Over the past 50 years, it has been developed a well-defined conceptual model of ADRS, characterised by a diffuse alveolar damage caused by an injury in the pulmonary endothelium and alveolar epithelium. It is defined as a sudden respiratory failure, with the presence of bilateral opacities in imaging studies (typically in chest radiographies and computed tomographies), pulmonary oedema not fully explained by cardiac failure or liquid overload and hypoxaemia with a PaO2/FiO2<300mmHg with a positive end expiratory pressure (PEEP)>5cm H2O. Its development has been described in the framework of numerous diseases and injuries, which are widely classified in pulmonary and extrapulmonary conditions; being pneumonia the most common risk factor to the development of this syndrome. Despite the advances in the management and prevention of ARDS, medical physicians are facing complications secondary to the treatment used, being the most characteristic ventilator induced lung injury, which not only increases lung damage but also has extrapulmonary repercussions, such as cardiac alterations.
Durante los últimos 50 años, se ha desarrollado un modelo conceptual bien definido del síndrome de distrés respiratorio agudo (SDRA), caracterizado patológicamente como daño alveolar difuso (DAD), causado por un insulto al endotelio capilar pulmonar y al epitelio alveolar. Se define como una falla respiratoria súbita, con presencia de opacidades bilaterales en los estudios de imagen (clásicamente radiografía o tomografía de tórax), oedema pulmonar no explicado totalmente por falla cardiaca o sobrecarga hídrica e hipoxemia con una PaO2/FiO2 ≤ 300mm Hg con presión positiva al final de la espiración (PEEP)>5cm H2O. Su desarrollo se describe en el marco de numerosas enfermedades y lesiones, las cuales son ampliamente clasificadas en origen pulmonar y sistémico (extrapulmonares); siendo la neumonía el factor de riesgo más común para desarrollar este síndrome. A pesar de todos los múltiples avances que se han tenido en el manejo y prevención del SDRA, el médico se enfrenta a complicaciones secundarias al tratamiento empleado, siendo la más característica la lesión asociada a ventilación mecánica (VILI), que no solo aumenta el daño pulmonar sino también tiene repercusiones extrapulmonares, como alteraciones a nivel cardiaco, entre otras.
Acute respiratory distress syndrome (ARDS) is a form of non-cardiogenic pulmonary oedema due to alveolar damage, secondary to an inflammatory process that may be of pulmonary or extrapulmonary origin.1 It is defined as sudden respiratory failure, with bilateral opacities on imaging studies (classically chest X-ray or a CT scan), pulmonary oedema not completely explained by heart failure or fluid overload and hypoxaemia with a PaO2/FiO2≤300mmHg with positive end-expiratory pressure (PEEP) >5cm H2O.2 This review is intended to provide healthcare staff who treat patients with ARDS with a simple way to reinforce and update their knowledge with recent information.
The first reports date back to World War II; however, it was not until 1967 that David Ashbaugh et al. reported on a cohort of 272 patients receiving respiratory support (oxygen or mechanical ventilation), 12 of whom had severe dyspnoea, tachypnoea, refractory hypoxaemia, cyanosis, decreased distensibility and bilateral pulmonary infiltrates on chest X-ray. In patients who died, histopathology studies reported heavier lungs, with atelectasis, interstitial oedema and alveolar oedema, as well as hyaline membranes. This clinical picture is similar to that of acute respiratory distress syndrome in newborns.3,4
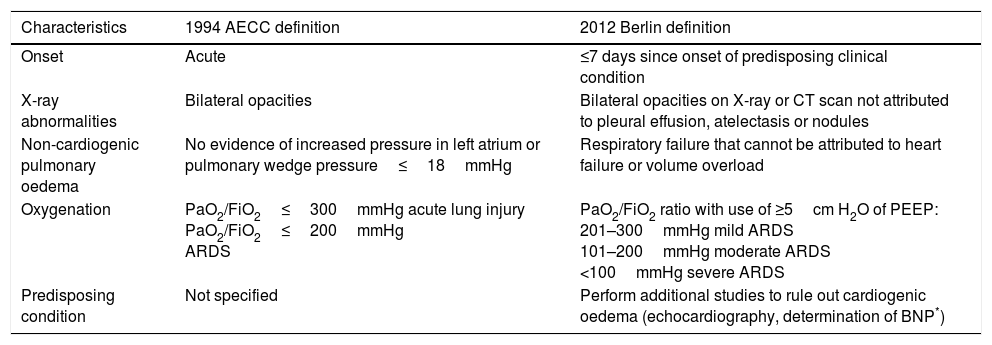
