Thyroid dysfunction affects negatively emotional stability and worsens the clinical course of bipolar affective disorder. The main stabiliser used in this illness, lithium carbonate has numerous effects on the physiology of the thyroid, with the most significant being the inhibition of thyroid hormone release that may occur at therapeutic levels. These dysfunctions have also been reported most frequently in bipolar patients not undergoing treatment with lithium, and were not completely explained by the effects of this drug. Apart from the numerous medical complications and mood disturbances, the cognitive or perceptual system may also be affected. In fact, the presence of thyroid disease increases the rates of obsessive–compulsive disorder, phobias, panic disorder, major depressive disorder, cyclothymia, or bipolar disorder. In severe cases of hypothyroidism, the clinical symptoms and signs can be similar to a melancholic depression or dementia.
It is therefore important to know well all these possible complications in daily clinical practice. This review will cover the main thyroid dysfunctions present in bipolar patients, whether or not produced by treatment with lithium carbonate, and will provide a series of recommendations for clinical management.
Las alteraciones del funcionamiento de la glándula tiroidea influyen en la estabilidad afectiva repercutiendo negativamente en el curso clínico de la enfermedad bipolar. El principal estabilizador utilizado en este trastorno, las sales de litio, ejerce numerosos efectos sobre la fisiología del tiroides. La inhibición del recambio de la hormona tiroidea, que puede producirse con niveles terapéuticos de sales de litio, es el que tiene mayor relevancia clínica. Por otro lado, la disfunción tiroidea también parece ser más frecuente en pacientes bipolares no tratados con litio. Al margen de las numerosas complicaciones médicas y afectivas, también el sistema perceptivo o el cognitivo pueden verse afectados. De hecho, la presencia de una enfermedad tiroidea aumenta las tasas de trastorno obsesivo compulsivo, fobias, trastorno de pánico, trastorno depresivo mayor, ciclotimia o trastorno bipolar (TB). En casos de hipotiroidismo grave, la clínica puede ser semejante a una depresión melancólica o a una demencia.
Por ello, en la práctica clínica diaria, es importante conocer bien los efectos de las sales de litio sobre la función tiroidea. En esta revisión abordaremos las principales disfunciones tiroideas presentes en los pacientes bipolares, generadas o no por el tratamiento con sales de litio, y aportaremos una serie de recomendaciones para su manejo clínico.
Greater prevalence of alterations in the hypothalamus–pituitary–thyroid (HPT) axis have been found in patients with mood disorders compared to the general population.1 However, in most of the studies published an important number of patients in treatment with lithium or carbamazepine were included, which may explain the elevated prevalence found. At any rate, the association between bipolar disorder (BD) and thyroid dysfunction is not completely explained by the effects of these drugs. In a study carried out on patients with bipolar disorders not previously treated with these stabilisers, 9% of patients were found to have thyroid hypofunction, while the rate of primary hyperthyroidism in the general population reaches 3%.2
Hypothyroidism can mimic depressive symptoms and, in turn, some patients with affective disorders respond favourably to coadjuvant thyroid treatment. For that reason, some authors suggest that these patients could present anomalies in their thyroid metabolism not detected by standard tests normally used.3 In fact, up to 90% of patients with primary affective disorders have thyroid hormone levels in the eurothyroid range.4 Elevation of serum concentrations of total and free thyroxine (T4) with normal levels of triiodothyronine (T3) is the most frequent thyroid alteration found during the depressive phase of the disease, in comparison with controls and healthy subjects. The fact that more affective episodes and more serious depression are suffered during treatment with lithium has been associated in some studies with low levels of free T4 in bipolar patients.5
The mechanisms that underlie the association between thyroid pathology and affective disorders continue to be uncertain. The current hypothesis is that it could be due to the disruption of circadian rhythms or deregulation of the sensitivity of the catecholaminergic receptor associated with thyroiditis and hyperthyroidism.6
Consequently, the thyroid hormones seem to modulate the seriousness and development of depression, more than specific pathogenic role. This hypothesis is reinforced by the relationship found in some studies between thyroid function and the clinical course of BD, especially in cases of rapid cycling.7
Materials and methodsWe carried out a systematic search in the databases Pubmed, Medline and Embase (1965, April 2013). The search terms were: bipolar disorder OR Lithium, Thyroid, Hypothyroidism, Goitre and Hyperthyroidism. At the same time we performed a manual search and located additional articles using the references of the articles obtained. We obtained 578 studies that complied with the search criteria. The abstracts were reviewed and 125 were excluded for not being written in English or Spanish, or for involving other pathologies or objectives.
