without additives | 3 | n.a.* | 76 |
n.a.*: not applied.
The yield ranged from 71% to 76% for both, the raw bio-oil and the bio-oil with additives. The exception was the 6-hour treated bio-oil, which had a yield of 45% in the stabilization process with holding time of 12h.
The diameter of the fiber influences the stabilization process. Higher diameters form an outer barrier preventing diffusion of oxygen throughout the fiber.
Brodin et al. [14] observed a skin-core structure in oxidized lignin fibers. Thus, a rapid surface oxidation occurred and the formed skin prevents oxygen penetration into deeper layers.
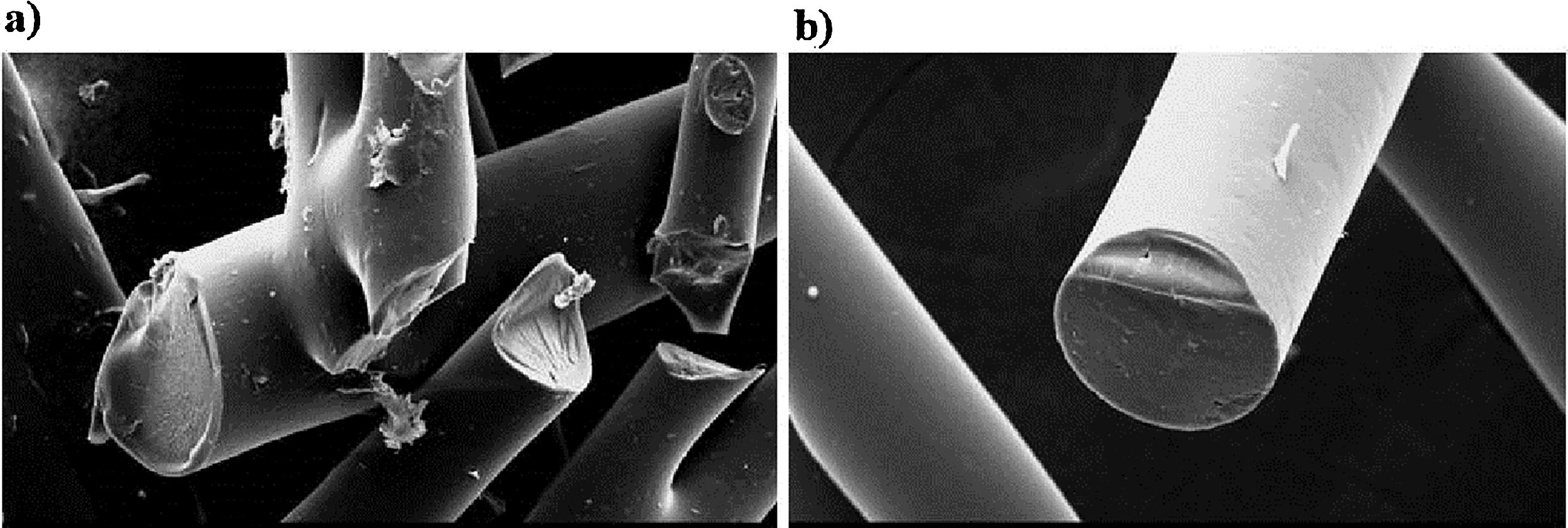
After oxidative stabilization the fibers diameter from the bio-oil additivated with furfuryl alcohol ranged from ∼57μm to ∼82μm. Fig. 2 shows SEM images of BO-AF fibers after stabilization showing that some fibers fused one to another.
The same behavior was observed for fibers additivated with kraft lignin with diameters ranging from ∼65μm to ∼81μm (figure not shown).
3.5CarbonizationCarbonization was conducted at 750°C and 1000°C under nitrogen atmosphere in order to finish the process and produce carbon fiber.
