To describe some epidemiological characteristics of malignant oral cancers in a population of the Oncology Portuguese Institute of Porto (IPO-PORTO).
MethodsAfter consulting databases of the IPO-PORTO database, we selected all patients diagnosed with oral cancer (C00 to C06) between 1 January 2004 and 31 December 2009, with histological confirmation. Data were analyzed according district of residence, gender, age, topographic location of the tumor, histological type, degree of differentiation, and stage (TNM).
ResultsDuring this retrospective descriptive study, 1041cases were reported in both genders. Men were more affected than women in a 3:1 proportion. The number of diagnosed oral carcinoma cases increased in the 5th decade of age (19.69%±2.42%) and progressively decrease after the 7th (18.35%±2.35%). The most affected region was the tongue (C01+C02) (43.51%±3.01%), followed by the floor of the mouth (C04) (17.48%±2.31%). The most prevalent histological entity was squamous cell carcinoma (93.37%±10.90%) in grade I (44.20%±5.86%). Regarding TNM stages, the most frequent was stage IV (42.00%±3.89%).
ConclusionThe epidemiological profile of oral malignant neoplasms found in our study is in accordance with the existing literature. More epidemiological studies, as well as awareness and prevention programs, should be conducted in this area in Portugal.
Descrever algumas características epidemiológicas das neoplasias orais malignas numa população do Instituto Português de Oncologia do Porto (IPO-Porto).
MétodosApós consulta da base de dados do IPO-Porto, foram selecionados todos os pacientes com diagnóstico de neoplasia maligna da cavidade oral (C00-C06) com confirmação histológica, diagnosticados entre um de janeiro de 2004 e 31 de dezembro de 2009. Os dados foram analisados por distrito de residência, género, idade, localização topográfica do tumor, tipo histológico, grau de diferenciação e estádio (TNM).
ResultadosNeste estudo retrospetivo e descritivo obtiveram-se no total 1.041 casos de ambos os géneros. O género masculino é mais afetado comparativamente ao feminino, numa proporção de 3:1. Verifica-se um aumento dos casos diagnosticados a partir dos 40 anos (19,69±2,42%) e um declínio progressivo a partir dos 70 (18,35±2,35%). A região mais afetada foi a língua (C01+C02) (43,51±3,01%), seguindo-se a região do pavimento da boca (C04) (17,48±2,31%). O tipo histológico mais prevalente foi o carcinoma de células escamosas (93,37±10,90%); de entre estes, a maioria é grau I(44,20±5,86%). Relativamente ao estádio, a maioria dos casos encontravam-se no estádio IV (42,00±3,89%).
ConclusãoO perfil epidemiológico das neoplasias orais malignas da cavidade oral que encontramos é semelhante a outros estudos descritos na literatura. Mais estudos epidemiológicos, bem como programas de sensibilização e campanhas de prevenção, deveriam ser realizados nesta área em Portugal.
Oral cancer is a significant component of the global burden of cancer (4% of all cancer cases), and together with the pharyngeal cancer, represents the sixth most common neoplasm worldwide.1–6 Oral cancer involves the lip and different regions of the oral cavity such as tongue, floor of the mouth, gums, and palate, thus representing a heterogeneous group of tumors with similar risk factors.3,5,7,8 The most common histological type, is the squamous cell carcinoma (SCC), which develops from the stratified squamous epithelium of the mucosa and represents approximately 90% of cases.3,4,9 In most cases, oral cancer appears after the age of 40 years, and frequency increases considerably until the 65 years of age, affecting more frequently men than women, in proportions ranging between 2:1 and 3:1.4,10
There are several risk factors involved in the etiology of oral cancer, of which tobacco use (either smoking or chewing) and alcohol consumption are the most significant ones, and they act synergistically when used together.1,4–6,11–14 The International Agency for Research on Cancer has confirmed that tobacco causes oral cancer.1,7 Other studies have shown that an excessive consumption of alcoholic beverages causes nutritional deficiencies (especially of vitamins A, C and E), which appears to contribute to oral carcinogenesis.1,12,13 Other associated etiologic factors are exposure to ionizing radiation, immunosuppression, viral infections (HIV, HPV), genetic or familial predisposition, and poor oral hygiene.1,6,11–13
The lower lip is the region most frequently affected by oral cancer, followed by the lateral borders of the tongue and the floor of the mouth.15,16 Oral cancer appears as a patch of variable color (usually white or reddish), a hardened mass, or an ulcer that does not heal.6,15,16 Its lesions are frequently asymptomatic in early stages and, become progressively painful.6,11
In the coming decades, the World Health Organization (WHO) predicts a continuing increase in oral cancer diagnosis.11
Therefore, epidemiological research is of utmost importance to characterize the individuals at risk, and prepare and plan social policies and future health programs.
The present study aims to describe some epidemiological characteristics of malignant oral cancers in a population of the Portuguese Institute of Oncology of Porto (IPO-Porto). Thus, this study intends to answer the following research hypothesis: “What is the epidemiological profile of malignant oral cancers in the population of the IPO-Porto?”.
MethodsThe data used in this retrospective and descriptive epidemiological study were collected from the IPO-Porto database.
The sample selection was based on the following inclusion criteria: every individuals (of both genders and any age), diagnosed with of malignant neoplasm of the oral cavity (C00–C06) between 1 January 2004 and 31 December 2009, with histological confirmation. All other cases were excluded.
The evaluated parameters were district of residence, gender, age, topographic location of the tumor, histological type, degree of differentiation and stage.