We have divided this article into various subsections, which appear as indicated below.
Thyroid autoimmunity and lithium saltLithium affects many aspects of cellular and humoral immunity, both in vitro and in vivo. However, controversy exists over whether it is capable of inducing thyroid autoimmunity on its own. It is known that lithium affectation of the thyroid can occur without the presence of thyroid autoimmunity, and that the prevalence of specific thyroid antibodies among patients treated with lithium varies in different studies. In fact, there are 2 important factors, age and gender, that influence the incidence of thyroid autoimmunity. This is greater in women and the range of greatest risk is middle age.8
Some studies have found high prevalence of antithyroid antibodies in patients with affective disorders that receive treatment with lithium. It seems that this salt can speed up the development of existing thyroiditis, as can be seen in the increase in the rate of circulating antibodies. Lithium is thought to be incapable of stimulating the production of de novo antibodies in humans, but it has been demonstrated that administering lithium salt can be associated with an increase in the antibody rate in patients that already had elevated antibodies at the commencement of treatment.9 This situation may imply a greater risk of developing hypothyroidism while this treatment is received. However, the study of this matter is complex, given that prevalence of antiperoxidase antibodies varies depending on the sensitivity and specificity of the measurement method used, as well as gender and age, as already commented. In addition, the interpretation of results can be affected by the fact that individuals with known thyroid disease or those at greater risk of suffering it are included more frequently in studies. Bocchetta et al. found a transient elevation of thyrotropin, or thyroid-stimulating hormone (TSH), in 116 bipolar patients in treatment with lithium followed for 2 years. They also observed that the risk of developing hypothyroidism was greater in women with elevated antithyroid antibodies and that exposition to lithium could represent an additional risk factor for these patients.10 Other studies have not found greater prevalence of antithyroid antibodies in patients with affective disorders treated with lithium as compared to the general population, normal controls or controls with other psychiatric disorders.11
It has also been hypothesised that autoimmune thyroid disease, with elevated antiperoxidase antibodies as a marker, can be a potential endophenotype for BD. Autoimmune thyroiditis is more related to genetic vulnerability to develop BD than with the process of the disease itself.
In short, it is not clear if lithium is the direct cause of the formation of thyroid antibodies. Some transversal studies found greater prevalence of antibodies in patients treated with lithium, a fact that has not been confirmed in more recent studies. Other studies demonstrated that lithium only induced a small increase in antibody titre.8 This would suggest that the pathogenesis of hypothyroidism induced by lithium might be either autoimmune or mediated by direct action of lithium on hormone secretion.
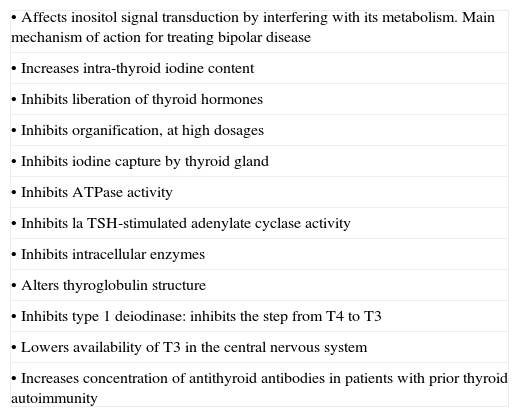
Effects of lithium on thyroid physiologyLithium salt is concentrated in the thyroid at levels 3 or 4 times greater than in plasma and its antithyroid effects are well documented (Table 1), although the mechanisms responsible for these effects are complex. Lithium salt inhibits both the cellular processes mediated by cyclic adenosine monophosphate (AMP) and the action of the enzyme inositol monophosphatase, thus blocking the inositol phosphate pathway. Although these 2 effects explain the intracellular alterations, the mechanism as a whole is not completely understood.
