To evaluate the effect of ethanol solutions as post-polymerization treatment on the shear bond strength and the surface free energy of acrylic reline resins.
MethodsThree reline resins (Kooliner, Ufi Gel Hard and Probase Cold) were manipulated and attached to 150 parallelepipeds denture base resin previously aged. Constructed specimens of each resin were randomly divided into control group (left untreated) or experimental groups subjected to different treatments: immersion in water or ethanol solutions 20, 50 or 70% at 55°C for 10min (n=10). Shear bond strength was tested and the failure mode was assessed. Surface free energy was calculated by determining the contact angle and estimated by the Wilhelmy plaque technique (n=5). Data were analyzed using Kruskal–Wallis and Mann–Whitney tests with Bonferroni correction (alfa=0.05).
ResultsProbase Cold showed higher values (p<0.001) in shear bond strength than other resins. There were no statistically significant differences (p=0.378) in shear bond strength between post-polymerization treatments. Kooliner showed lower values (p<0.001) in surface free energy than other resins. Considering the post-polymerization treatment groups, there were no statistically significant differences (p>0.05) in surface energy.
ConclusionsEthanol solutions as post-polymerization treatments did not deteriorate the bond strength of acrylic reline resins to denture base and neither their wettability.
Avaliar o efeito do tratamento pós-polimerização com soluções de etanol na resistência adesiva a tensões de corte e na energia de superfície de resinas acrílicas de rebasamento.
MétodosCento e cinquenta paralelepípedos de resina de base protética, previamente envelhecidos, foram unidos a uma de 3 resinas de rebasamento (Kooliner, Ufi Gel Hard e Probase Cold). Os espécimes de cada resina foram aleatoriamente distribuídos por 5 grupos conforme o tratamento pós-polimerização: controlo (sem tratamento), imersão em água ou em soluções aquosas de etanol a 20, 50 ou 70% a 55°C durante 10 minutos (n=10). Foram realizados testes de resistência adesiva e o tipo de falha foi determinado. A energia de superfície foi calculada através da determinação dos ângulos de contacto pela técnica da placa de Wilhelmy (n=5). Os resultados foram analisados com testes Kruskal–Wallis e Mann–Whitney com correção Bonferroni (alfa=0,05).
ResultadosOs valores de resistência adesiva obtidos com Probase Cold foram estatisticamente superiores (p<0,001) aos valores encontrados nas restantes resinas testadas. Não foram encontradas diferenças estatisticamente significativas (p=0,378) entre os valores de resistência para os diferentes tratamentos realizados. Kooliner apresentou valores de energia de superfície estatisticamente inferiores (p<0,001) aos das outras resinas. Entre os diferentes tratamentos pós-polimerização, não foram encontradas diferenças estatisticamente significativas (p>0,05) de energia de superfície.
ConclusõesAs soluções de etanol como tratamento pós-polimerização não afetam a adesão entre as resinas de rebasamento e a resina para base da prótese, nem a molhabilidade das mesmas.
In clinical practice, removable prostheses may require periodic relining with autopolymerizing acrylic reline resins. It can be done in laboratory (indirect technique) or directly in mouth (direct technique).1–5
During the polymerization of the acrylic resins, the conversion of monomers to polymers is never complete and some unpolymerized monomers remain within the material.6–9 These residual monomers can affect the mechanical and physical properties of the biomaterial or cause undesirable biological reactions when leached to the oral environment.7,8 Post-polymerization treatments that decrease the residual monomer content have become relevant.7 Recent studies showed that immersion of acrylic resins in water at high temperatures8,10,11 or submitting it to microwave radiation6,12–14 were effective treatments to reduce residual monomer. With the same goal, it has been proposed the immersion of polymeric materials in ethanol.15,16 Since water immersion treatment is dependent on temperature,11 promoting an additional polymerization of the resins and a decrease of residual monomer content,7,12,17 possible benefits of the interaction between ethanol aqueous solutions and temperature have been suggested.7
Under experimental conditions, a post-polymerization treatment based on a combination approach of ethanol–water solutions and temperature (55°C) for 10min, enables the reduction of the monomer content and cytotoxicity of acrylic reline resins.7 In this study, it was also showed that microhardness and flexural strength were not affected by the proposed treatments.7 However, there are other surface properties that are crucial for adequate performance of dentures. The effect of these post-polymerization treatments on the bond strength between acrylic reline resins and denture base and the surface free energy of the reline resins has not been investigated.
Adequate bond strength between the denture base and reline resins is essential for successful clinical performance.4,5,18 A weak bond can result in adhesive failure under low stress4,5 that could result in debonding between the two materials and gap formation with ingress of bacteria and fungus and promote staining.4,5,19,20
Surface free energy strongly influences the wettability of relining materials which is one of the most important factor that influences the denture retention.21 Also, along other surface properties such as hardness and roughness, surface free energy contributes to the adherence, bonding and colonization of fungal species.22–26
The purpose of this study was to evaluate the effect of post-polymerization treatment with several ethanol solutions on the shear bond strength (SBS) between acrylic reline resins and a denture base resin, and on the surface free energy of the reline resins, according to the following null hypotheses: (1) the acrylic reline resins used do not influence the SBS to denture base resin; (2) post-polymerization treatment does not affect the adhesion of reline resins to denture base; (3) there are no differences between the surface free energy of the acrylic reline resins studied; and (4) the surface free energy of reline resins is not affected by the post-polymerization treatment.