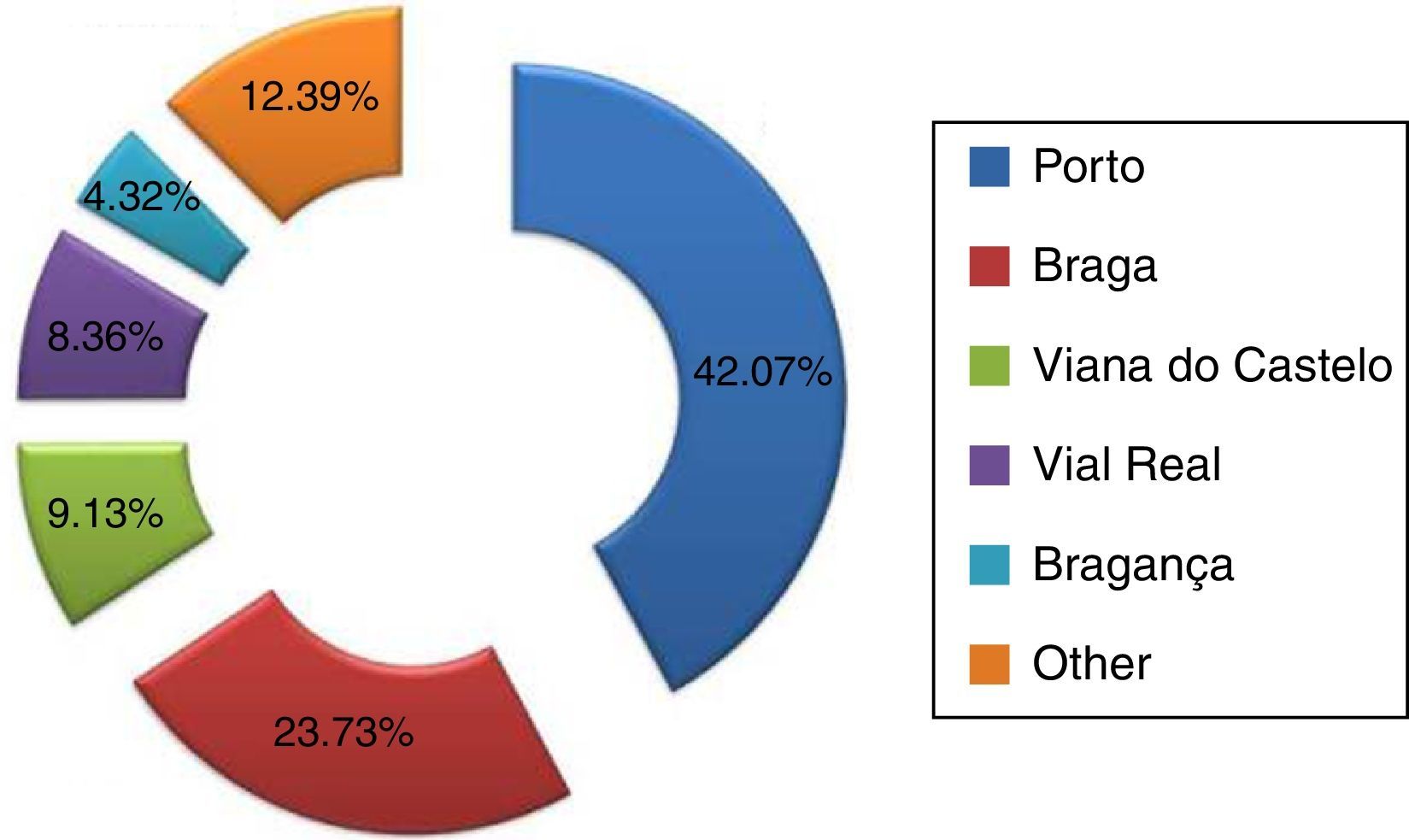
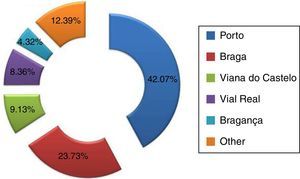
According to their district of residence, the sample was distributed by the following categories: Braga, Bragança, Porto, Viana do Castelo, Vila Real, and other (corresponding to other districts of Portugal).
Given the wide range of ages in the sample for the “age” paremeter, the sample was distributed by nine age groups with intervals of 10 years each, from 20–29 to ≥100.
The classification of the tumors’ topography and morphology, as well as their degree of differentiation, was based on the 3rd edition of the International Classification of Diseases for Oncology (ICD-O 3rd edition).8 We considered the anatomical locations of the lip (C00) and the oral cavity, which includes tongue (C01+C02), gum (C03), floor of the mouth (C04), palate (C05), and other regions of the oral cavity (C06). Malignant neoplasms of the salivary glands (C07–C08), oropharynx (C09, C10 and C14), and nasopharynx and hypopharynx (C11–C13) were not considered.
According to the tumor's degree of differentiation, the sample was divided into five groups: grade I (well differentiated); grade II (moderately differentiated); grade III (poorly differentiated); grade IV (undifferentiated/anaplastic), and undetermined.
The sample study according to the tumors’ stage was based on the staging system proposed by the Union for International Cancer Control (UICC), denominated TNM Classification of Malignant Tumors – 7th Edition. Thus, the “stage” parameter included six groups: stage 0 (TIS N0 M0), stage I (T1 N0 M0), stage II (T2 N0 M0), stage III (T3 N0 M0; T1 or T2 or T3 N1 MO), stage IV (any T4, or N2 or N3, or any M1), and undetermined.9
The collected data were stored in a database created in the Microsoft Excel® software (2007® version), to be statistically analyzed. Categorical variables were described as absolute frequencies (n) and relative frequencies (%), and continuous variables were described based on their mean and standard deviation. The statistical analysis was conducted using the Statistical Package for the Social Sciences – SPSS® software (version 23). A 95% confidence level was applied throughout the statistical analysis, and the chi-square hypothesis test was used to support the association of some variables.