Preliminary tests were carried out to explore the residence time and temperature. The results showed that the carbonization temperature is a more significant factor than the residence time of the sample inside the oven. Generally, highest this temperature, greatest weight loss and consequently lowest yield. The spun fibers from bio-oil without additives did not carbonize at 1000°C into long carbon fiber, even after 2 and 12h of stabilization. Carbonization resulted in crumbled filaments with very low yield after volatilization of the most part of the fibers. Both additives used – furfuryl alcohol and kraft lignin – allowed the carbonization at highest temperature resulting in carbon fiber.
Nevertheless, kraft lignin additive resulted in carbonized fibers with lower yield than FA additive, probably because FA is more reactive to bio-oil than lignin producing thermoset polymers less volatile at high temperatures.
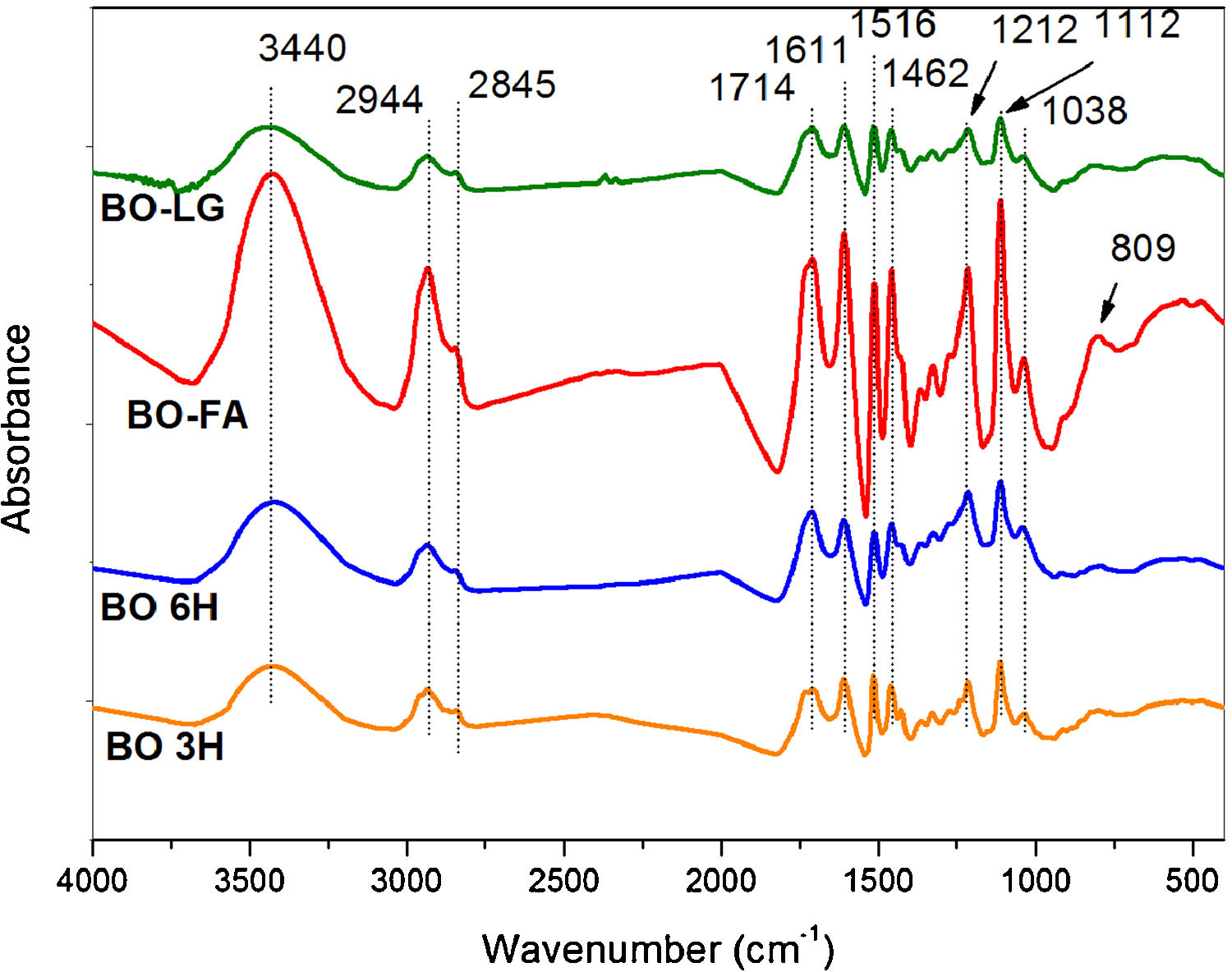
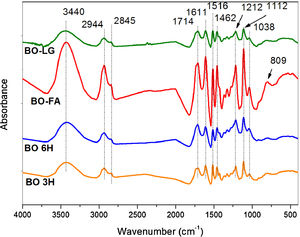
3.6Infrared and 13C NMR spectroscopyThe FTIR spectra (Fig. 3) show that all samples before carbonization have the same chemical functionalities since furfuryl alcohol in one of the components of the bio-oil and kraft lignin resulted from chemical degradation oh the lignin from eucalyptus –the origin of the bio-oil.