In 1994, the American-European Consensus Conference (AECC) recommended that patients with risk factors for ARDS, with hypoxaemia of sudden onset (PaO2/FiO2≤300mmHg regardless of the use of PEEP) and a chest X-ray consistent with bilateral pulmonary oedema, should be identified as having acute lung injury (ALI), whereas patients with PaO2/FiO2≤200mmHg should be identified as having ARDS. In both groups, patients were excluded if there was evidence of hypertension of the left atrium and/or pulmonary artery wedge pressure >18mmHg.5 Subsequently, in 2012, an expert group from the European Society of Intensive Care Medicine developed the Berlin definition, which focused on four points—reliability, feasibility, validity and objective evaluation—and proposed three exclusive categories for ARDS based on degree of hypoxaemia: mild (PaO2/FiO2 of 200–300mmHg), moderate (PaO2/FiO2 of 100–200mmHg) and severe (PaO2/FiO2≤100mmHg), without taking into account the auxiliary criteria from the prior definition.
The expert group removed the concept of acute lung injury and replaced it with the concept of mild ARDS. It also added the stipulation of a PEEP≥of at least 5cm H2O in patients with mechanical ventilation.6 Therefore, according to the Berlin definition (Table 1), ARDS is a form of diffuse acute lung injury that occurs in patients with a predisposing risk factor.
Definition of ARDS (AECC* 1994 vs Berlin 2012).
| Characteristics | 1994 AECC definition | 2012 Berlin definition |
|---|---|---|
| Onset | Acute | ≤7 days since onset of predisposing clinical condition |
| X-ray abnormalities | Bilateral opacities | Bilateral opacities on X-ray or CT scan not attributed to pleural effusion, atelectasis or nodules |
| Non-cardiogenic pulmonary oedema | No evidence of increased pressure in left atrium or pulmonary wedge pressure≤18mmHg | Respiratory failure that cannot be attributed to heart failure or volume overload |
| Oxygenation | PaO2/FiO2≤300mmHg acute lung injury PaO2/FiO2≤200mmHg ARDS | PaO2/FiO2 ratio with use of ≥5cm H2O of PEEP: 201–300mmHg mild ARDS 101–200mmHg moderate ARDS <100mmHg severe ARDS |
| Predisposing condition | Not specified | Perform additional studies to rule out cardiogenic oedema (echocardiography, determination of BNP*) |
The term acute lung injury (ALI) ended up being relegated to acute respiratory failure that does not meet the criteria of the Berlin definition,6 which encompasses a long list of syndromes, diseases and aetiologies. However, it is nearly indistinguishable on a molecular and histopathological level,7 establishes the clinical precursor to ARDS and has a treatment, course and prognosis similar to mild ARDS.8,9
A well defined conceptual model of ARDS has been developed in the last 50 years. ARDS is pathologically characterised as diffuse alveolar damage (DAD), caused by an insult to the pulmonary capillary endothelium and alveolar epithelium that results in increased permeability and subsequently protein-rich interstitial and alveolar oedema, atelectasis and damage to the lung parenchyma.3
AetiologyThe development of ARDS is described as part of many diseases and lesions, which are broadly classified as of pulmonary origin or of systemic origin. Pneumonia is the most common risk factor for developing the syndrome and, together with ARDS due to bronchoaspiration, it has the highest associated mortality rate, whereas ARDS due to trauma has the lowest mortality rate.1 Direct or pulmonary causes, apart from pneumonia, include inhalation of toxins, pulmonary contusion and pulmonary vasculitis, whereas indirect or systemic causes include sepsis of non-pulmonary aetiology, pancreatitis, extrathoracic trauma, burns, fat embolism, massive blood transfusion, surgical procedures and anything that triggers a systemic inflammatory response.2,10
Unfortunately, ventilator-induced lung injury (VILI) significantly contributes to the development and exacerbation of the syndrome. Damage results from complex interaction between several mechanical forces acting on lung structures such as type I and II pneumocytes, endothelial cells, macrophages, peripheral airways and the extracellular matrix (ECM) during mechanical ventilation.11 It is pathologically characterised by inflammatory cell infiltrate, hyaline membrane, increased vascular permeability and pulmonary oedema. This phenomenon is called VILI.12 It occurs by several mechanisms, such as: volutrauma (excessive stretching of the lung), barotrauma (excessive increase in lung pressures) and atelectrauma (secondary to repetitive opening and closing of the alveoli).1 All these mechanisms generate a biological reaction in response to mechanical forces that is called biotrauma (release of pro-inflammatory cytokines, recruitment of leukocytes and onset of a local inflammatory process).13