The literature research was conducted using the National Library of Medicine's PubMed/MEDLINE database. The research included the period between 20 October 2010 and 5 April 2011 and the following keywords were used: squamous cell carcinoma, etiology, epidemiology, and oral cancer.
Our study was approved by the Ethics Commitee of the Faculty of Dental Medicine of the University Porto (FMDUP).
ResultsA total of 1041 cases of carcinoma of the oral cavity (C00–C06) were obtained, of which 807 cases (77.52%) were diagnosed in men and 234 (22.48%) in women. The most representative district in the sample was the Porto district, with a total of 247 cases (42.07%) (Figure 1).
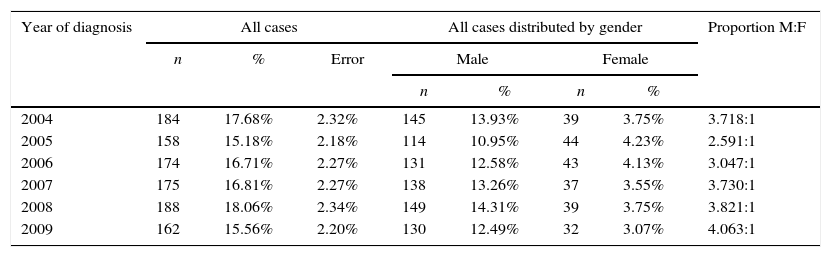
In the studied period (2004–2009), there was no statistical evidence indicating that the number of diagnosed cases would vary between years (p value=0.548>5%) (Table 1). The same finding was detected when analyzing the sample according to gender throughout the six years (p value=0.483>5%), %; i.e., there is no statistical evidence that the number of diagnosed cases in both genders develops differently throughout the studied years (Table 1). However, men were more affected by oral cancer than women, in a 3:1 proportion (Table 1). However, men were more affected by oral cancer than women, in a 3:1 proportion (Table 1).
Distribution of the sample by year of diagnosis and gender.
| Year of diagnosis | All cases | All cases distributed by gender | Proportion M:F | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | Error | Male | Female | ||||