FTIR spectra of bio-oil after heat treatments of polymerization for 2h at 200°C of BO-LG (bio-oil additivated with kraft lignin); BO-FA (bio-oil additivated with furfuryl alcohol); BO 6H (bio-oil without additives heat treated for 6h) and BO 3H (bio-oil without additives heat treated for 3h).
The peaks of the spectra (Fig. 3) indicate the presence of phenolic compounds, carboxylic acids, sugars, aldehyde functional groups and ketones, as well as alcohols (Table 5).
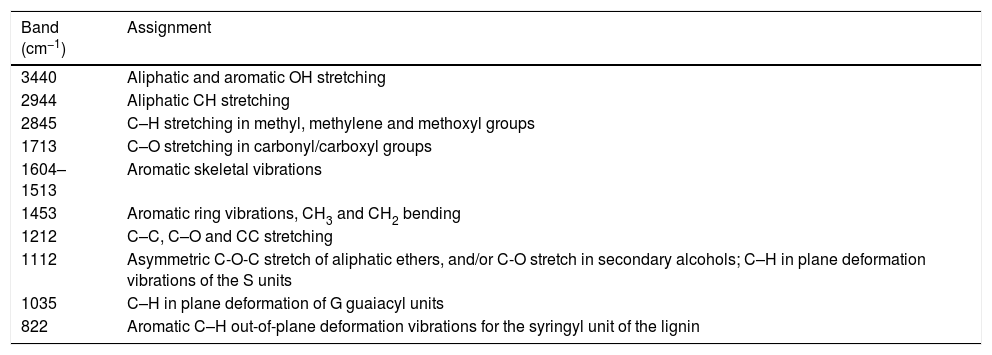
FTIR assignment correspondence [9,16,18].
| Band (cm−1) | Assignment |
|---|---|
| 3440 | Aliphatic and aromatic OH stretching |
| 2944 | Aliphatic CH stretching |
| 2845 | C–H stretching in methyl, methylene and methoxyl groups |