The pathogens most commonly responsible for the syndrome are: bacteria (Streptococcus pneumoniae, Haemophilus influenza, Enterobacteriaceae, Staphylococcus aureus, Legionella pneumophila, Chlamydia pneumoniae, Mycoplasma pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia), viruses (influenza A and B and cytomegalovirus), fungi (Pneumocystis jirovecii and Aspergillus fumigatus) and parasites (Toxoplasma gondii).14
EpidemiologyDespite all the advances made in ARDS management and prevention, especially with regard to lung protection, the syndrome has an incidence of 34–75 patients per 100,000 people in the United States, and an average of 150,000 cases of ARDS occur per year in the United States. This incidence is similar to the yearly incidence of cancer in that country. It should be noted that these data are underestimated, especially in less developed health systems, where there are limitations for meeting the criteria of the current definition of ARDS. It is estimated that 7% of patients admitted to the intensive care unit (ICU) and 16% of those who receive mechanical ventilation have ARDS.
The LUNGSAFE study15 reported that ARDS was more common than is reported, finding a prevalence of 10.4% of ICU admissions in 50 countries on 5 different continents and a mortality rate of 34.9% for mild ARDS, 40.3% for moderate ARDS and 46.1% for severe ARDS. Other studies have indicated that 80% of deaths due to ARDS in adults occurred in the second or third week following the onset of symptoms. In epidemiological studies, based on the 1994 definition of ARDS, the strongest predictors of mortality have been: age, underlying medical condition, degree of lung damage, extrapulmonary organ dysfunction and sepsis. Some reports have shown an incidence of ARDS in patients with sepsis of 6–7%. Only a small percentage of patients die of hypoxaemia. However, lung damage seems to be a predisposing factor for an inflammatory response resulting in multiple organ dysfunction and death.16,17
Concerning the prevalence and incidence of ARDS in Mexico, no recent epidemiological records are available. However, the database of the Adult Intensive Care Unit (AICU) at Hospital Juárez de México (HJM) from 2011 to 2015 recorded 1401 admissions, 229 of which met ARDS criteria, equating to 16.3% of all admissions, and reported a mortality rate of 48% and a mean stay of 12.7 days, more than double the average stay on the AICU.18 In 2013, Mikkelsen et al. conducted an epidemiological study in 778 patients with severe sepsis. Of these patients, 6.2% (48) had ARDS on enrolment in the study, 0.9% met criteria for ARDS on admission to the emergency department and 8.9% met criteria for ARDS on admission to the ICU, and this was associated with a 4 times greater risk of mortality.18
In 2015, Fuller et al. reported an incidence of ARDS of 14.7% after admission to the emergency department; alveolar protection measures were taken in only 46.7% of cases. These data indicate that most patients are not admitted to hospital with ARDS, but rather develop it during their stay in the emergency department and hospitalisation, secondary to the ventilation management that they undergo, since ventilation with alveolar protection parameters is uncommon in these patients during their stay outside of the ICU.19
PathophysiologyAfter pulmonary insult, alveolar inflammatory injury is described in 3 phases: exudative, proliferative and fibrotic. The essential pathophysiology of ARDS starts with the exudative phase and immune cell-mediated destruction of the barriers of the alveolar–interstitial–endothelial epithelial complex. Pulmonary microvascular permeability increases, with the passage of water from the capillaries to the alveoli, which allows plasma proteins and cell contents to flood the interstitium and airspace.