| n | % | n | % | |||||
| 2004 | 184 | 17.68% | 2.32% | 145 | 13.93% | 39 | 3.75% | 3.718:1 |
| 2005 | 158 | 15.18% | 2.18% | 114 | 10.95% | 44 | 4.23% | 2.591:1 |
| 2006 | 174 | 16.71% | 2.27% | 131 | 12.58% | 43 | 4.13% | 3.047:1 |
| 2007 | 175 | 16.81% | 2.27% | 138 | 13.26% | 37 | 3.55% | 3.730:1 |
| 2008 | 188 | 18.06% | 2.34% | 149 | 14.31% | 39 | 3.75% | 3.821:1 |
| 2009 | 162 | 15.56% | 2.20% | 130 | 12.49% | 32 | 3.07% | 4.063:1 |
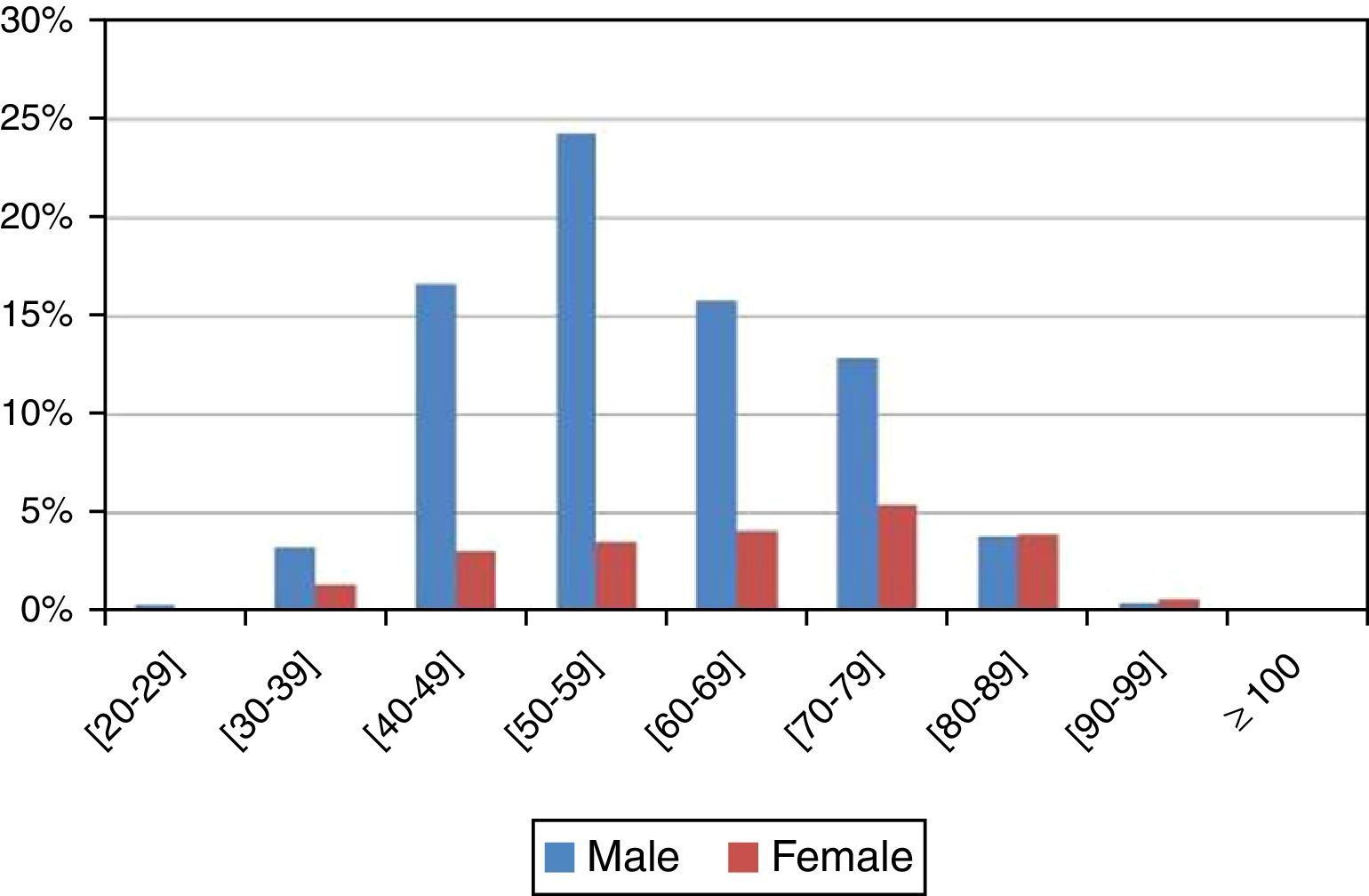
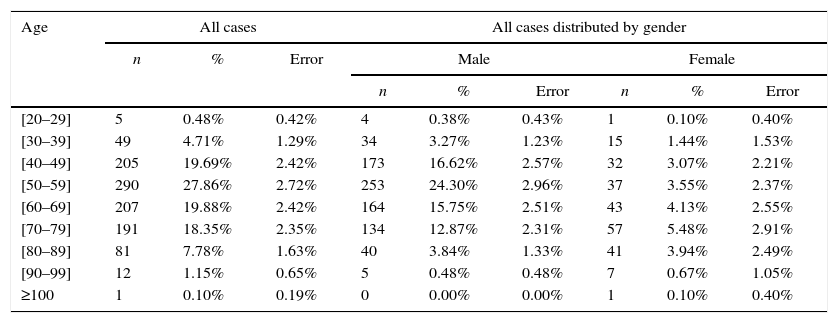
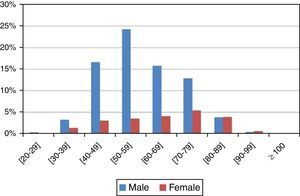
The average age was 60 years (standard deviation, 13.645). In men, diagnosed cases increased progressively after the age of 40 years (16.62%±2.57%), were more frequent between the 50 and 60 years of age (24.30%±2.96%), and decreased after the 70 years of age (15.75%±2.51%). On the other hand, in women, that finding was not observed (Figure 2 and Table 2). Statistically significant differences were found between gender and age (p value <0.001<5%).
Distribution of the sample by age and gender.
| Age | All cases | All cases distributed by gender | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | Error | Male | Female | |||||
| n | % | Error | n | % | Error | ||||
| [20–29] | 5 | 0.48% | 0.42% | 4 | 0.38% | 0.43% | 1 | 0.10% | 0.40% |
| [30–39] | 49 | 4.71% | 1.29% | 34 | 3.27% | 1.23% | 15 | 1.44% | 1.53% |
| [40–49] | 205 | 19.69% | 2.42% | 173 | 16.62% | 2.57% | 32 | 3.07% | 2.21% |