| 1713 | C–O stretching in carbonyl/carboxyl groups |
| 1604–1513 | Aromatic skeletal vibrations |
| 1453 | Aromatic ring vibrations, CH3 and CH2 bending |
| 1212 | C–C, C–O and CC stretching |
| 1112 | Asymmetric C-O-C stretch of aliphatic ethers, and/or C-O stretch in secondary alcohols; C–H in plane deformation vibrations of the S units |
| 1035 | C–H in plane deformation of G guaiacyl units |
| 822 | Aromatic C–H out-of-plane deformation vibrations for the syringyl unit of the lignin |
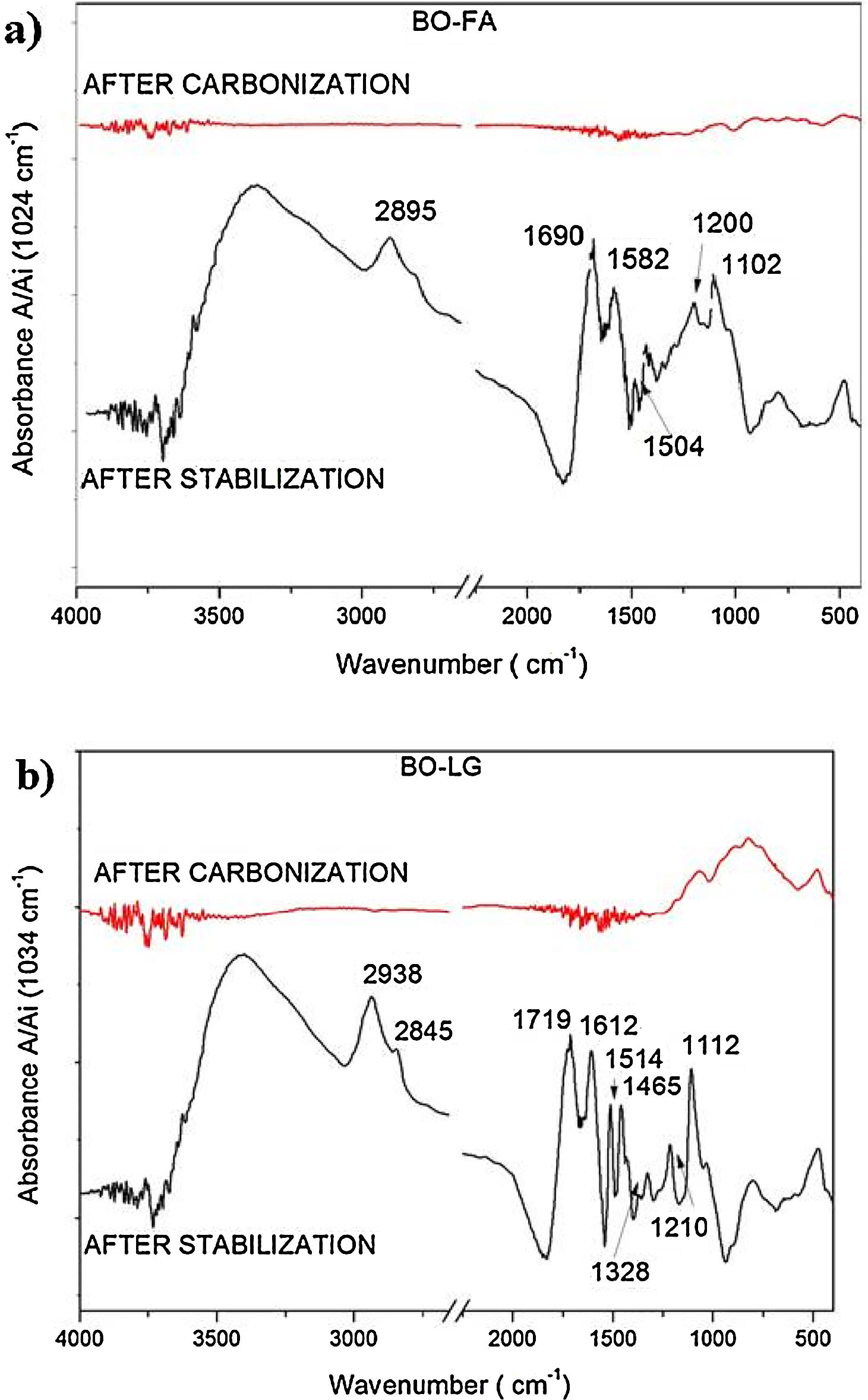
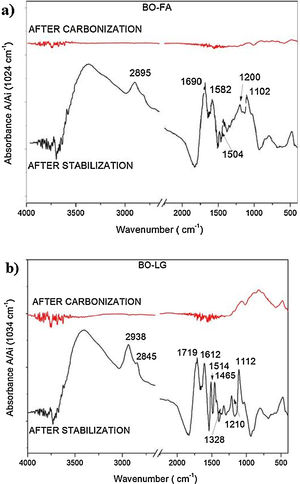
The oxidative stabilization did not significantly increase the peaks relative to oxygen moieties [Fig. 4(a) and (b) compared to Fig. 3] since the pristine bio-oil is already quite oxidized.