Classically, ARDS is recognised as a disease mediated by neutrophils; however, alveolar neutrophilia may occur with no need to increase vascular permeability. Neutrophils and macrophages are recruited by the inflammatory site and give rise to the propagation of the initial insult. All inflammatory exudate generates physical interaction with surfactant, initially causing dysfunction, followed by progressive epithelial damage and loss of surfactant production, which prevents alveolar permeability. The loss of epithelial ion channels damages the generation of osmotic forces, which are needed to return the fluid to the interstitium.
This damage described plus the development of hyaline membrane and decreased pulmonary distensibility result in interruption of gas diffusion. Alveolar vascular damage also occurs with increased permeability due to abnormalities in vasomotor tone (vasoconstriction and vasodilation) and presence of microthrombi. The resulting pulmonary hypertension increases right ventricular afterload. This right ventricular dysfunction may be exacerbated with mechanical ventilation and fluid overload. This combination of epithelial and alveolar damage worsens the imbalance between ventilation and perfusion and loss of hypoxic pulmonary vasoconstriction, leading to refractory hypoxia.
The proliferative phase involves attempts at recovery, with restoration of type II pneumocytes and subsequent differentiation of type I pneumocytes. Regeneration of the functional epithelial layer enables clearance of the exudate within the interstitium, and cell residue is removed by inflammatory cells. Vasomotor tone returns to normal and the microthrombi are removed; pulmonary hypertension also decreases. Shunts are reduced, leading to improved oxygenation, which progresses slowly, through recovery of pulmonary distensibility.
The third and final phase is the fibrotic phase, which occurs inconsistently and consists of failure to remove collagen and alveolar remodelling, which was established at the start of the injury, combined with the development of cystic changes, thereby limiting functional recovery. Diffuse alveolar damage is the pathognomonic pathological finding in ARDS. It is defined by the presence of hyaline membrane and may be detected through lung biopsy and autopsy. However, it is non-specific and may occur in the absence of acute respiratory distress syndrome, just as ARDS may occur in the absence of diffuse alveolar damage.1,20
Clinical signs and symptomsPatients with ARDS have dyspnoea of acute onset, which is the syndrome's main characteristic, as well as cough, tachypnoea and cyanosis. Arterial blood gases document hypoxaemia refractory to supplementary oxygen and present clinical and biochemical signs particular to the triggering disease. These patients, due to the severity of their inflammatory response, may quickly progress to signs and symptoms of multiple organ dysfunction.3–10 In patients on mechanical ventilation, it tends to present with the need for increased levels of FiO2, to maintain arterial oxygen saturation >90%, increased airway pressures in volume-controlled modes or reduced tidal volume in pressure-controlled modes. There may be reduced ETCO2 levels, increased dead space, patient–ventilator asynchrony or simply no possibility of progression and withdrawal of mechanical ventilation.