| [50–59] | 290 | 27.86% | 2.72% | 253 | 24.30% | 2.96% | 37 | 3.55% | 2.37% |
| [60–69] | 207 | 19.88% | 2.42% | 164 | 15.75% | 2.51% | 43 | 4.13% | 2.55% |
| [70–79] | 191 | 18.35% | 2.35% | 134 | 12.87% | 2.31% | 57 | 5.48% | 2.91% |
| [80–89] | 81 | 7.78% | 1.63% | 40 | 3.84% | 1.33% | 41 | 3.94% | 2.49% |
| [90–99] | 12 | 1.15% | 0.65% | 5 | 0.48% | 0.48% | 7 | 0.67% | 1.05% |
| ≥100 | 1 | 0.10% | 0.19% | 0 | 0.00% | 0.00% | 1 | 0.10% | 0.40% |
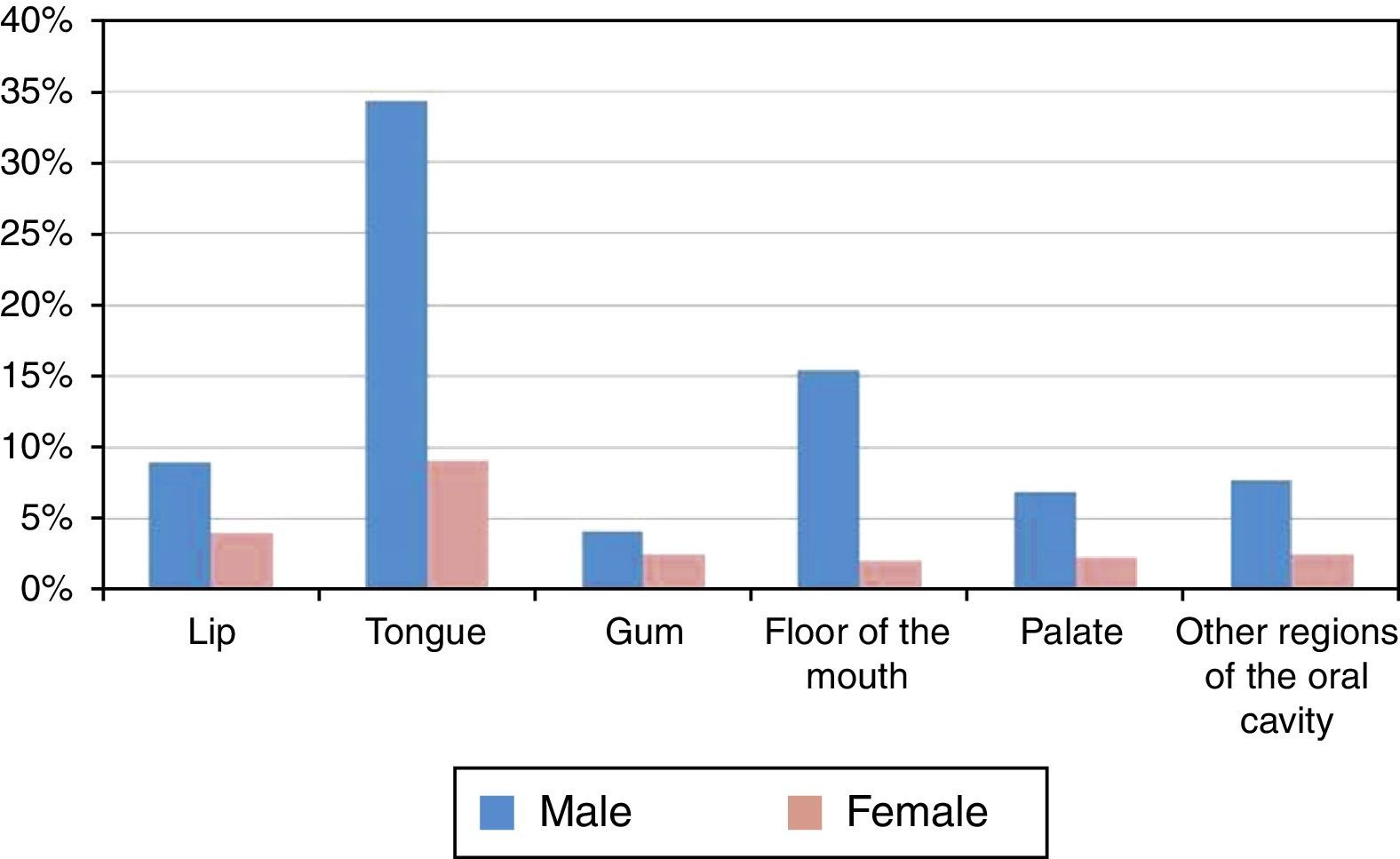
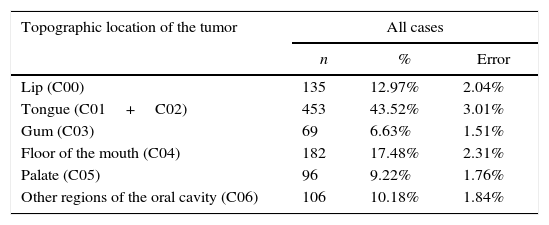
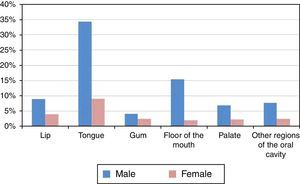
The most affected region of the oral cavity in for both genders, was the tongue (C01+C02), with a total of 453 cases (43.52%±3.01%). The least affected regions were the palate (C05) and the gum (C03), with 96 (9.22%±1.76%) and 69 cases (6.63%±1.51%) respectively (Figure 3 and Table 3).
Distribution of the sample by topographic location of the tumor.
| Topographic location of the tumor | All cases | ||
|---|---|---|---|
| n | % | Error | |
| Lip (C00) | 135 | 12.97% | 2.04% |
| Tongue (C01+C02) | 453 | 43.52% | 3.01% |
| Gum (C03) | 69 | 6.63% | 1.51% |
| Floor of the mouth (C04) | 182 | 17.48% | 2.31% |
| Palate (C05) | 96 | 9.22% | 1.76% |
| Other regions of the oral cavity (C06) | 106 | 10.18% | 1.84% |
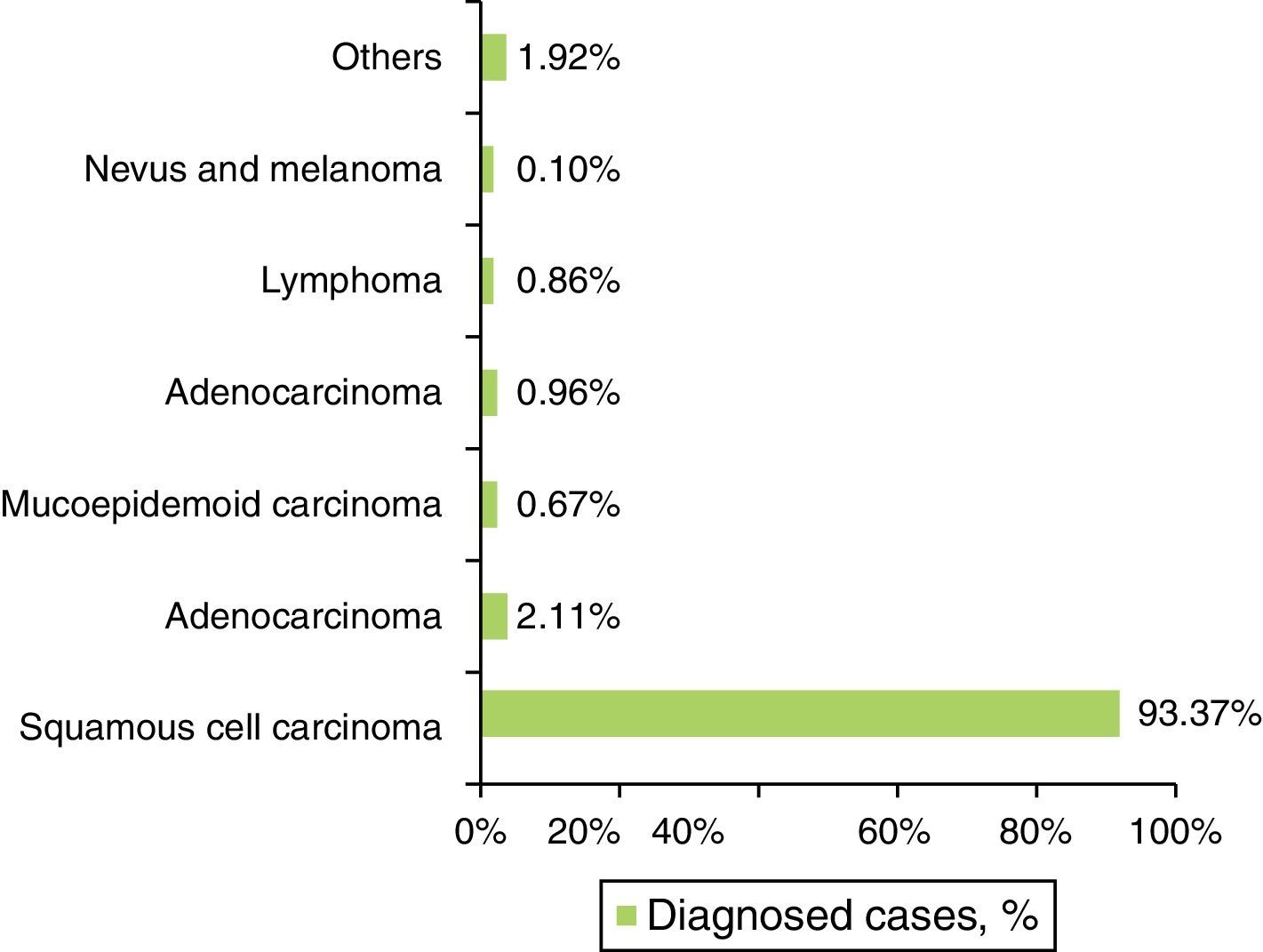
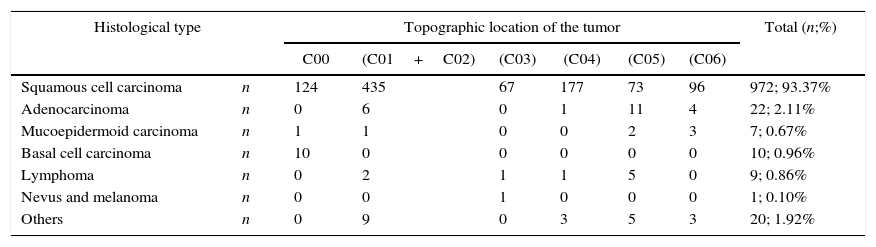
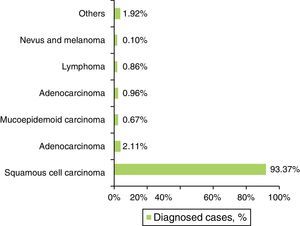
