This study assesses the potential eligibility of patients admitted to a psychiatric hospitalisation unit to take part in the major clinical trials based on schizophrenia treatment in clinical practice (CATIE, CUtLASS and EUFEST).
Materials and methodsA retrospective evaluation by consulting the medical records of 241 subjects (59.8% males and 40.2% females, mean age 39.7±13.0 years), admitted consecutively over one year to psychiatric hospitalisation unit with a diagnosis of schizophrenia or another psychosis. The influence of the factors involved in the non-eligibility in each of the clinical trials is analysed using logistic regression analysis.
ResultsOnly 20.7%, 22.3%, and 22.5% of patients with schizophrenia or another psychosis would be eligible to participate in the CATIE, CUtLASS and EUFEST studies, respectively. The main factors involved in the non-eligibility were polytherapy with anti-psychotics (2 or more) (Odds Ratio (OR): 7.64, 95% confidence interval (CI): 3.06–19.06, P<.001), mental retardation (OR: 16.67, 95% CI: 1.75–166.67, P=.014), and resistance, intolerance or contraindication to any of the anti-psychotics of the study (OR: 3.68, 95% CI: 1.13–11.99, P=.030).
ConclusionsThree out of every four patients with schizophrenia or another psychosis admitted to a psychiatric hospitalisation unit are not represented in the major clinical trials on schizophrenia treatment.
Este estudio pretende evaluar la potencial elegibilidad para la participación en los principales ensayos clínicos de tratamiento de esquizofrenia basados en la práctica clínica (CATIE, CUtLASS y EUFEST) de los pacientes ingresados en una unidad de hospitalización de psiquiatría.
Material y métodosEvaluación retrospectiva, mediante consulta de la historia clínica, de los 241 sujetos (59,8% varones y 40,2% mujeres, edad 39,7±13,0 años), ingresados de forma consecutiva a lo largo de un año en una unidad de hospitalización de psiquiatría con diagnóstico de esquizofrenia u otra psicosis. La influencia de los factores implicados en la no elegibilidad en cada uno de los ensayos clínicos se analizó mediante análisis de regresión logística.
ResultadosUn 20,7, un 22,3, y un 22,5% de los pacientes con esquizofrenia u otra psicosis serían elegibles para participar en los estudios CATIE, CUtLASS y EUFEST, respectivamente. Los principales factores implicados en la no elegibilidad fueron la politerapia con antipsicóticos (2 o más) (Odds Ratio (OR): 7,64, intervalo de confianza (IC) 95%: 3,06–19,06, p<0,001), el retraso mental (OR: 16,67, IC 95%: 1,75–166,67, p=0,014) y la resistencia, intolerancia o contraindicación a alguno de los antipsicóticos del estudio (OR: 3,68, IC 95%: 1,13–11,99, p=0,030).
ConclusionesTres de cada cuatro pacientes con esquizofrenia u otra psicosis ingresados en una unidad de hospitalización de psiquiatría no están representados en los grandes ensayos clínicos de tratamiento de esquizofrenia.
Pharmacological treatment of patients with schizophrenia and other psychoses earns its scientific support mainly from clinical trials (randomised treatment assignment, double-blind, for both the patient and the researcher). The methodological guarantees of these trials tend to reinforce the internal validity of the results, but at the expense of their external validity and the possibility of generalising the results to the clinical population.1,2 This has been the topic of analysis in various articles that emphasise the importance of external validity in clinical research. These articles also support performing clinical trials that are closer to patients’ daily reality.3–5
To this end, performing large pragmatic clinical treatment trials for schizophrenia has been promoted in recent years, based on standard clinical practice. This practice also serves to minimise external validity deficiencies while maintaining internal validity. This type of trial also broadens inclusion criteria and restricts those of exclusion to incorporate the largest number of patients possible, based on the reality of the population from which the subject samples that participate in the clinical trials are selected.6
The main clinical treatment trials, based on clinical practice, for schizophrenia and other psychoses are the following studies:
- (1)
CATIE7 (randomised double-blind clinical trial, comparing the effectiveness–in terms of discontinued treatment for any illness–of different antipsychotics [olanzapine, risperidone, quetiapine, ziprasidone and perphenazine] during an 18-month follow-up for treating 1493 patients referred from 57 institutions in the United States, with chronic schizophrenia [2 or more episodes], funded by the US National Institute of Mental Health).