Effects of lithium on the hypothalamus–pituitary–thyroid axis.
| • Affects inositol signal transduction by interfering with its metabolism. Main mechanism of action for treating bipolar disease |
| • Increases intra-thyroid iodine content |
| • Inhibits liberation of thyroid hormones |
| • Inhibits organification, at high dosages |
| • Inhibits iodine capture by thyroid gland |
| • Inhibits ATPase activity |
| • Inhibits la TSH-stimulated adenylate cyclase activity |
| • Inhibits intracellular enzymes |
| • Alters thyroglobulin structure |
| • Inhibits type 1 deiodinase: inhibits the step from T4 to T3 |
| • Lowers availability of T3 in the central nervous system |
| • Increases concentration of antithyroid antibodies in patients with prior thyroid autoimmunity |
T4, thyroxine; T3, triiodothyronine.
In addition, lithium salt is implicated in the possible stabilisation of thyroid microtubules, with the consequent influence of thyroid hormone release. The salt is also implicated in the transformation of T4–T3 in the neurons and in the periphery. It alters the structure of thyroglobulin as well, and can provoke an exaggerated TSH response following stimulation with thyrotropin-releasing hormone (TRH).7
Hypothyroidism and bipolar diseaseHypothyroidism is the clinical picture produced by a decrease in the activity of the thyroid gland. Thyroid hormones (T4 and T3) mainly regulate metabolic actions and are essential for the performance of most of the functions of the organism. For that reason, hypothyroidism is characterised by an overall decrease in organic activity: metabolic, neuronal, cardiovascular, digestive and so on. When levels of thyroid hormones drop, as happens in hypothyroidism, TSH secretion increases to try to stimulate thyroid function.
Hypothyroidism can present various levels of seriousness and biochemical abnormalities. In general it is characterised by low serum concentrations of free T4 and high concentrations of TSH. Decreased plasma T4 is related with a greater number of affective episodes and major depressive symptoms.5 In subclinical hypothyroidism, the serum level of TSH is elevated (>5μU/L) but that of free T4 is normal (0.7–1.4ng/dL), and clinical symptoms are absent. Response to treatment is more favourable in these cases than in established clear hypothyroidism.