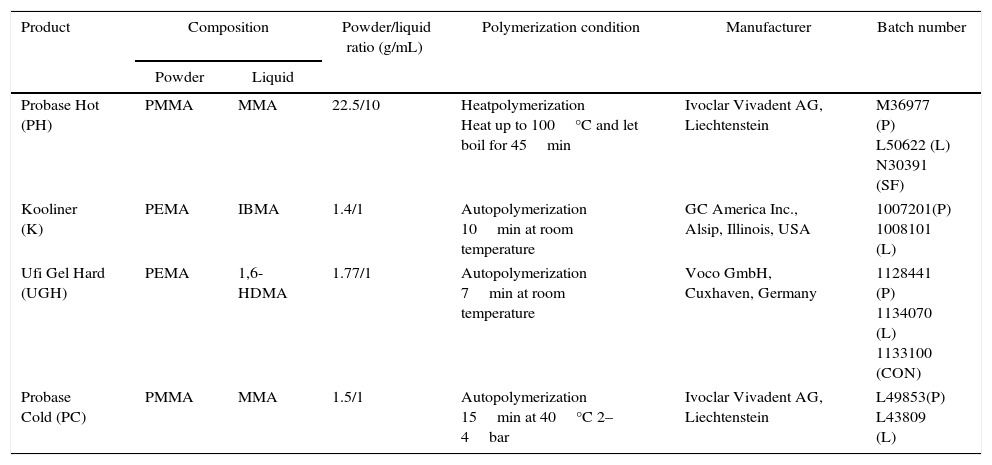
Materials and methodsMaterials used in this study included one heat-polymerizing denture base resin, Probase Hot, and three autopolymerizing acrylic reline resins, Kooliner, Ufi Gel Hard and Probase Cold. Two of the relining materials could polymerized in mouth (direct technique) and the other should polymerized under laboratory conditions (indirect technique) (Table 1).
Materials used in the study.
| Product | Composition | Powder/liquid ratio (g/mL) | Polymerization condition | Manufacturer | Batch number | |
|---|---|---|---|---|---|---|
| Powder | Liquid | |||||
| Probase Hot (PH) | PMMA | MMA | 22.5/10 | Heatpolymerization Heat up to 100°C and let boil for 45min | Ivoclar Vivadent AG, Liechtenstein | M36977 (P) L50622 (L) N30391 (SF) |
| Kooliner (K) | PEMA | IBMA | 1.4/1 | Autopolymerization 10min at room temperature | GC America Inc., Alsip, Illinois, USA | 1007201(P) 1008101 (L) |
| Ufi Gel Hard (UGH) | PEMA | 1,6-HDMA | 1.77/1 | Autopolymerization 7min at room temperature | Voco GmbH, Cuxhaven, Germany | 1128441 (P) 1134070 (L) 1133100 (CON) |
| Probase Cold (PC) | PMMA | MMA | 1.5/1 | Autopolymerization 15min at 40°C 2–4bar | Ivoclar Vivadent AG, Liechtenstein | L49853(P) L43809 (L) |
P=powder, L=liquid, SF=separating fluid, CON=conditioner, PMMA=polymethylmethacrylate, MMA=methylmethacrylate, PEMA=polyethylmethacrylate, IBMA=isobutylmethacrylate, HDMA=hexanedioldimethacrylate.
A modified flasking technique was used to make 150 denture base parallelepipeds (12mm×10mm×6mm) according to manufacturer's instructions (Table 1). To remove irregularities, their sides were grounded in a rotational grinding and polishing machine (DAP-U, Struers, Denmark) with 600-grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., Lake Bluff, IL).27,28 All specimens were submitted to a standardized thermal cycling aging procedure (2500 cycles, 5–55°C) (Refri 200-E, Aralab, Cascais, Portugal) to simulate 3 months of intraoral condition.29
Using the same grinding and polishing machine, surfaces of denture base specimens were finished to a 3mm thickness, to simulate the preparation of the denture base to be relined. The thickness was confirmed with digital micrometer (Mitutoyo Digimatic, Mfg. Co., Ltd. Tokyo, Japan) with precision ±0.01mm.
The denture base specimens were randomly divided into three groups, corresponding to the three different acrylic reline resins. A perforated adhesive tape (Glossy White Film EA, Xerox) was positioned on the center of the surface of denture base providing a customized and uniform bonding area (3mm in diameter). As recommended by the manufacturer, specific adhesive was applied on this area and let dry for 30s after Ufi Gel Hard was used. With the Kooliner or Probase Cold specimens, the bonding areas were wetted with the corresponding monomer. Then, a silicon mold with a circular hole (5mm internal diameter×3mm height) was placed on the adhesive tape and filled with acrylic reline resin. Each acrylic reline resin was mixed and applied according to the manufacturer's instructions (Table 1). Polymerization of direct relining materials was carried out at 37°C to simulate the temperature of the oral cavity during the specific time. A pressure device (Ivomat, Ivoclar Vivadent, Liechtenstein) was used to maintain the indirect relining material under 40°C and 2–4bar for 15min.