- (2)
CUtLASS8 (randomised double-blind clinical trial, including 227 patients with schizophrenia cared for at United Kingdom public mental health centres, comparing improvement in quality of life after a change in antipsychotics [first generation versus second generation] due to an inadequate response or the appearance of adverse events, funded by the UK National Health Service).
- (3)
EUFEST9 (randomised double-blind clinical trial, including 498 patients with first episode of schizophrenia, schizophreniform psychosis or schizoaffective disorder, assigned randomly to a low dose of haloperidol or of a second generation antipsychotic [amisulpride, olanzapine, quetiapine and ziprasidone] and subject to 1-year follow-up, to assess effectiveness in terms of discontinued treatment for any illness, funded by Astra Zeneca, Pfizer and Sanofi-Aventis).
However, despite the improvement in external validity,6 the samples of these large clinical trials present some important differences from the standard clinical population. One of these differences is the disproportion of male patients in clinical trials compared to the clinical population that the evaluated samples represent (males are generally overrepresented in trials).10,11
These data have encouraged the study of the representation of patients included in clinical trials. In general, comparing clinical and sociodemographic variables between participants in clinical trials of schizophrenia treatment and non-participating patients (treated according to standard practice) has not produced any relevant differences between the 2 groups, except the higher frequency of suicide ideation and disorganised thought and increased severity of negative symptomatology in subjects that do not participate in the clinical trials.1,12 However, standard exclusion criteria in clinical trials, like antipsychotic combination therapy or mental retardation, were not considered in these comparisons. Furthermore, these comparisons were made before the large pragmatic clinical trials CATIE, CUtLASS and EUFEST were published.
The objective of the present study was evaluating whether psychiatric in patients with diagnoses of schizophrenia or other psychoses would be eligible for participation in the main clinical treatment trials for schizophrenia.7–9
Materials and methodsThe sample for the study was composed of all the short-term psychiatric inpatients at the University of Albacete Hospital Complex (CHUA in Spanish) between 1 May 2009 and 1 May 2010, with a primary clinical diagnosis of schizophrenia or another psychosis. The population area that CHUA covers includes 399,191 inhabitants (data from 2003, http://jccm.es/sanidad/salud).
Diagnoses were made on the basis of the clinical judgments of the practitioners who cared for each of the patients, according to DSM-IV-TR classification criteria.
The sample consisted of 241 subjects (144 males [59.8%] and 97 women [40.2%]) with a mean age (±standard deviation) of 39.7±13.0 years. Among them, 145 patients (60.2%) had a diagnosis of schizophrenia, 35 subjects (14.5%) schizoaffective disorder, 8 patients (3.3%) chronic delusional disorder, 21 patients (8.7%) schizophreniform psychosis and 32 patients (13.3%) non-specified psychotic disorder.