The spectra of carbonized samples of bio-oil added with kraft lignina and furfuryl alcohol (Fig. 4) showed a reduction of aliphatic chains. In accordance with Prauchner et al. [10] the carbonization restructure the aliphatic side chains to aromatic chains. Li et al. [19] found similar results for carbonized PAN fibers.
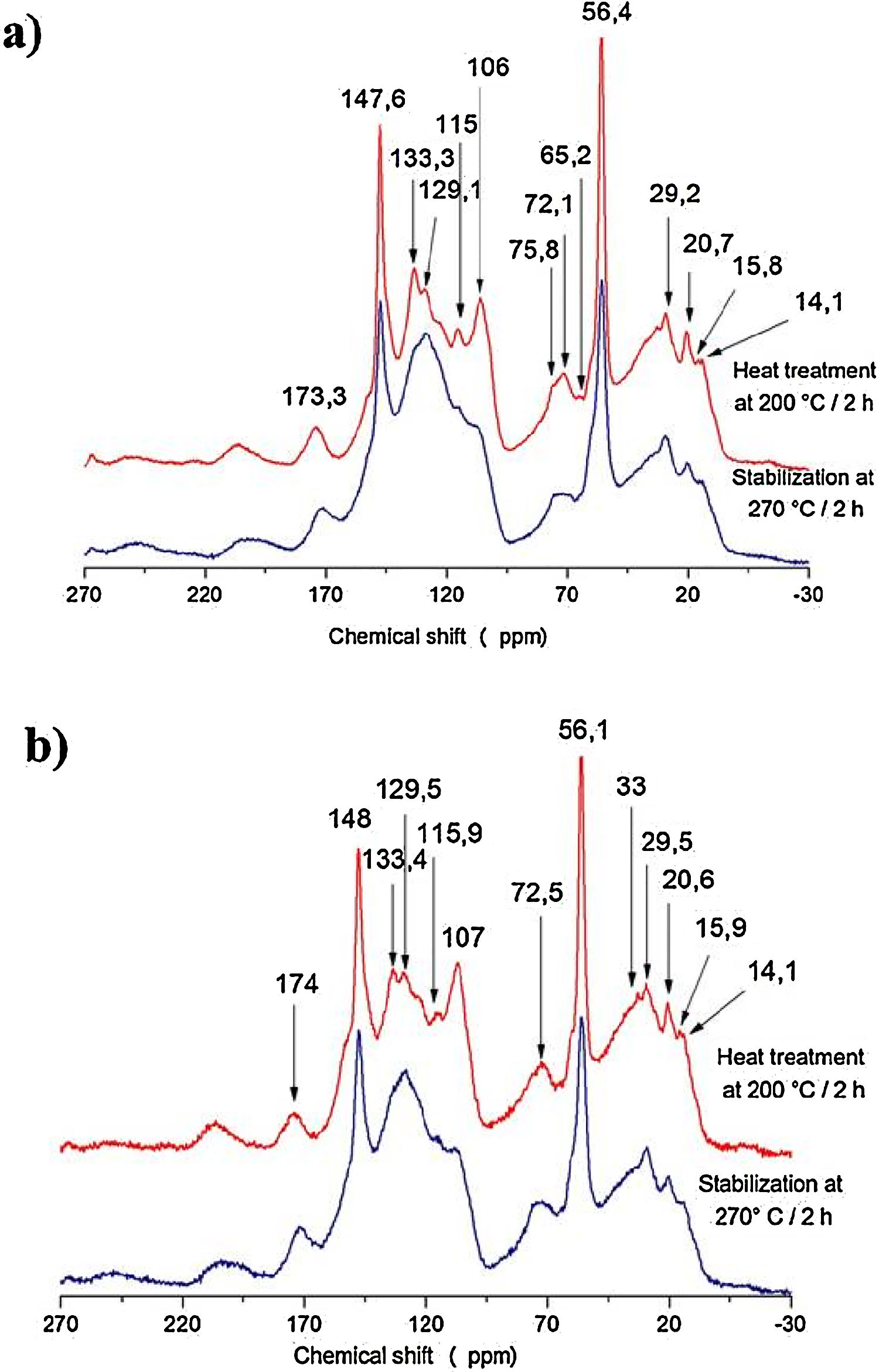
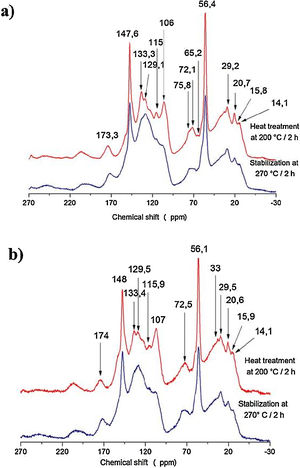
The samples of bio-oil added with kraft lignin and furfuryl alcohol (Fig. 5) heat treated and stabilized were investigated by 13C NMR spectroscopy to obtain additional chemical structural information.