DiagnosisARDS is diagnosed when the criteria of the Berlin definition are met. This definition focuses on 3 basic aspects—feasibility, reliability and validity—and establishes three mutually exclusive grades of severity of ARDS based on degree of hypoxaemia, based in turn on PaO2/FiO2 values: mild (PaO2/FiO2 200–300mmHg), moderate (PaO2/FiO2 100–200mmHg) and severe (PaO2/FiO2<100).6,21
Differentiating diseases and conditions similar to ARDS remains a matter of great significance. Currently, just a few biomarkers are available for this purpose. For example, brain natriuretic peptide (BNP), determination of extravascular lung water and lung ultrasound are used to differentiate between ARDS and hydrostatic pulmonary oedema, although their usefulness is debated.21
As mentioned above, the syndrome may have various aetiologies, and suitable treatment of the underlying cause is crucial for improving the outcome. Therefore, suitable determination of the underlying cause will be reflected in the patient's prognosis. Although the diagnostic criteria for ARDS were updated some years ago through the Berlin definition, they still have certain limitations. In a patient with hypoxaemia (PaO2<60mmHg, PaO2/FiO2<100mmHg and SaO2<88%)22 who does not respond to management with supplementary oxygen, sequential imaging studies, whether simple chest X-ray or a CT scan, should be performed within 7 days of the onset of the predisposing clinical disease. These studies enable disease course and severity to be measured and estimated, and data on aeration, oedema and potential for recruitment to be collected. Bilateral ground-glass opacities, which may be interstitial or alveolar, are observed on chest imaging.22
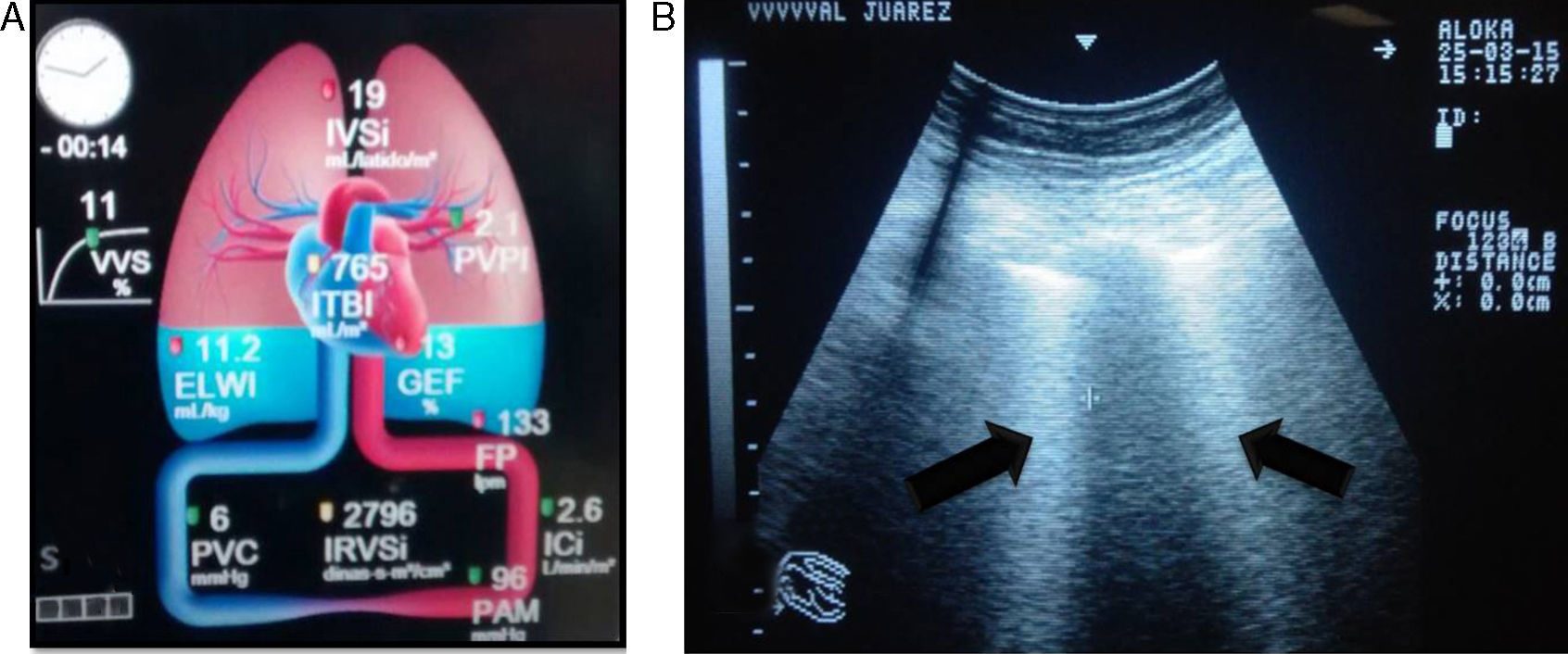
The differential diagnosis between cardiogenic pulmonary oedema (CPO) and ARDS is not easy. The accuracy of portable chest X-ray for detecting lung abnormalities consistent with ARDS is significantly limited. Measuring the extravascular lung water index (EVLWi) and pulmonary vascular permeability index (PVPI) using a transpulmonary thermodilution method seems to be a useful quantitative tool for diagnosing ARDS in patients with hypoxaemic respiratory failure and radiological opacities. In one study, a PVPI value of 2.6–2.85 provided a definitive diagnosis of ALI/ARDS (specificity 0.90–0.95), and a value <1.7 ruled out a diagnosis of ALI/ARDS (specificity 0.95).23
Ultrasound is considered the “stethoscope of the ICU”; it facilitates the diagnosis of ARDS, which presents with a predominant B profile in both hemithoraces, with a non-uniform distribution not dependent on severity on pulmonary ultrasound. This is called “alveolar–interstitial syndrome”, and it still lacks validity for diagnosing ARDS.24,25 Pleural thickening, subpleural consolidations, decrease in or abolition of pulmonary sliding and, in early stages, lung consolidation in dependent regions may also be observed.26
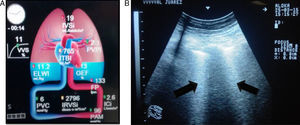