The SCC was the most prevalent histological type in every location of the oral cavity (C00–C06) in both genders, with 972 cases (93.37%±10.90%) (Figure 4 and Table 4). Identification of other histological types was scarce, representing 69 cases in total (6.63%±10.90%) (Figure 4 and Table 4). The basal cell carcinoma was only identified in the lip (C00). The palate (C05), unlike the gum region (C03), was the region with the widest variety of identified histological types (Table 4).
Distribution of the sample by histological type and topographic location of the tumor.
| Histological type | Topographic location of the tumor | Total (n;%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C00 | (C01+C02) | (C03) | (C04) | (C05) | (C06) | |||
| Squamous cell carcinoma | n | 124 | 435 | 67 | 177 | 73 | 96 | 972; 93.37% |
| Adenocarcinoma | n | 0 | 6 | 0 | 1 | 11 | 4 | 22; 2.11% |
| Mucoepidermoid carcinoma | n | 1 | 1 | 0 | 0 | 2 | 3 | 7; 0.67% |
| Basal cell carcinoma | n | 10 | 0 | 0 | 0 | 0 | 0 | 10; 0.96% |
| Lymphoma | n | 0 | 2 | 1 | 1 | 5 | 0 | 9; 0.86% |
| Nevus and melanoma | n | 0 | 0 | 1 | 0 | 0 | 0 | 1; 0.10% |
| Others | n | 0 | 9 | 0 | 3 | 5 | 3 | 20; 1.92% |
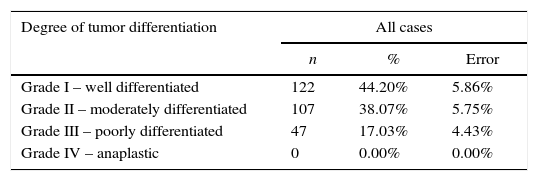
The degree of differentiation and the stage (TNM) of the tumor were analyzed taking into account only the 972 cases diagnosed with SCC. The sample analysis according to the degree of differentiation revealed that cases were well differentiated – grade I (44.20%±5.86%). However, the degree of tumor differentiation was undetermined in more than half of the studied cases (71.60%±2.83%) (Table 5).