The 50 constructed specimens of each reline resin were randomly divided into five groups (n=10) determined by post-polymerization treatment: 5mL of water or ethanol/water solutions of 20, 50 and 70% (by volume) at 55±2°C in closed plastic flasks for 10min. Control specimens of the reline resins were exposed to dry conditions at room temperature (no treatment).
After submitted to the post-polymerization treatment, specimens were stored in distilled water at 37±2°C for 48±2h in an incubator (Memmert, Schwabach, Germany) before SBS tests.
SBS was determined using a universal test machine (Instron model 4502, Instron Ltd, Bucks, England) with 1kN load cell and a crosshead speed of 1mm/min until fracture.
Fracture surfaces were analyzed with a stereomicroscope (EMZ-8TR, Meiji Techno Co. Lda, Saitama, Japan) and the failure mode was classified by 2 independent observers as: adhesive, if the failure occurred at the adhesive interface; cohesive, when failure occurred within acrylic reline resin; or mixed, when a combination of adhesive and cohesive failure was observed.
For the determination of surface free energy, 25 rectangular specimens of Kooliner, Ufi Gel Hard and Probase Cold (24mm×18mm×1mm) were obtained from cured strips in rectangular metal molds. The edges of each sample were polished manually with 600-grit silicon carbide paper (Carbimet Paper Discs, Buehler Ltd., Lake Bluff, IL). The specimens were randomly divided into the same five groups (n=5) of post-polymerization treatment and were stored in distilled water at 37±2°C for 48±2h in an incubator (Memmert, Schwabach, Germany) before measuring the contact angle and surface free energy.