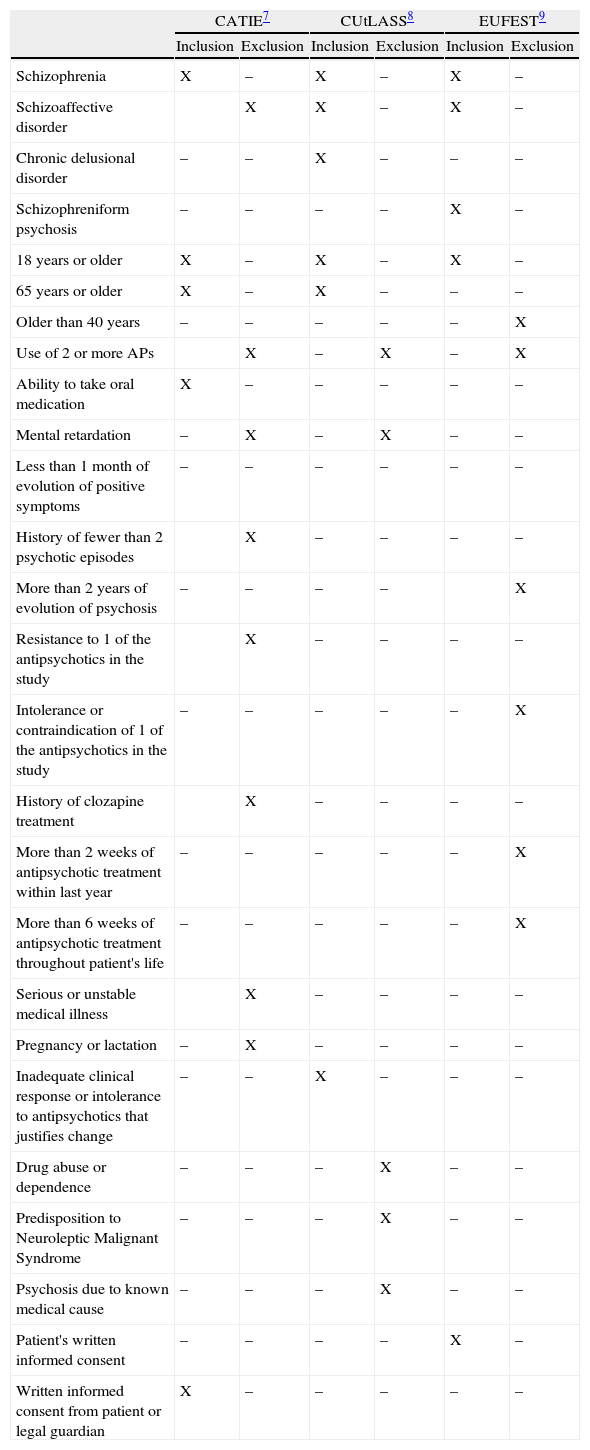
Information was collected retrospectively by consulting clinical histories to check whether or not patients complied with the inclusion or exclusion criteria for the 3 main pragmatic clinical trials of schizophrenia treatment published to date.7–9 Due to the retrospective nature of information collection, the criteria for granting informed consent could not be assessed. Involuntary admittance does not necessarily involve the inability to grant consent for participation in research studies.13 Consequently, following an inclusive criterion and in an effort not to incur speculative assumptions, we decided to consider that all patients would consent to participating in the trials. Table 1 summarises the inclusion and exclusion criteria for each of these studies.
Inclusion and exclusion criteria for the clinical trials in CATIE, CUtLASS and EUFEST.
| CATIE7 | CUtLASS8 | EUFEST9 | ||||
| Inclusion | Exclusion | Inclusion | Exclusion | Inclusion | Exclusion | |
| Schizophrenia | X | – | X | – | X | – |
| Schizoaffective disorder | X | X | – | X | – | |
| Chronic delusional disorder | – | – | X | – | – | – |
| Schizophreniform psychosis | – | – | – | – | X | – |
| 18 years or older | X | – | X | – | X | – |
| 65 years or older | X | – | X | – | – | – |
| Older than 40 years | – | – | – | – | – | X |
| Use of 2 or more APs | X | – | X | – | X | |
| Ability to take oral medication | X | – | – | – | – | – |
| Mental retardation | – | X | – | X | – | – |
| Less than 1 month of evolution of positive symptoms | – | – | – | – | – | – |
| History of fewer than 2 psychotic episodes | X | – | – | – | – | |
| More than 2 years of evolution of psychosis | – | – | – | – | X | |
| Resistance to 1 of the antipsychotics in the study | X | – | – | – | – | |
| Intolerance or contraindication of 1 of the antipsychotics in the study | – | – | – | – | – | X |
| History of clozapine treatment | X | – | – | – | – | |
| More than 2 weeks of antipsychotic treatment within last year | – | – | – | – | – | X |
| More than 6 weeks of antipsychotic treatment throughout patient's life | – | – | – | – | – | X |
| Serious or unstable medical illness | X | – | – | – | – | |
| Pregnancy or lactation | – | X | – | – | – | – |
| Inadequate clinical response or intolerance to antipsychotics that justifies change | – | – | X | – | – | – |
| Drug abuse or dependence | – | – | – | X | – | – |
| Predisposition to Neuroleptic Malignant Syndrome | – | – | – | X | – | – |
| Psychosis due to known medical cause | – | – | – | X | – | – |
| Patient's written informed consent | – | – | – | – | X | – |
| Written informed consent from patient or legal guardian | X | – | – | – | – | – |
The study was approved by the Ethics and Clinical Research Committee of the University of Albacete Hospital Complex.
Statistical analysis: a description of frequencies and an analysis of logistic regression (dependent variable: eligible for each trial, yes/no) demonstrated the influence of participation criteria (inclusion and exclusion) on the non-eligibility of patients in each of the 3 clinical trials. The level of significance was fixed at P<.05. Analysis of the data was performed with the SPSS program for Windows, version 16.0.