A shift was observed at 174ppm (carboxyl and carbonyl groups); at 100-150ppm (aromatic units and syringyl and guaiacyl units) and a main shift at 56ppm that means the presence of methoxyl groups. Around 30–10ppm occurs methyl radicals linked groups with methylene, carbonyl (esters) and alkenes. A shift at 129ppm becomes more evident after the stabilization heat treatment for the two samples spectra [Fig. 5(a) and (b)], indicating a higher amount of aromatics in the structure as the temperature increases from 200°C to 270°C. The vanishing of the peaks at 133ppm in the two spectra after the stabilization treatment [Fig. 5(a) and (b)] indicates demethoxylation of the syringyl with consequent increasing of guaiacyl (peak 147ppm) in accordance with Melkior et al. [20].
The cellulose derivatives are completely degraded as shown by the lacking of the signals at 76ppm, 72ppm and 65.2ppm after the stabilization with heat treatment [Fig. 5(a) and (b)]. In this case, the increase of temperature restructures the aromatic rings to condensed structure.
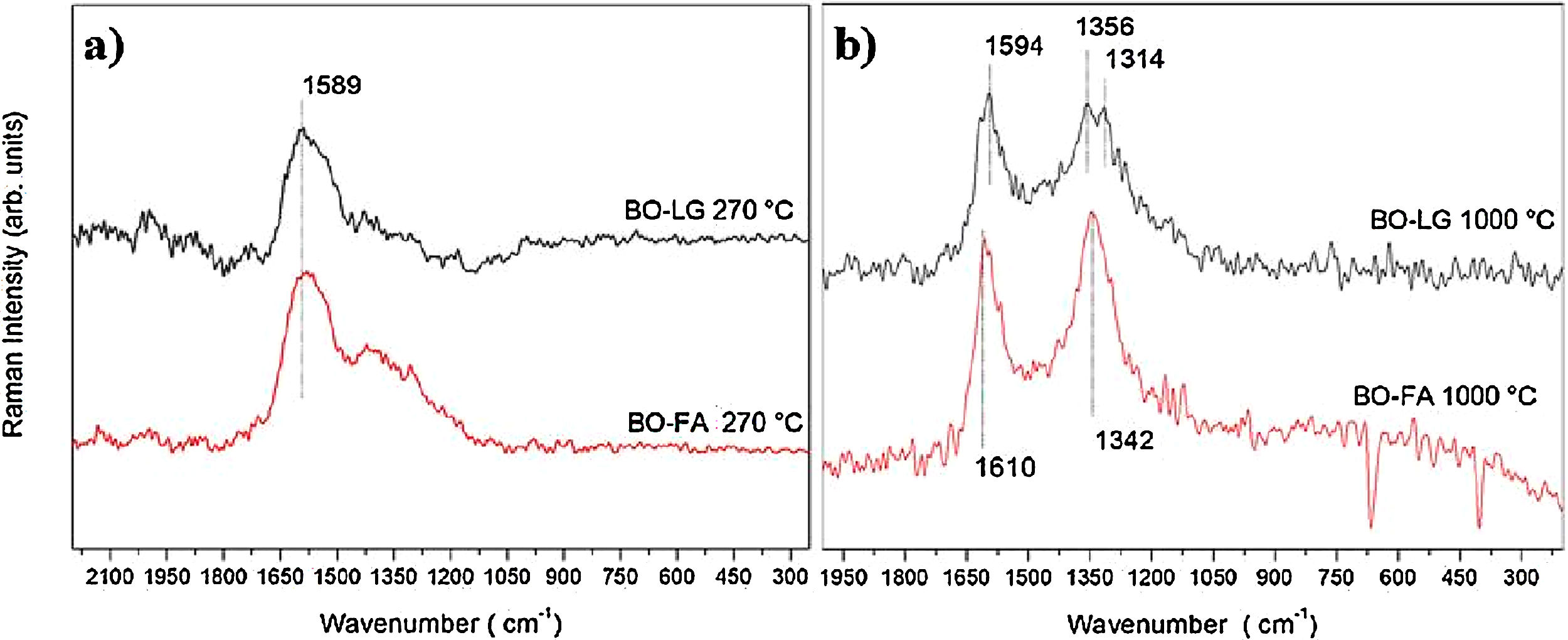
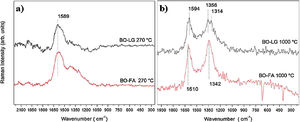
3.7Raman spectroscopyThe samples of bio-oil added with kraft lignin and furfuryl alcohol (Fig. 6) stabilized and carbonized were investigated by Raman spectroscopy to characterize crystalline or amorphous structure.
The spectra of Fig. 6(a) shows a band at 1589cm−1, that means that stabilized fibers at 270°C presented a ordered carbon structure, common behavior of graphitic materials. This band is attributed for sp2 bonds between carbon in aromatic rings, organized repetitively and within defined and limited dimensions.
When this repeatability of the aromatic chains is broken, disorder occurs in the structure, characterized by the presence of peaks around 1350cm−1.
The spectra of Fig. 6(b) of the samples carbonized at 1000°C showed two bands. One at 1594–1610cm−1, called the G-band, and another at 1342–1356cm−1, called the D-band. D band represents disorder in graphitic structures. This disorder is also attributed to condensed aromatic rings but they are distorted in amorphous carbon structures.
The Raman spectra corroborate with the FTIR and 13C VACP-MAS NMR spectra that showed condensation of the aromatic rings as the thermal treatment temperature increased.