Assays were made with a Kruss K12 tensiometer (Kruss GMBH, Hamburg, Germany) using the Wilhelmy Plate method by immersing plates into the test liquids, water and 1,2-propanediol, at a speed of 3mm/min, at 25±0.1°C. Advancing contact angles were used for surface energy (γ) estimation of the BC matrices, as well as its dispersive (γd) and polar components (γp) based on the harmonic mean method.30 At least five plates were independently tested. Equations for surface tension estimation were solved using the equation handling KRUSS-software program: contact angle measuring system K12 (version 2.05).
Data were analyzed using SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA). As normal distribution was not verified (Shapiro–Wilk test, p<0.001), data were submitted to nonparametric tests according to Kruskall–Wallis method followed by multiple comparisons using Mann–Whitney tests with Bonferroni correction. In all statistical tests, it was considered the 5% level of significance (α=0.05).
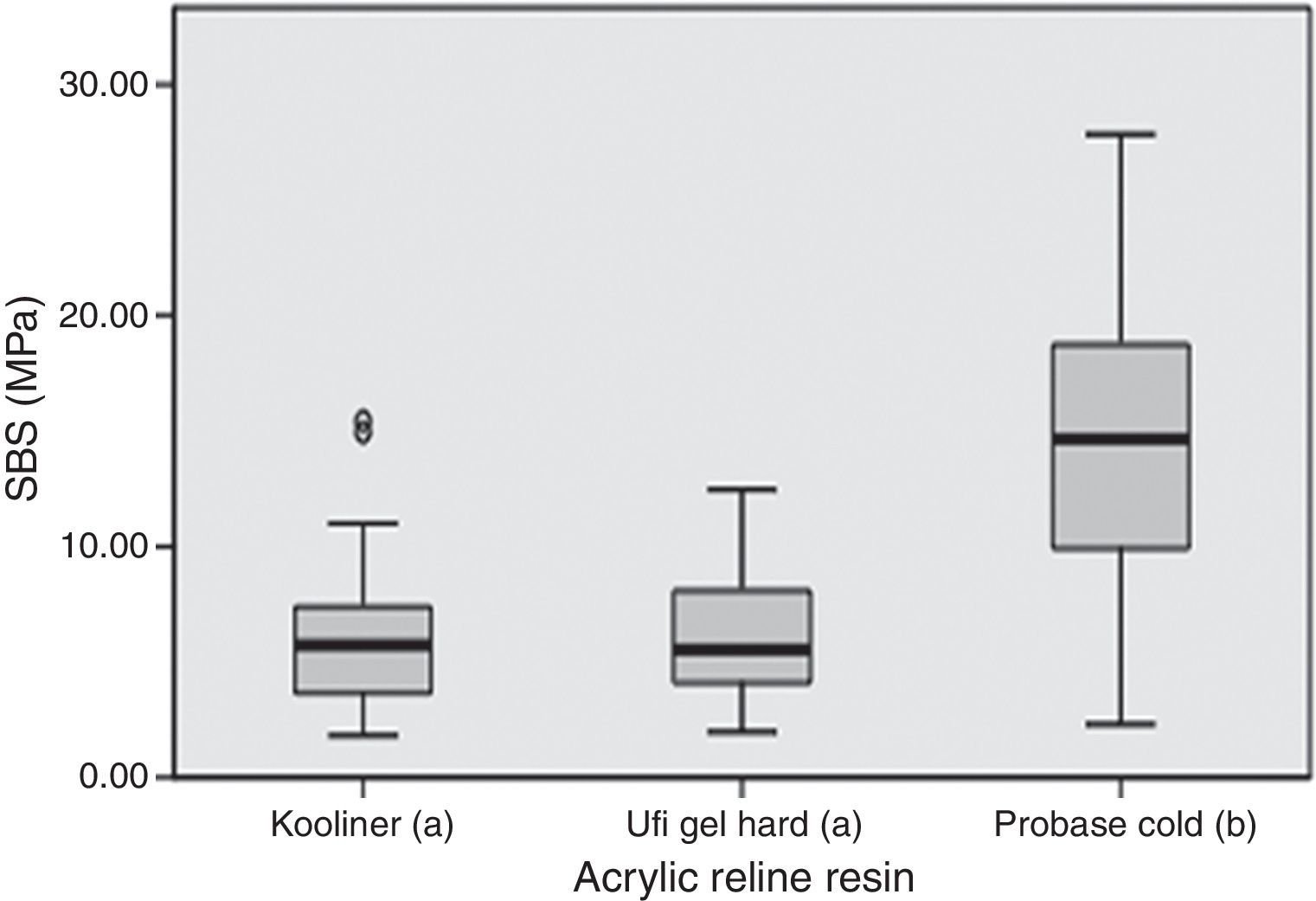
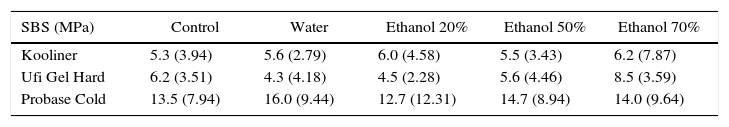
ResultsSBS ranged between 4.3MPa, observed in Ufi Gel Hard specimens with water post-polymerization treatment, and 16.0MPa found in Probase Cold specimens with the same treatment (Table 2). Only adhesives failures were observed.
SBS median (interquartile range) according to the three acrylic reline resins and the five post-polymerization treatments.
| SBS (MPa) | Control | Water | Ethanol 20% | Ethanol 50% | Ethanol 70% |