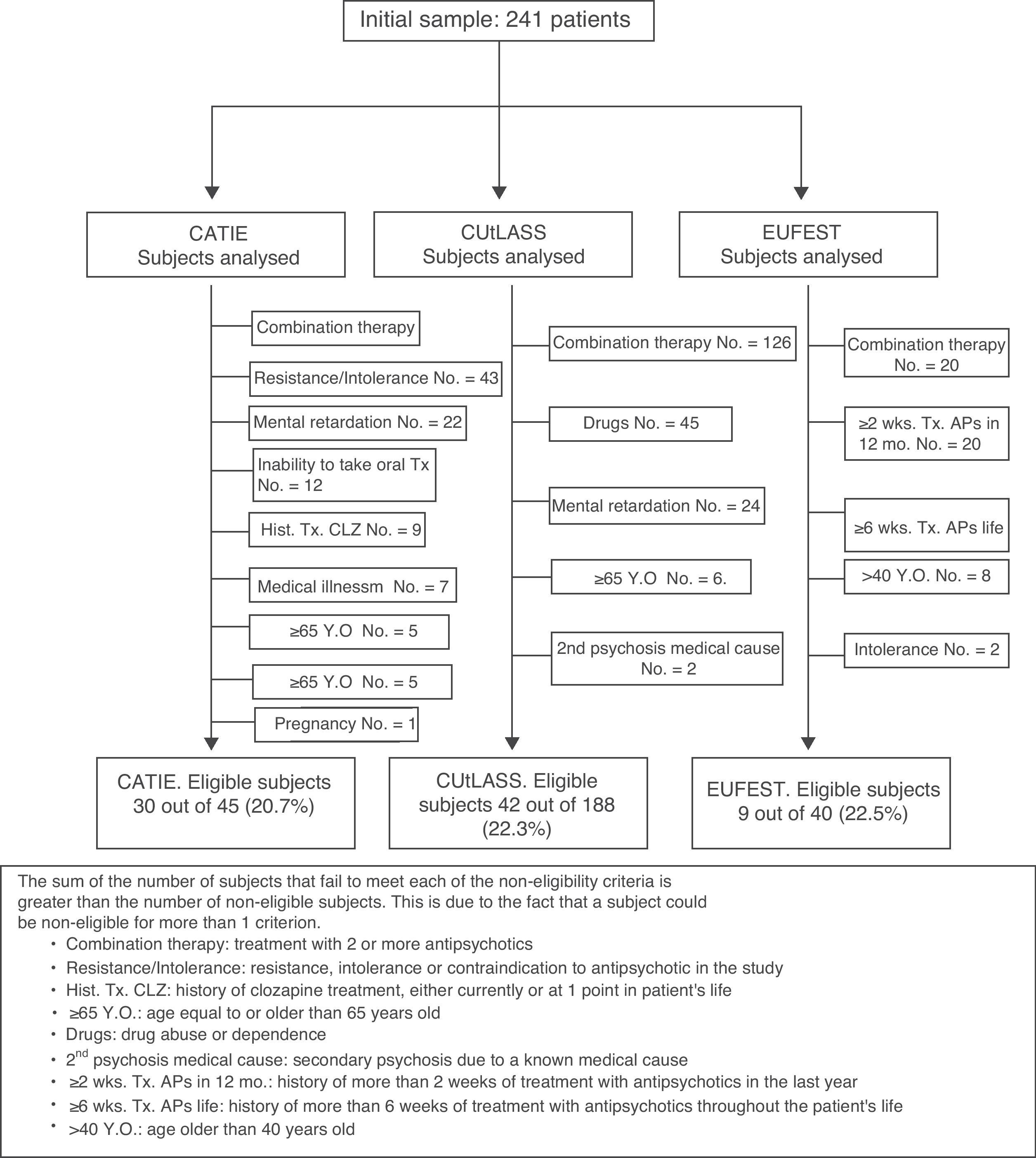
Fig. 1 shows a chart of the subjects analysed to calculate the percentage of patients eligible for each clinical trial.
ResultsCATIE eligibilityOf the 241 subjects included in the study, 145 (60.2%) had a schizophrenia diagnosis. Since the CATIE7 study included patients with a schizophrenia diagnosis, the analysis of eligibility for CATIE was exclusively performed in the group of patients from our sample with this diagnosis.