Hypothyroidism rates in patients with bipolar disorders not treated with lithium is around 9.2–10.8% compared with 28–32% in patients treated.12,13 According to a 10-year longitudinal study, the annual rate of subclinical hypothyroidism in patients treated with lithium is 1.7%.14 The male/female ratio is estimated to be approximately 1:5.15
An important factor in this gender-based difference in prevalence is the autoimmune thyroiditis that appears 7 times more frequently in females, with an increase in incidence from middle-age on.16
More than a third of the patients treated with lithium carbonate can experience transient elevation of TSH and approximately 10% suffer from persistent hypothyroidism, especially if there is underlying autoimmune thyroiditis.17 In a population of 1705 patients treated with lithium for the first time, a hypothyroidism rate of 5.65 per 100 persons and per year was found, which represents almost 6% of the patients that were following this treatment. This indicates that the frequency with which hypothyroidism developed in these patients was double that expected in the general population.18 Compensatory mechanisms are normally put into action upon beginning therapy with lithium to prevent alteration of thyroid function; however, these mechanisms can be annulled by various risk factors for the development of hypothyroidism, such as pre-existing autoimmunity, iodine deficit or genetic susceptibility.8
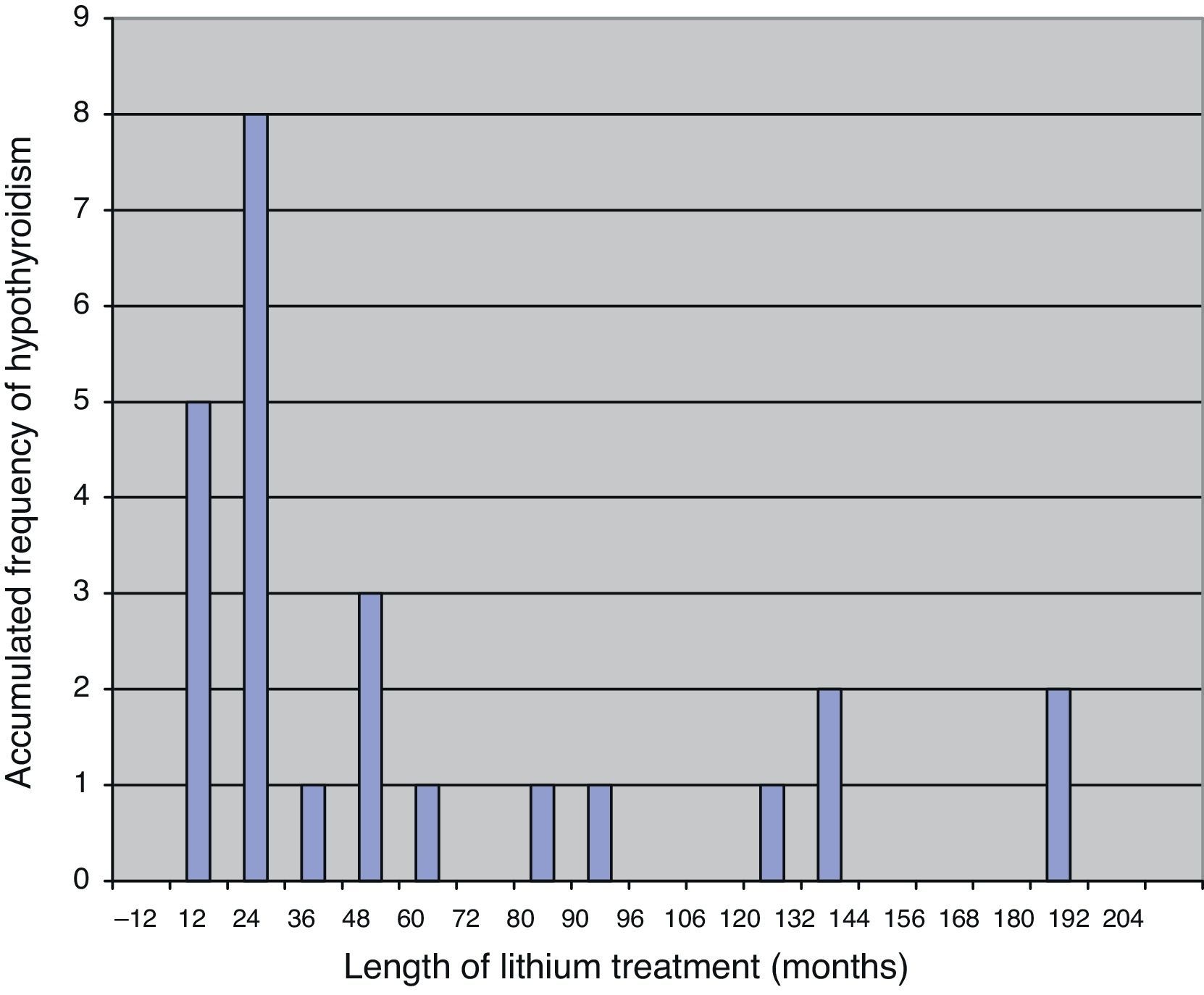
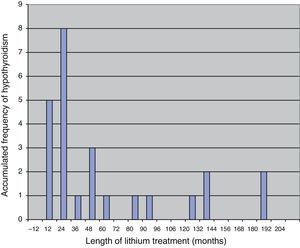
Most patients are diagnosed with hypothyroidism in the first few years after initiating treatment with lithium, with a mean length of treatment with this drug from commencement to diagnosis of hypothyroidism of some 18 months.19 The fact that hypothyroidism appears after a relatively short period of treatment suggests that there must be an individual susceptibility, with a certain genetic predisposition.20 In Fig. 1 we present the results of a sample of 25 patients over 65 years of age who developed subclinical or clinical hypothyroidism during treatment with lithium, in which 17 of them were diagnosed in the first 3½ years of treatment.21
Time until the diagnosis of hypothyroidism in 25 patients after initiating treatment with lithium salt.