|---|---|---|---|---|---|
| Kooliner | 5.3 (3.94) | 5.6 (2.79) | 6.0 (4.58) | 5.5 (3.43) | 6.2 (7.87) |
| Ufi Gel Hard | 6.2 (3.51) | 4.3 (4.18) | 4.5 (2.28) | 5.6 (4.46) | 8.5 (3.59) |
| Probase Cold | 13.5 (7.94) | 16.0 (9.44) | 12.7 (12.31) | 14.7 (8.94) | 14.0 (9.64) |
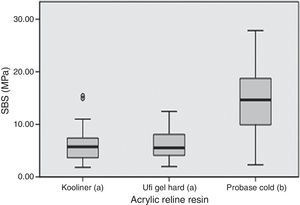
SBS was significantly (p<0.001) influenced by the acrylic reline resin used (Figure 1). Probase Cold specimens yielded higher (p<0.001) bond strength than the specimens made with Kooliner or Ufi Gel Hard. However, no significant differences were found (p=0.378) between SBS observed among the several post-polymerization treatments (Figure 2).
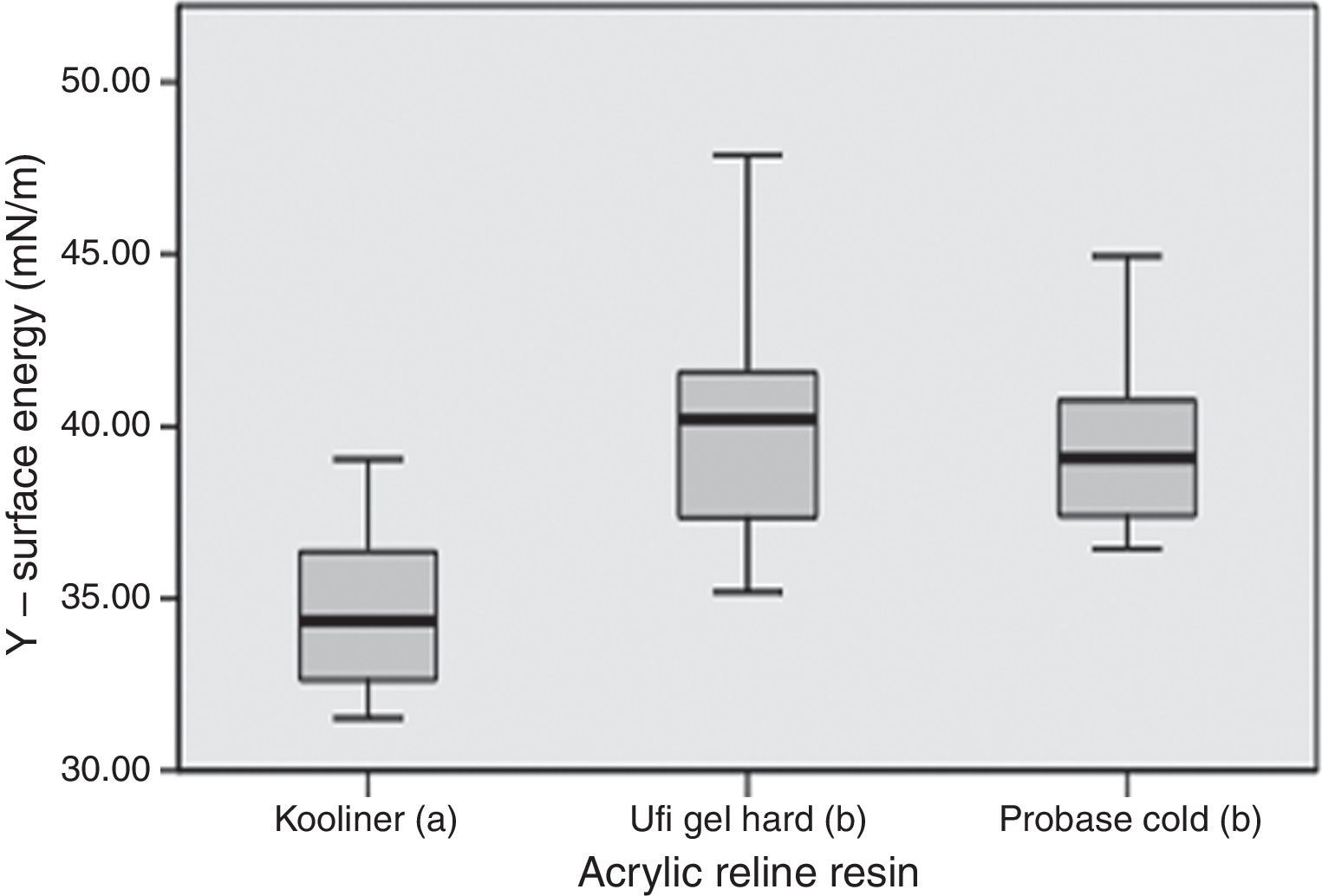
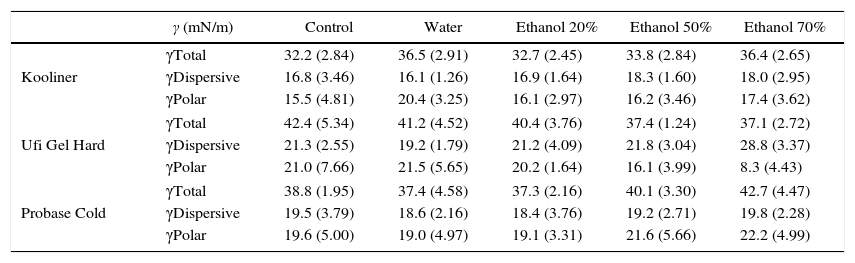
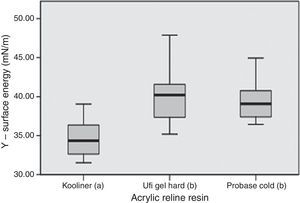
Total surface free energy ranged between 32.2mN/m, observed in Kooliner control group, and 42.7mN/m for Probase Cold specimens submitted to ethanol 70% post-polymerization treatment (Table 3).
Surface free energy median (interquartile range) according to the three acrylic reline resins and the five post-polymerization treatments.
| γ (mN/m) | Control | Water | Ethanol 20% | Ethanol 50% | Ethanol 70% | |
|---|---|---|---|---|---|---|
| Kooliner | γTotal | 32.2 (2.84) | 36.5 (2.91) | 32.7 (2.45) | 33.8 (2.84) | 36.4 (2.65) |
| γDispersive | 16.8 (3.46) | 16.1 (1.26) | 16.9 (1.64) | 18.3 (1.60) | 18.0 (2.95) | |
| γPolar | 15.5 (4.81) | 20.4 (3.25) | 16.1 (2.97) | 16.2 (3.46) | 17.4 (3.62) | |
| Ufi Gel Hard | γTotal | 42.4 (5.34) | 41.2 (4.52) | 40.4 (3.76) | 37.4 (1.24) | 37.1 (2.72) |