Of these 145 subjects (105 males, 40 females, age 39.6±12.8 years), 30 (20.7%) were eligible for the CATIE study. According to the participation criteria, non-eligibility was conditioned by: antipsychotic combination therapy (100 patients, 70.0%); resistance, intolerance or contraindication to 1 of the antipsychotics in the study (43 patients, 29.7%); mental retardation (22 subjects, 15.2%); inability to take medication orally (12 subjects, 8.3%): serious or unstable medical illness (7 patients, 4.8%); being 65 years old or older (5 subjects, 3.4%); first episode of psychosis (4 patients, 2.8%); and pregnancy or lactation (1 patient, 0.7%).
Gender (P=.211) and age (P=.229) were not correlated with eligibility for the CATIE study.
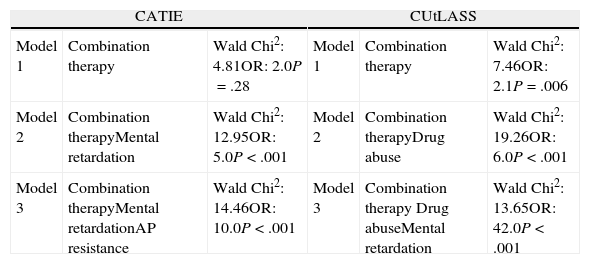
The binary logistic regression analysis showed that the best predictive model of non-eligibility for the CATIE study included the following criteria: combination therapy, mental retardation and resistance, intolerance or contraindication to 1 of the antipsychotics in the study (Wald chi-squared statistic: 14.46; Odds Ratio [OR]: 10; P<.001). The rest of the participation criteria did not present any improvements to the model. Table 2 details the prediction models of binary logistic regression.
Prediction models of clinical trial non-eligibility.
| CATIE | CUtLASS | ||||
| Model 1 | Combination therapy | Wald Chi2: 4.81OR: 2.0P=.28 | Model 1 | Combination therapy | Wald Chi2: 7.46OR: 2.1P=.006 |
| Model 2 | Combination therapyMental retardation | Wald Chi2: 12.95OR: 5.0P<.001 | Model 2 | Combination therapyDrug abuse | Wald Chi2: 19.26OR: 6.0P<.001 |
| Model 3 | Combination therapyMental retardationAP resistance | Wald Chi2: 14.46OR: 10.0P<.001 | Model 3 | Combination therapy Drug abuseMental retardation | Wald Chi2: 13.65OR: 42.0P<.001 |
AP resistance: resistance, intolerance or contraindication to 1 of the antipsychotics in the study.
The CUtLASS8 study included patients with diagnoses of schizophrenia, schizoaffective disorder or chronic delusional disorder. Consequently, the eligibility analysis for the CUtLASS study was performed exclusively in the group of 188 patients (78.0%) from our sample with 1 of these diagnoses (145 with schizophrenia, 35 with schizoaffective disorder and 8 with chronic delusional disorder).
Of these 188 subjects (122 males, 66 females, 40.8±12.8 years old), 42 (22.3%) were eligible for the CUtLASS study. According to the participation criteria, non-eligibility was conditioned by: antipsychotic polytherapy (126 patients, 67.0%), mental retardation (24 subjects, 12.8%), being 65 years old or older (6 subjects, 3.2%), drug abuse or dependence (45 patients, 23.9%) and secondary psychosis due to a known medical cause (2 subjects, 1.1%).
Being female was correlated with eligibility for the CUtLASS study. Of the 42 eligible subjects, 24 (57.1%) were female, while among the 146 non-eligible patients, 42 (28.8%) were females (chi-squared: 11.53, P=.001). Age (P=.893) was not correlated with CUtLASS study eligibility.
Binary logistic regression analysis showed that the best predictive model for non-eligibility for the CUtLASS study included the following criteria: combination therapy, drug abuse or dependence and mental retardation (Wald chi-squared statistic: 13.65; OR: 42; P<.001). The rest of the participation criteria did not present any improvements to these models. Table 2 details the prediction models of binary logistic regression.