The relationship between the subtype of BD and altered thyroid function has also been studied. Some authors have found that type II bipolar patients, not treated with lithium or carbamazepine, show significantly lower levels of TSH than type I bipolar patients.12
The clinical presentation of hypothyroidism secondary to treatment with lithium salt is no different from that due to other causes of hypothyroidism (Table 2). Apart from the normal signs and symptoms, it can also cause neurocognitive deficits that especially affect memory. In fact, hypothyroidism has traditionally been included in the differential diagnosis of the dementias that appear in the elderly.
Clinical manifestations of hypothyroidism.
| Nervous system | Somnolence, difficulty concentrating, memory loss, psychomotor slowness, depression, cerebellar ataxia, headache |
| Musculoskeletal system | Arthromyalgia, decrease in strength |
| Cardiovascular system | Bradycardia, muffled heart sounds, pericardial effusion, lowered cardiac output, palpebral and pretibial oedema without pitting |
| Respiratory system | Dyspnoea, pleural effusion, obstructive sleep apnea–hypopnea syndrome, hypoventilation |
| Skin and nails | Dry, cold, flaky skin, nail fragility, loss of hair and brows |
| Digestive system | Constipation, abdominal distension |
| Energy metabolism | Decreased energy output and O2 consumption, intolerance to cold, weight increase |
| Goitre | Present depending on aetiology |
| Gonad function | Oligomenorrhoea, infertility, abortions |
Clinical implications of hypothyroidism in BD. The clinical relevance of hypothyroidism in BD is especially important. In a study carried out with a group of 65 type I bipolar patients in depressive phase, with values near the lower limit of normality for T4 and at the upper limit for TSH, the patients were found to present more prolonged periods until reaching remission and a slower response to acute treatment.22 In another similar study, the conclusion was reached that, in patients with thyroid dysfunction, remission of an acute episode was longer and tended to present more depressive symptoms during maintenance treatment.23 Low T3 levels and high TSH levels have also been significantly associated with more frequent affective episodes, greater severity of manic symptoms in manic patients treated with lithium, females and longer duration of the disease.13
In summary, the psychiatric symptoms most linked to hypothyroidism are depression and cognitive problems. There are a few cases described of mania or hypomania, but they are practically anecdotal. In a retrospective review of 18 patients, Evans et al. found that the appearance of organic mania shortly after initiating substitutive treatment with thyroid hormone was more frequent in women and was associated with the coexistence of psychotic symptoms, personal or family history of psychiatric disease and with treatment with levothyroxine (LT4) at dosages over 150μg/day. The authors suggested that rapid administration of levothyroxine could increase the sensitivity of the catecholaminergic receptor sharply, precipitating a hypercatecholaminergic state and thus causing manic symptoms in these patients.23
Goitre and bipolar diseaseThe increase in the size of the thyroid gland from lithium salt was observed shortly after its introduction. Back in 1968, Schou et al. indicated an annual incidence of goitre of 4% in patients under maintained treatment with lithium as compared to an incidence of 1% in the general population of the same geographic area.24
Due to the inhibition of thyroid hormone secretion by lithium, an increase in the concentration of TSH is produced that stimulates the growth of the thyroid. In addition to this effect, lithium activates tyrosine-kinase and alters the intracellular transduction signal and the function of the insulin-like growth factor (IGF). All of this favours cellular proliferation. The moment when goitre appears is uncertain. It can be produced from the commencement of therapy with lithium or appear months or years later.9
The goitre rates found are variable, as they depend on various factors such as population sample, length of therapy, observer experience or diagnosis method. While some groups have not found greater incidence of goitre in patients treated with lithium salt, others place it at 30–60% of patients.25 In a Danish transversal study on 100 patients, using ultrasound methods to measure thyroid volume, goitre was detected in 40% of patients treated for 1–5 years; furthermore, it was detected in 50%, if they had received treatment for more than 10 years, in comparison with the 16% found in the control subjects.26 In another German study with similar results, on patients who followed long-term therapy with lithium, regular ultrasound use was recommended to detect thyroid increase because of its superior efficacy to palpation.27