| γDispersive | 21.3 (2.55) | 19.2 (1.79) | 21.2 (4.09) | 21.8 (3.04) | 28.8 (3.37) | |
| γPolar | 21.0 (7.66) | 21.5 (5.65) | 20.2 (1.64) | 16.1 (3.99) | 8.3 (4.43) | |
| Probase Cold | γTotal | 38.8 (1.95) | 37.4 (4.58) | 37.3 (2.16) | 40.1 (3.30) | 42.7 (4.47) |
| γDispersive | 19.5 (3.79) | 18.6 (2.16) | 18.4 (3.76) | 19.2 (2.71) | 19.8 (2.28) | |
| γPolar | 19.6 (5.00) | 19.0 (4.97) | 19.1 (3.31) | 21.6 (5.66) | 22.2 (4.99) | |
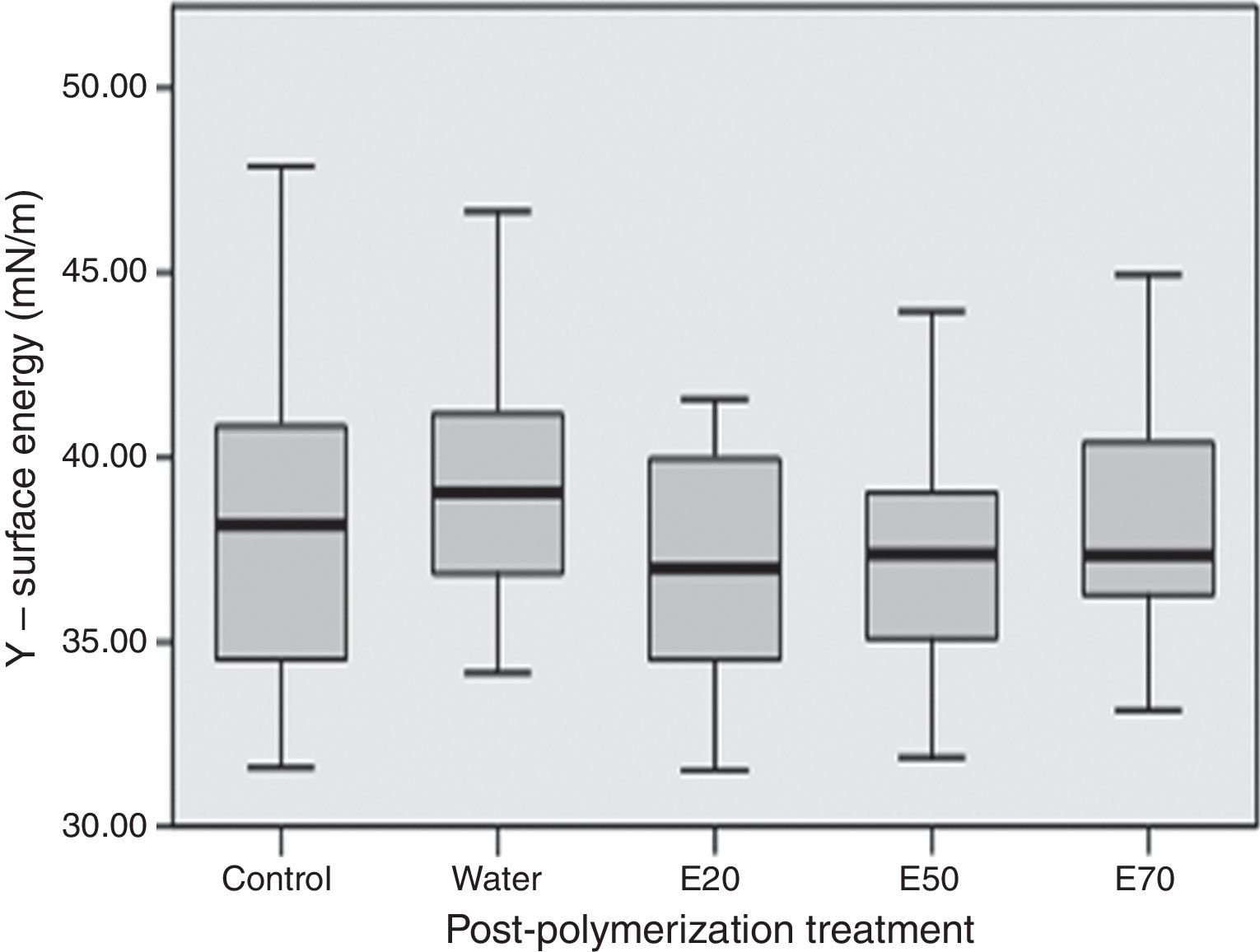
Statistically significant (p<0.001) differences were found between acrylic reline resins (Figure 3). Kooliner's total surface free energy and polar component was significantly (p<0.001) lower than Ufi Gel Hard and Probase Cold, and no differences were found (p>0.05) between these two. No differences between post-polymerization treatments were found when total surface free energy was studied (p>0.05) (Figure 4).
Since ethanol post-polymerization treatment was considered an easy and effective treatment to reduce residual monomer in polymeric biomaterials and therefore to decrease potential toxicity,7 it was important to test the effects of this treatment on the unstudied properties of acrylic reline resins, bond strength to the denture base and the surface free energy.
In order to full field this requirement, one objective of the present work was to evaluate the effect of ethanol solutions as post-polymerization treatment on the bond strength between three acrylic reline resins (Kooliner, Ufi Gel Hard and Probase Cold) and a denture base resin (Probase Hot).