The Raman spectra still shows that in the stabilization stage, the carbon fiber presented an ordered or graphitic structure. However, that does not mean that at 270°C the fiber actually had graphite structure. There may have been an orderly orientation of the structure of carbon atoms, oxygen and hydrogen. After carbonization, at 1000°C, this ordered orientation is undone and appears disorderly and amorphous [21–25].
4ConclusionsBio-oil from fast pyrolysis was transformed into carbon fiber by means of heat treatments. Polymerization heat treatment is required to increase the molecular weight, which occurs through condensation reactions of the phenolic compounds, removal of low molecular weight fractions, volatile compounds and water. In additional, adequate fluidity for the melt spinning is achieved.
The melt spinning were carried out resulting fibers with large diameters. A nozzle used with big diameter (0.45mm) to pull the fibers was not small enough to be compensate by the stretching due to winding speed.
The heat-treated bio-oil with additives, such as furfuryl alcohol and kraft lignin formed long yarns, while raw bio-oil only formed small broken filaments. The subsequent oxidative stabilization process was necessary to render the fibers infusible for carbonization. The bio-oil yarns with furfuryl alcohol and kraft lignin after stabilization were subsequently transformed into carbon fiber. The crude bio-oil wires did not stabilize and remained just ash after the carbonization process at 1000°C.
Characterization by FTIR, 13C VACP-MAS NRM and Raman revealed that the heat-treated bio-oil presented a great amount of oxygenated groups and that mainly the aromatization of the samples was verified after the carbonization process.
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council of Technological and Scientific Development) and Embrapa Florestas for financially supporting this work, and we also thank Complexo de Laboratórios Multiusuários e Laboratório de Sinterização da Universidade Estadual de Ponta Grossa (Complex Multiuser Laboratories and Sinter Laboratory of the State University of Ponta Grossa, Paraná, Brazil).