Distribution of the sample by degree of tumor differentiation.
| Degree of tumor differentiation | All cases | ||
|---|---|---|---|
| n | % | Error | |
| Grade I – well differentiated | 122 | 44.20% | 5.86% |
| Grade II – moderately differentiated | 107 | 38.07% | 5.75% |
| Grade III – poorly differentiated | 47 | 17.03% | 4.43% |
| Grade IV – anaplastic | 0 | 0.00% | 0.00% |
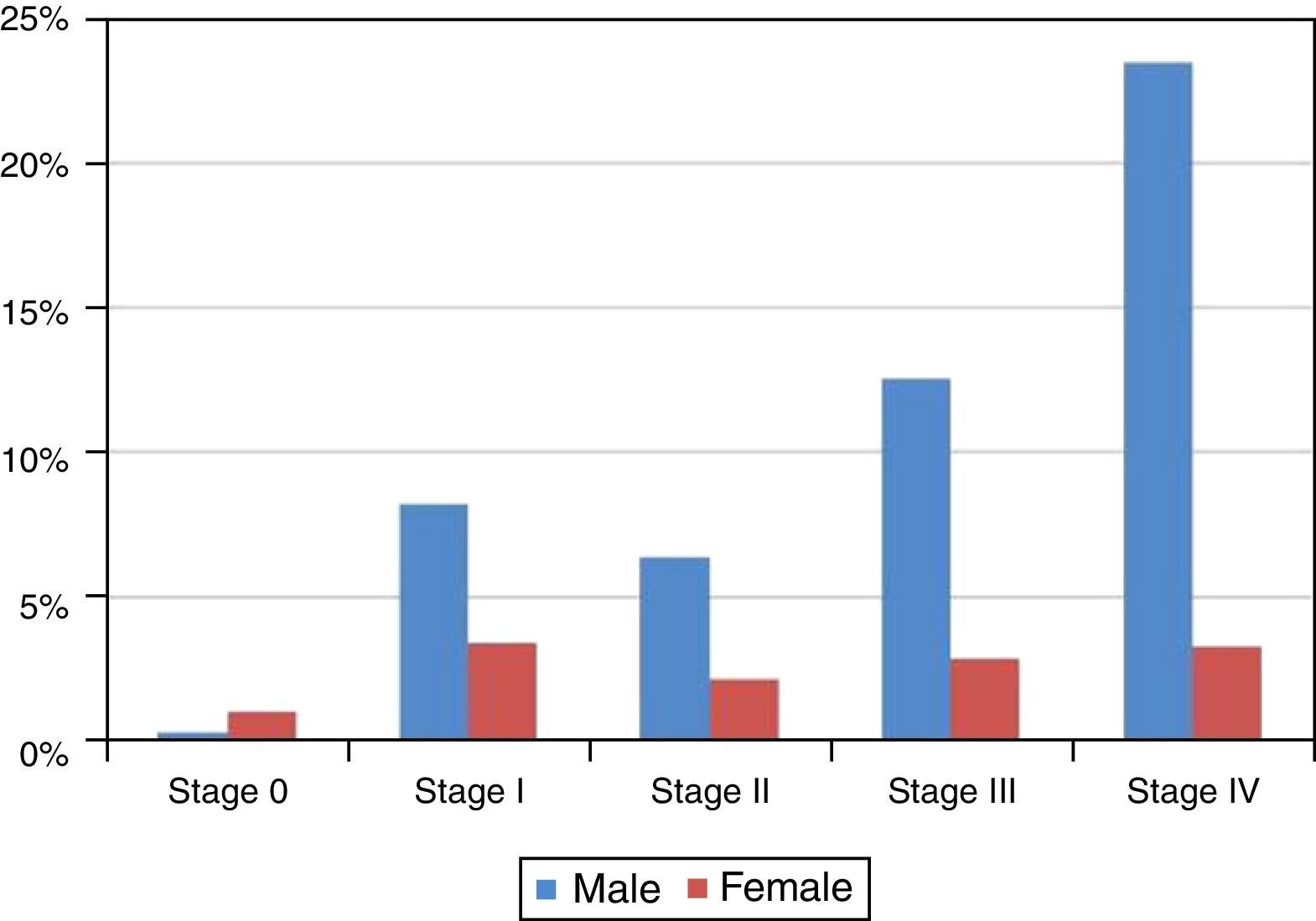
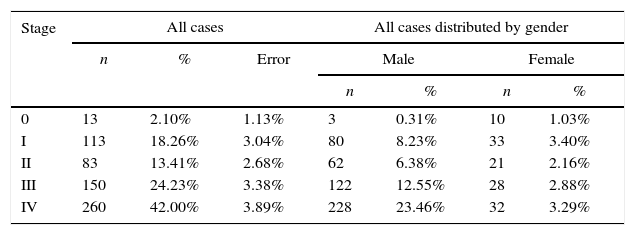
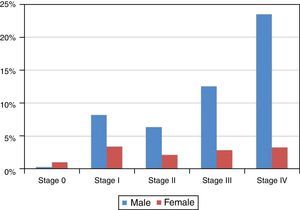
Although 36.32%±3.02% of the studied cases, the tumor stage was undetermined, as shown in Figure 5 and Table 6 most of the diagnosed cases in both genders concerned stages III (24.23%±3.38%) and IV (42.00%±3.89%) (Table 6). Only in stage 0 was the number of diagnosed cases higher in women than in men (Figure 5 and Table 6).