Thyroid hypofunction and rapid cyclingThe figures on prevalence of rapid cycling in patients with bipolar disorders are inconsistent. Prevalence of 16.3% has been estimated, with a range of 12–24%.28 Likewise, the strength of association between thyroid abnormalities and the development of rapid cycling are uncertain. Most of the studies include patients who have received long-term preventative treatment with lithium, have a retrospective design, lack a healthy control group or have a majority of women. Some studies show an association between hypothyroidism and rapid cycling, such as Bauer et al., who found 60% with patients with hypothyroidism in a sample of 30 rapid cycling patients.29 However, in general, transversal studies on unmedicated patients with rapid cycling have not found abnormalities in basal TSH and T4 levels. It is postulated that the rapid cycling patients are unable to express thyroid alterations until physiological changes take place from what are called “antithyroid stressors”, which include drugs such as lithium. A latent hypofunction in the HPT system may explain why high dosages of T4 added to lithium can reverse the pattern of rapid cycling.30
Thyroid hypofunction and mixed statesStudies on the relationship between thyroid dysfunction and the presence of mixed states are less conclusive than those carried out on rapid cycling. Some have been incapable of finding this association.31 Other studies find significantly lower T4 levels and higher TSH levels in mixed conditions compared with pure manias not associated with exposition lithium; likewise, they find higher antithyroid antibodies in these patients in comparison with uni- or bipolar groups.32
HyperthyroidismThere is a hypersecretion of T4 and/or T3 in hyperthyroidism of thyrotoxicosis, with consequently elevated plasma levels. Anxiety, mood lability, depression and insomnia frequently accompany hyperthyroidism. It can also be associated with, although less frequently, manic episodes or even mixed conditions,33 delirium and hallucinations. What happens most frequently is that the patients who suffer a manic episode in relation with the hyperthyroidism already have an underlying mood disorder or family history of affective disorder.34 Symptoms such as anxiety or mania are mediated by beta-adrenergic hyperactivity.
The condition of thyroid hyperfunction may be due to autoimmune thyroid disease (Graves’ disease), toxic nodular goitre or silent thyroiditis. The most common aetiology for the hyperthyroidism that appears in patients treated with lithium is subacute granulomatous thyroiditis. In an important retrospective review, Miller and Daniels found that silent thyroiditis and hyperthyroidism have a much higher incidence in patients exposed to lithium than in the general population.35 Lithium probably masks the underlying hyperthyroidism as it reduces thyroid hormones and, when this therapy is stopped, the hyperthyroidism appears.
Given that lithium is a potent inhibitor of thyroid hormone release, using lithium was put forward in treating thyrotoxicosis from primary hyperthyroidism. However, in a recent review, Lehman and Lee warned that lithium could induce hypercalcaemia and hyperparathyroidism, conditions that are especially dangerous in the elderly. For these reasons, calcaemia should be determined before initiating therapy with lithium salt and at least annually thereafter.36
Prognostic implications of thyroid dysfunctionThere are clinical data that confirm the effect of alterations in thyroid function at the prognostic level in bipolar patients. More rapid response to antidepressant treatment and shorter hospital stay have been described in bipolar depression with elevated levels of free T4.37 It seems that the levels of the thyroid hormone in the low-normal range or lower can lead to a suboptimum response to treatment. In fact, lower serum levels of free T4 and higher levels of TSH are significantly associated with worse response to treatment in bipolar patients during the depressive phase.22 Response to lithium can also be modified, so high levels of T3 predict better response to lithium and less probability of depressive relapses during the first years.38 In addition, patients treated with lithium that require an intervention for a depressive phase have significantly elevated levels of TSH in comparison with subjects that do not need such an intervention.39
Management/practical considerationsBefore initiating prophylaxis with lithium, assessment of thyroid function is recommended for all patients; the assessment should include plasma levels of TSH and antithyroid antibodies. Determination of free T4 should be added in cases in which TSH is suppressed (to rule out central hyperthyroidism or hypothyroidism) or elevated (to confirm the presence of hypothyroidism). Once treatment with lithium salt has commenced, the analytic determinations to study thyroid functions should be repeated every year. When there is subclinical hypothyroidism (TSH≥5mU/mL and ≤10mU/mL with normal free T4), shorter intervals are recommended: every 4–6 months. It can also be advisable to control thyroid function every 6 months in some patients in which certain factors making them more sensitive to the inhibitory effects on lithium on the thyroid concur40 (Table 3). The patient should be referred to an endocrinology consultation if the TSH concentrations are repeatedly abnormal or if goitre or thyroid nodules are detected.8
Factors that increase sensitivity to the inhibitory effects of lithium on the thyroid gland.