EUFEST eligibilityThe EUFEST9 included patients with their first diagnostic episode of schizophrenia, schizoaffective disorder and schizophreniform psychosis. Of the 241 subjects from the total sample, 55 (22.8%) were admitted for their first psychotic episode. Of these 55 patients, 40 complied with 1 of the diagnostic criteria (15 with schizophrenia, 6 with schizoaffective disorder and 19 with schizophreniform psychosis), while 15 received a non-specified psychotic diagnosis. Consequently, our eligibility analysis for the EUFEST study was performed exclusively in the group of 40 patients from the sample that complied with the diagnostic criteria for schizophrenia, schizoaffective disorder or schizophreniform psychosis. These patients also had less than 2 years of evolution from the start of their psychosis (this time criterion was applied because it was used in the EUFEST study to define the first episode).
Of these 40 subjects (22 males, 18 females, 31.4±11.8 years old), 9 (22.5%) were eligible for the EUFEST study. According to the participation criteria, non-eligibility was conditioned by: antipsychotic combination therapy (20 patients, 50.0%), more than a 2-week history of antipsychotic treatment in the previous year (20 patients, 50.0%), more than a 6-week history of antipsychotic treatment throughout life (16 patients, 40.0%), being older than 40 years (8 subjects, 20.0%) and intolerance or contraindication to 1 of the antipsychotics in the study (2 patients, 5.0%). Gender (P=.970) and age (P=.200) were not correlated with EUFEST study eligibility.
The binary logistic regression analysis did not find any significant prediction model of non-eligibility for the EUFEST study.
Eligibility for the clinical trials (CATIE, CUtLASS or EUFEST)The eligibility analysis for any of the 3 studies showed that of the 241 initial subjects, 34 (14.1%) could not be analysed, as they did not comply with the basic participation criteria for any of the studies (e.g. schizophrenia diagnosis for the CATIE study). Of the 207 analysable patients, 56 subjects (27.1%) would be eligible to participate in at least 1 of the clinical trials. The percentage of eligible subjects fell to 23.2% if the total 241 patients admitted with psychosis were considered (56/241).
The logistic regression analysis revealed that the criteria involved in non-eligibility were: combination therapy (Wald chi-squared statistic: 18.97; OR: 7.64; 95% confidence interval (CI): 3.06–19.06; P<.001), mental retardation (Wald chi-squared statistic: 5.98; OR: 16.67; 95% CI: 1.75–166.67; P=.014) and resistance, intolerance or contraindication to 1 of the antipsychotics in the study (Wald chi-squared statistic: 4.68; OR: 3.68; 95% CI: 1.13–11.99; P=.030).
A comparison between eligible subjects for the CATIE and CUtLASS studies showed that of the 145 analysable subjects (since the number of analysable subjects for eligibility for the CATIE study was 145 and all the analysable subjects for the CATIE study were also analysable for the CUtLASS study), a total of 110 patients (75.9%) were not eligible for either of the 2 trials, 23 (15.9%) were eligible for both, 5 (3.4%) were eligible for the CUtLASS study but not the CATIE and, finally, 7 (4.8%) were eligible for the CATIE study but not the cutlass. Most subjects thus coincided in their eligibility (133, 91.7%) (P<.001) for the CATIE and CUtLASS studies.
DiscussionThe data in this study revealed that of the psychiatric in patients with schizophrenia or other psychoses in our hospital, only 27.1% would be eligible to participate in at least 1 of the clinical trials (CATIE, CUtLASS or EUFEST).
The main factors implicated in non-eligibility were antipsychotic combination treatment (treatment with 2 or more antipsychotics), mental retardation and resistance, intolerance or contraindication to 1 of the antipsychotics in the study.
Clinical guides to treatment of schizophrenia do not recommend antipsychotic combination therapy. If used, it is only after multiple monotherapy trials have been attempted, including clozapine treatment in monotherapy.14–16 However, combination therapy as a standard practice is on the rise in clinical settings all over the world.17–20 In various published studies, the prevalence of antipsychotic combination therapy is between 7 and 90%.17,21–28 This wide range is due to the fact that antipsychotic combination therapy varies considerably depending on the variables, such as a schizophrenia diagnosis (combination therapy is more frequent in patients with this diagnosis than in those with other psychoses),23 the institution being analysed (more frequent in inpatient than in outpatient environments),23,24 duration of illness (more combination therapy correlates with longer duration)25 or the region where the study is performed (higher rates of combination therapy have been reported in Asian countries: as high as 90% in Japan).26,27
In our sample, 62.0% of the patients received treatment with antipsychotic combination therapy. This percentage, although high, is congruent with data published in other studies performed in Spain: 51% in a hospital unit in Zaragoza21 or 73% in a unit in Badajoz.28
Antipsychotic combination therapy has been correlated with increased appearance of adverse effects29 and increased spending on pharmaceuticals.30 Unlike monotherapy, it has not been correlated with significant clinical improvement.29,31,32 Nevertheless, a recent meta-analysis found data suggesting, although not conclusively, that in some situations combination can be preferable to monotherapy, due to higher clinical effectiveness and a lower rate of ceasing medication.19 Of course, combination therapy hinders the analysis of the results concerning the efficiency of each drug, which limits the possibility of generalising the results to other scenarios.30 In any case, it proves fundamental to recognise that the main clinical treatment trials for schizophrenia leave out a very significant part of the population treated in clinical practice with antipsychotic combination therapy.