Distribution of the sample by stage of tumor and gender.
| Stage | All cases | All cases distributed by gender | |||||
|---|---|---|---|---|---|---|---|
| n | % | Error | Male | Female | |||
| n | % | n | % | ||||
| 0 | 13 | 2.10% | 1.13% | 3 | 0.31% | 10 | 1.03% |
| I | 113 | 18.26% | 3.04% | 80 | 8.23% | 33 | 3.40% |
| II | 83 | 13.41% | 2.68% | 62 | 6.38% | 21 | 2.16% |
| III | 150 | 24.23% | 3.38% | 122 | 12.55% | 28 | 2.88% |
| IV | 260 | 42.00% | 3.89% | 228 | 23.46% | 32 | 3.29% |
The study sample showed that there was only one case diagnosed in women for every three cases diagnosed in men, which indicates a proportion of 3:1. This disproportion in gender has been observed in numerous studies and justified an increased consumption of tobacco and alcohol by men.4,5 However, according to some authors, this proportion has become less pronounced due to an increased exposure to these factors by women.4,10
Other authors state that the difference ratio between genders can not be explained taking into account only the lifestyle of women, as carcinogenesis mechanism is complex and variable, and both genders have some characteristics in common, but women have a better defense mechanism due to their special hormonal characteristics.17–20
Less than 10% of cases, in general are family related, which means that over 90% are primarily caused by environmental influences, and oral cancer is no exception.6,7 Risk factors include smoking, alcohol consumption, and chronic and viral infections, these can lead to a wide range of genetic and molecular changes according to the intensity and duration of the stimulus.6,21,22 The average age of diagnosis in our study was 60 years, which may be justified by the fact that oral cancer arises as a result of the interaction of lifestyle and genetic susceptibility.4,6,7
However, about 6% of oral cancer cases occur in young patients (aged below 45 years), and, although this incidence is increasing, only some cases have been initially documented in Scotland and Denmark.4,5,23 Currently, that incidence has become a common finding in many countries in the European Union and some regions of the United States of America.5,23 Recent evidence has revealed that there is a subgroup of patients (about 25%) who are young adults and are not exposed to the traditional risk factors, however other factors may be involved in the process, such as infections.24–26 According to some authors, oral cancer in young patients may be a distinct disease from that occurring in older patients, with different etiology and progression.23–26 Some cases of oral cancer in young patients submitted to transplants have been reported where they had consequent chronic immunodeficiency, which may be a predisposing cause of oral cancer; however, the incidence of oral cancer in these patients appears to be very low.26 Women's hormonal defenses may explain the appearance of oral cancer at older ages comparison with men.19 The consumption of tobacco and alcoholic beverages from an earlier age in men may also be one of the causes for that difference.27
Oral cancer is a heterogeneous disease, occurring in various regions and sub-regions.28 In the published literature, few studies have identified the same oral regions and sub-regions.29,30
However, we were able to compare our study results with four published studies and verified that the final results are quite similar.28,29,31,32,11
In our study, the SCC was the most representative histological type in every location of the oral cavity (C00 to C06), and there was no predilection for any anatomical region, which is consistent with the literature.11,29
The basal cell carcinoma was identified only in the lip region (C00). This finding is consistent with the fact that it is the most common type of skin cancer, and 85% of its cases occur in the head and neck as a result of chronic exposure to ultraviolet radiation.11,16,23,29
The mucoepidermoid carcinoma is the most common malignant tumor of the salivary glands, and affects mostly the minor salivary glands. This malignancy appears typically on the palate but may also appear in other locations of the oral cavity such as the retromolar area and the oral mucosa.16,33,34 Similarly, the adenocarcinoma is a malignant tumor that originates in the glandular epithelium and affects mainly the minor glands of the palate and other regions of the oral cavity such as the retromolar area and the oral mucosa.16,33,34 Therefore, the palate region (C05) is the region of the oral cavity with a wider variety of identified histological types.
Lymphoma is a general term for a group of malignancies arising in the lymphoreticular system. In the oral cavity, lymphomas represent 5% of all malignancies and approximately 85% of their lesions involve the palate.20,29 The results of our study are thus in agreement with the literature since the majority of lymphomas was diagnosed in the palate. We also found that lymphomas appeared more frequently at younger ages (around 20 years) than other histological types, which is consistent with the study of Epstein JB, et al. (2001), who found 361 cases of patients with lymphoma in the oral cavity.35
In the literature, most of the reported SCC were grade I or II, thus maintaining a similar structure to that of the tissue of origin.16 In our study, most of the diagnosed cases were well differentiated (grade I) followed by moderately differentiated (grade II). These results are in accordance with several published studies.9,36,37
Regarding the tumors’ stage, studies by IU Doobaree et al.,31 Costa A de et al.,38 and Huang et al.37 reported that most cases were in stages III and IV, which is consistent with our results.31,37,38
ConclusionThe epidemiological profile of malignant oral tumors in the IPO-Porto sample is characterized by men having more diagnosed cases than women, in a 3:1 proportion.
In the study period (2004–2009), there was no statistical evidence indicating that the number of diagnosed cases would vary between years.
The number of diagnosed cases increased after the 40 years of age (19.69%±2.42%) and progressively decreased after the 70 years of age (18.35%±2.35%).
The most affected regions were the tongue (C01+C02) and the floor of the mouth (C04), and we verified that injuries in those regions were diagnosed at late stages (III and IV), which worsens the prognosis of the patient.
The results obtained in this study are similar to those of studies found in the literature.
It would be interesting to conduct further studies on the same subject that would include a follow-up of these patients and the assessment of other variables. More epidemiological studies should be conducted in this area in Portugal, thus enabling the development and planning of social policies and future health programs.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.