| • Radiation over thyroid glands |
| • Autoimmune thyroid disease |
| • Family history of thyroid disease |
| • Tobacco use: greater frequency of autoimmune thyroiditis in smokers |
| • Gender: greater risk of autoimmune thyroiditis in women |
| • Treatment with valproate: possible mild inhibitory effects |
Assessing thyroid function is especially important in rapid cycling patients, those resistant to treatment, mixed episodes, and patients who are psychotic or being treated with lithium, above all if they are women.41
At a practical level, it is important to consider that, after commencing therapy with lithium, plasma TSH levels increase in the majority of patients although these levels are usually kept within normal range. After 2 or 3 months, the serum concentration of TSH decreases practically to the levels prior to treatment with lithium, with accompanying normal plasma T4 levels.42 Transient elevation of the levels of total or free T4 can also be detected in some patients with mania shortly after hospitalisation; these levels gradually descend until normal a few weeks after treatment, as the clinical symptoms remit. It has been suggested that this elevation is directly related to the severity of the symptoms and that the decrease of these levels is linked to better prognosis.43
With respect to multi-drug therapy (a standard practice in bipolar patients), a significant interaction between the use of lithium and valproate has been described; that is, a positive lineal association between the number of stabilisers and the risk of hypothyroidism. That is why being cautious when combining mood stabilisers in hypothyroid patients or those at greater risk of being so is recommended.44
There is a lack of unanimity among clinicians as to the management of hypothyroidism diagnosed after initiating treatment with lithium salt. In general, although the consensus recommends not treating patients with subclinical hypothyroidism with plasma TSH levels lower than 10mU/mL,45 treatment for these patients could be justified in some cases, above all if there is goitre and/or elevated values of antithyroid antibodies.46 In clinical practice, it was more standard before to withdraw lithium treatment if hypothyroidism appeared. At present, the trend is to maintain the treatment, supplementing it with LT4. The presence of abnormalities in thyroid function should generally not constitute a contraindication for treatment with lithium; likewise, administration of this drug should not be interrupted if a patient develops thyroid abnormalities. At any rate, any decision should be individualised and should consider the efficacy of lithium as a stabiliser and as a reducer of suicide.47 The adverse effects on the thyroid gland are normally reversible when treatment is discontinued or thyroid substitution therapy is initiated.
In the cases in which substitute treatment with LT4 is unnecessary, a regime of dosage of 1–1.5μg/kg/day (some 100μg day) can be used initially in young patients without any cardiovascular risks. In elderly patients or those with cardiovascular risk, the initial dosage should be less: 12.5–25μg/day with increments of approximately 25μg/day every 14–28 days until the appropriate dosage is reached. It is not useful to perform any analytical assessment until at least 6 weeks have passed from the commencement of treatment, or after modifying a dosage, because this is the minimum time needed to reach stable plasma hormone levels. The goal of substitute treatment is to achieve thyroid hormone levels within the normal range with non-stimulated TSH. It has also been postulated that, in patients in whom thyroid and psychiatric illnesses coexist, TSH levels should be lower than 3mUI/mL, and not the 5mUI/mL proposed for the population free from psychiatric pathology.48
Therapeutic management of lithium-induced goitre is the same as for that from any other aetiology. The sonogram is the method of choice for initial assessment of goitre. Diffuse increases of thyroid size orient towards drug aetiology. If there are recent signs of nodular increase or a dominant nodule in a multinodular goitre, this should be investigated through fine needle aspiration cytology, to rule out malignancy. Treating goitre with LT4 in the absence of hypothyroidism is debatable, although some authors even recommend prophylaxis with LT4 in all patients in which lithium treatment is initiated if they come from areas of endemic goitre.49 However, other authors advise against treatment with LT4 because of its possible adverse effects (hyperthyroidism, loss of bone mass and vascular risks) and the lack of proven results on goitre evolution. In addition, visible and/or palpable goitre can decrease in size over the course of its development, even without LT4 treatment and maintaining exposure to lithium.14 Resection would be indicated in nodular goitre of great size, especially when compressive symptoms are caused.