Regarding mental retardation (defined as an IQ below 70 with accompanying functional limitation and requiring support to carry out daily activities), 12.4% of inpatients with schizophrenia or another psychosis had mental retardation. The relationship between psychosis and mental retardation is well documented.33–35 Epidemiological studies show that the presence of mental retardation is associated with a 2–10 times higher risk of suffering some form of psychosis.36,37 However, there are little data concerning the prevalence of mental retardation in the population with psychosis. A retrospective observational study performed in Madrid, Spain, found a mental retardation frequency of 3.6% in a sample of patients with psychosis (most were not hospitalised).36 The inverse correlation (prevalence of psychosis in people with mental retardation) also suffers from a lack of data. A study carried out more than 20 years ago found high rates of psychosis in a population with mental retardation: 70.8% with schizophrenia among patients with serious mental retardation, 18.7% among those with moderate mental retardation, 2.1% among those with mild mental retardation and 8.3% among those not specified.38 These numbers, however, need to be replicated in new research. In any case, it is clear that mental retardation is associated with psychosis33–38 and can worsen the clinical evolution of psychosis.35 It is also clear that patients with mental retardation and psychosis are not represented in the clinical treatment trials for schizophrenia.
Regarding resistance, intolerance or contraindication to 1 of the antipsychotics in the clinical trials, 29.0% of the patients in our sample had a history of 1 of these circumstances. They would thus not be eligible for the CATIE study. Despite affecting almost a third of the sample, this percentage falls within the expected range of 20–45% for resistance to pharmacological treatment among patients with schizophrenia.14 In the CATIE study, 48.3% of the patients ceased treatment or were withdrawn from it due to intolerance or lack of effectiveness. Specifically, 29.0% of patients in the CATIE study “discontinued” treatment due to a lack of effectiveness and 19.3% due to intolerance.7
The main limitations of this study corresponded to its retrospective transversal design (without follow-up data) and the absence of data for non-hospitalised patients (since combination therapy–which contributes significantly to non-eligibility of patients in the clinical trials–is more frequent in inpatient environments).23,24 Another important limitation was that diagnoses were established based on clinical judgements of the professionals who cared for each patient, without confirmation through a structured or semi-structured interview. Despite this, it is relevant to mention that in standard clinical practice, diagnoses are not confirmed through additional interviews. Consequently, the methodology used in this case was closer to a naturalistic model of real practice.
On the whole, the data presented in this study show that large clinical treatment trials for schizophrenia have excluded a very significant segment of the population with this illness. A large part of research in this field is designed according to results of these trials. Furthermore, drug regulatory agencies make their decisions to approve treatments using 1 drug or another based on these results. Consequently, many of the patients who present greater drug prescription difficulties (hospitalised with combination therapy, with mental retardation or with resistance or intolerance to antipsychotics) are not represented in the samples of the main clinical treatment trials for schizophrenia.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed conformed to the ethical standards of the committee for responsible human experimentation and were in accordance with the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that they followed the protocols of their work centre regarding the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in said study.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interests to declare.
This study was made possible by the participation of members from the Albacete Group of Mental Health Research (ISAMA in Spanish).
We also wish to thank the Centre for Biomedical Research in the Mental Health Network (CIBERSAM in Spanish).
Please cite this article as: Iniesta E, et al. Elegibilidad de pacientes con esquizofrenia ingresados en una unidad de hospitalización psiquiátrica para participar en ensayos clínicos. Rev Psiquiatr Salud Ment (Barc.). 2012;5:71–8.