Shear bond strength test has been widely used in acrylic resins, since it represents a shear load directly to the reline-denture base polymer interface and therefore considered more accurate to what happens in the oral cavity compared to the tensile load test.5,27,31–34
In the present study, Probase Cold showed significant higher bond strength compared to the other resins. This result may be explained by the similar chemical composition of the indirect reline resin Probase Cold and the denture base resin, based on polymethylmethacrylate (PMMA) polymer and both having methylmethacrylate (MMA) as the monomer. Similar result was already found in earlier studies since PMMA based reline resin yielded a higher bond strength to PMMA based denture base resin than non-PMMA-based reline resin.27,35 Direct reline resins, like Kooliner and Ufi Gel Hard, have a different chemical composition than the denture base resin, since are based on polyethylmethacrylate (PEMA). These findings corroborate that bond strength is dependent on the chemical composition of both materials.18,27,31,33 Bonding of chemically activated reline resins to denture base resin seems to be achieved by penetration and diffusion of monomer into denture base resin polymeric matrix. As so, a monomer with smaller molecular weight (like MMA monomer with a molar mass of 100gmol−1 and present in Probase Cold) may be advantageous for bonding then a heavier monomer (like 1,6-HDMA monomer with a molar mass of 254gmol−1 present in Ufi Gel Hard).18 This fact suggests that greater crosslinking occurred between similar base materials. Another monomer with higher molecular weight than MMA is IBMA (142gmol−1), monomer available in Kooliner, which might have limited monomer penetration.27 This supported the theory that, when compared with conventional polymers based on methylmethacrylate, the bond strength of hard denture reline resins could not be so effective because of the low penetration of the monomers with relatively greater molecular weight.20,36 At this point, it may be concluded that the first null hypothesis of this study concerning the non-influence of the acrylic reline resins used on the SBS to denture base resin can be rejected.
Previous studies showed that surface properties, as hardness, are not influenced by post-polymerization treatments with hot water, microwave irradiation11,37 or ethanol solutions at high temperature.7 Similar results were obtained in the present study since no differences were found between post-polymerization treatments on bond strenght, which represents a relevant surface property. As so, the second null hypothesis of this study concerning that the post-polymerization treatments do not affect the adhesion of reline resins to denture base cannot be rejected.
Another way to characterize a solid surface is by its surface free energy values calculated by measuring the contact angle between the material and liquids with different polarity as water and 1,2-propanediol. Changes in the surface energy of the materials will directly impact their wettability. The retention and stability of removable dentures are dependent on the wettability of denture materials because it provides a condition in which saliva will easily spread over the surfaces.21,38,39
In the present study, the total surface free energy of Kooliner specimens had lower levels than Ufi Gel Hard and Probase Cold specimens. This can be possibly explained because of differences in the polymeric structure and polymerization of the resins. Kooliner undergoes a rapid polymerization reaction and solidifies quickly. It is likely that air voids are entrapped during mixing of the power and liquid components, which result in a porous structure on the surface.11,12,17 According to others studies, beyond the surface chemistry, wettability of a substrate is sensitive to the topographical texture40 and this parameter must be considered when surface free energy data are evaluated.41 Low contact angle indicates high surface free energy and good wettability and therefore the retention would be expected to be greater.21,41–43 As the contact angle increases, the surface free energy diminish and wettability decreases.21 Poor wettability showed in Kooliner specimens in the present study may lead to frictional problems and patient discomfort.21,44 At this point it may be concluded that the third hypothesis of this study which reflects no differences between materials can be rejected.
In the present study, there were no differences in surface free energy, and their components, between specimens submitted to ethanol solutions as post-polymerization treatment. On previous studies, hot water and microwave irradiation, showed no effect on these surface properties.11,37 This result is similar to those found by who demonstrated that ethanol does not considerably change the wettability properties of the PMMA polymer.16 Therefore, as there were no differences, none of the ethanol post-polymerization treatments evaluated in this study affect the lubrification around the relining denture. At this point it may be concluded that the fourth hypothesis of this study that surface energy is not affected by the post-polymerization treatments cannot be rejected.
Along with other surface properties such as hardness and roughness, surface free energy contributes to the adherence, bonding and colonization of fungal species. Oral candidiasis associated with prosthetic surfaces is by far considered the most common fungal infection in denture wearers and Candida albicans species being the primary etiological agent associated with this infection.22–26 The effect of the surface energy on Candida albicans adhesion to these materials remains to be investigated. Other parameters must be evaluated in the future, such as the surface roughness and microbiological assays. Furthermore, it is important to note that information obtained from this test is limited and in future this should be complemented with other surface analysis technique, the X-ray photoelectron spectroscopy (XPS), for example.
In respect to different ethanol post-polymerization treatments, the work of 2013 that proposed the immersion of Kooliner on 50% ethanol solution at 55°C during 10min and of Ufi Gel Hard on 20% ethanol at 55°C during 10min7 remains to be feasible, because not only enable the reduction of the monomer content and the biological effects, but also allows to maintain their properties, like microhardness, flexural strength, shear bond strength and surface free energy. This is a simple method and easy to achieve with equipment in a dental office to improve the biocompatibility of resins.
ConclusionsDespite there were some differences between the acrylic reline resins used, neither the bond strength nor the surface free energy were affected by the ethanol solutions studied.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank VOCO GmbH (Cuxhaven, Germany) for the donation of the Ufi Gel Hard material evaluated in this study.