In the case of hyperthyroidism, management is different, depending on the aetiology. As the first step, it is necessary to re-establish the euthyroid state so that secondary psychiatric symptoms remit. Antithyroid therapies, steroid treatment in some cases or even surgery can be indicated. Beta-adrenergic receptor antagonists, as well as antimanic drugs, can also be useful at times. In addition, as lithium inhibits hormone release from the thyroid glad, it can be used as adjuvant therapy in the management of severe hyperthyroidism together with thionamide.
With respect to using LT4 as a coadjuvant treatment to stabilisation of patients with affective disorders, although there are some data in favour in a subgroup of patients with chronic and refractory forms of BD, the evidence is still slight,7 given that there are no randomised controlled trials and the number included in studies published is too small. This strategy should consequently be considered only as a last resort for patients who have not responded to other treatments. If it is used, it should be initiated with a dosage of 50–100μg/day and should be increased by 25–50μg/day per week, up to a maximum of 500μg/day. Response to the treatment is normally evident within the first 2 weeks. If the patient responds, the treatment can be continued for a few months; if the patient does not respond, the T4 should be suppressed progressively because sudden discontinuance can lead to drug-related hypothyroidism. Although recent reviews indicate maximum dosages of up to 500μg/day,7 we tend to reach lower dosages in daily practice. In addition, special caution should be taken in endocrine or cardiovascular disorders and in elderly patients. Administration is not recommended during pregnancy.
Future lines of investigationAt present, neuroimaging and genetic studies attempt to explain the connection between dysfunction in the HPT axis and mood disorders. With respect to neuroimaging, a recent study in which positron emission tomography (PET) scans of the brain were performed on hypothyroid patients with thyroid substitution treatment found an association between improved clinical status during therapy and the restoration of metabolic activity in brain areas that are an integral part of affect and cognition regulation.50 Some of these findings would explain the therapeutic effects of thyroid hormones in bipolar patients. Although genetic studies are still in a preliminary stage, the findings suggest that anomalies in the HPT axis may be potential endophenotypes for BD. It has also been suggested that autoimmune thyroiditis, with an elevation of antibody titre as markers, could be an endophenotype for BD and could be related with a genetic susceptibility to develop BD.51
ConclusionsThe link between thyroid dysfunction and mood disorders is a demonstrated fact that is relevant in clinical practice. The etiopathogenesis, clinical course, prognosis and treatment of BD can be influenced by an alteration in the HPT axis.
Lithium is still the stabilising treatment of choice in bipolar patients, even though it can cause various thyroid anomalies. After treatment with lithium is initiated, the majority of patients develop compensatory mechanisms that prevent the appearance of clinically relevant thyroid alteration. However, these mechanisms can become blocked in some cases from the existence of additional risk factors, whether intrinsic, of genetic origin or environmental (iodine deficiency, some types of diet or tobacco use). In the end, clinical consequences occur. The most frequent of these, hypothyroidism and goitre, are fundamentally produced by inhibition of thyroid hormone release.
Even minimum alterations of the HPT axis can affect the prognosis of BD. For that reason, it is important for the physician to monitor thyroid function carefully and to consider prescribing thyroid supplement when necessary. Treatment with lithium salt has undoubted benefits and therapy with LT4 for hypothyroidism is effective. Consequently, the administration of lithium should not be interrupted as a general rule in the face of the detection of thyroid hypofunction in the patient with BD.
Genetic and neuroimaging studies are currently being carried out in this field. Future lines of research should focus on the objective of identifying, using genetic markers, the bipolar patients that can benefit most from treatment of the alterations detected in the HPT axis.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sierra P, Cámara R, Tobella H, Livianos L. ¿Cuál es la relevancia real y el manejo de las principales alteraciones tiroideas en los pacientes bipolares?. 2014; 7:88–95.