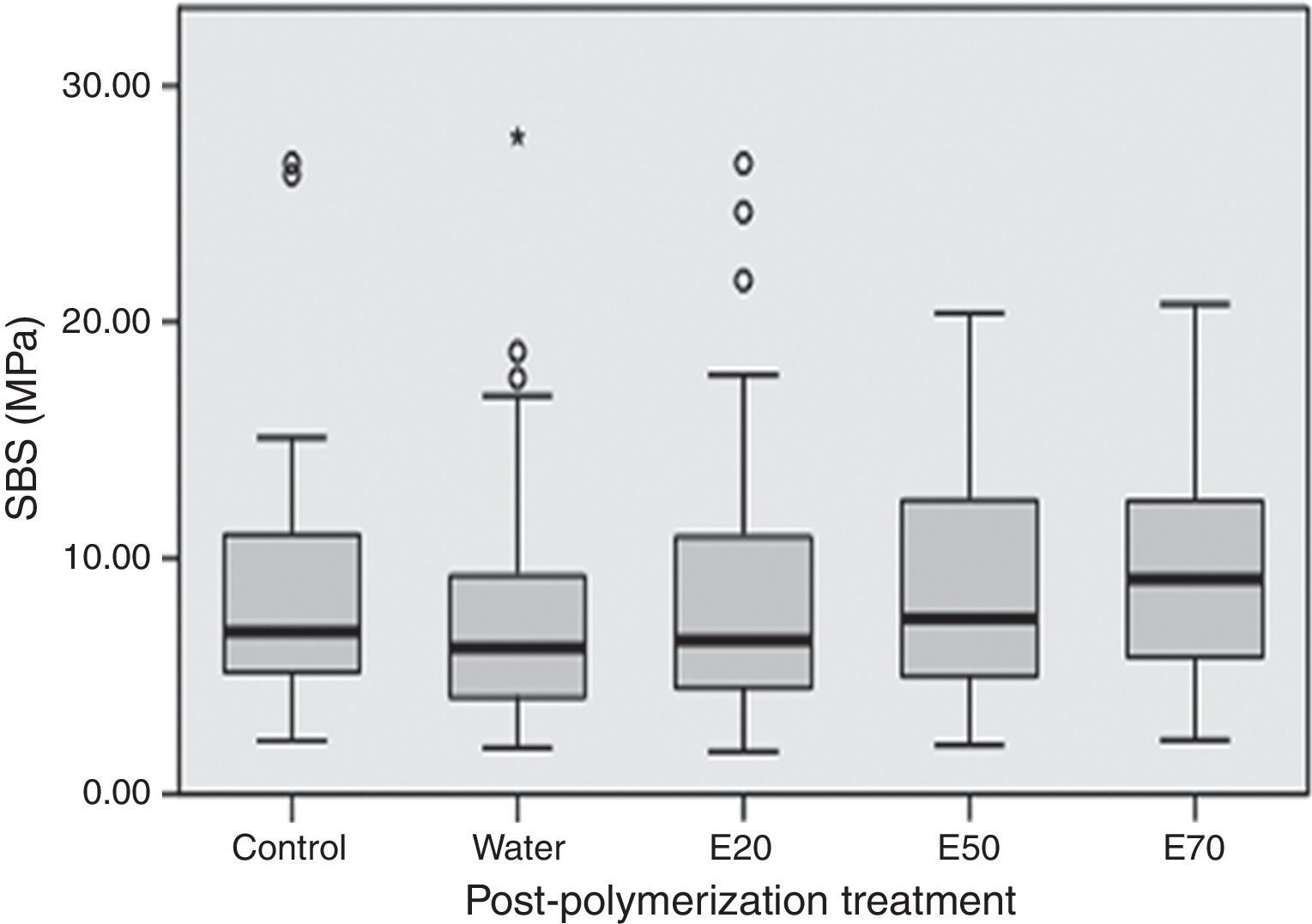
![Influence post-polymerization treatment on the shear bond strength (MPa). No significant differences were found between groups (p=0.378). [E20 – ethanol/water solution of 20%; E50 – ethanol/water solution of 50%; E70 – ethanol/water solution of 70%]. Influence post-polymerization treatment on the shear bond strength (MPa). No significant differences were found between groups (p=0.378). [E20 – ethanol/water solution of 20%; E50 – ethanol/water solution of 50%; E70 – ethanol/water solution of 70%].](https://static.elsevier.es/multimedia/16462890/0000005700000004/v1_201612030115/S1646289016301844/v1_201612030115/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Influence post-polymerization treatment on the total surface free energy (mN/m). No significant differences were found between groups (p=0.499). [E20 – ethanol/water solution of 20%; E50 – ethanol/water solution of 50%; E70 – ethanol/water solution of 70%]. Influence post-polymerization treatment on the total surface free energy (mN/m). No significant differences were found between groups (p=0.499). [E20 – ethanol/water solution of 20%; E50 – ethanol/water solution of 50%; E70 – ethanol/water solution of 70%].](https://static.elsevier.es/multimedia/16462890/0000005700000004/v1_201612030115/S1646289016301844/v1_201612030115/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)