The extravascular lung water index (EVLWi) reflects the degree of pulmonary oedema, which may be measured through transpulmonary thermodilution systems (e.g. volume view or pulse contour cardiac output [PiCCO] monitor) or with ultrasound, where pulmonary B-lines are indicative of pulmonary oedema and, in critical patients, are considered a good predictor of EVLWi, through determining the quantity and confluence of B-lines in three segments of each hemithorax,24,25 thereby allowing ARDS to be differentiated from cardiogenic pulmonary oedema. EVLWi (normal value 3–7ml/kg) has been shown to be an independent prognostic marker in acute respiratory distress syndrome.27 Central venous pressure (CVP) has little correlation with the volaemic state and/or response to fluid in patients. Therefore, its routine use offers no greater benefits (Fig. 1).
The PaO2/FiO2 ratio is a measure of oxygenation that allows the severity of acute respiratory distress syndrome to be classified as acute, mild, moderate or severe. Although it is easy to calculate, it may be imperfect, due to its variability with different PEEP and tidal volume (Vt) levels. The oxygenation index (OI) is an indicator based on the relationship between PaO2, FiO2 and mean pressure (Pmean) of the airway ([Pmean×FiO2×100]/PaO2). It may be higher than the PaO2/FiO2 ratio, since it takes ventilation variables into account. However, to date, no predictive value has been documented in adult patients.28
Bronchoalveolar lavage (BAL) enables sampling of the alveolar space and aids in identification of the infectious cause of ARDS and/or diagnosis of haemorrhage or malignancy. Lung biopsy remains the gold standard for diagnosing diffuse alveolar damage. However, low specificity for the clinical diagnosis of ARDS has been observed with DAD. Therefore, at present, open lung biopsy is reserved for cases of uncertain diagnosis with no response to medical management. If pneumonia is a triggering factor for ARDS, the pathogen responsible for the infection must be identified early. The local epidemiological context and the patient's travel history, recent hospitalisations, history of exposure to or contact with sick people and immunocompetence must also be considered. An initial systematic microbiological evaluation should be performed to detect potential pathogens and suitably direct patient management.14
The clinical entities that may be confused with ARDS should be differentiated. Therefore, the differential diagnosis of ARDS should be against congestive heart failure and acute pulmonary oedema, idiopathic pulmonary fibrosis (usually interstitial pneumonitis), organised cryptogenic pneumonia, non-specific interstitial pneumonitis, Wegener's granulomatosis, diffuse alveolar haemorrhage, Goodpasture syndrome, pneumonitis due to hypersensitivity, acute eosinophilic pneumonia, and drug-induced lung disease (amiodarone and bleomycin).23
TreatmentSpecific treatment is intended to correct the aetiology of ARDS or its triggering disease. In severe cases, it is associated with refractory hypoxaemia. Therefore, early identification and management of hypoxaemia is indispensable. Supportive treatment of ARDS is focused on pharmacologic and non-pharmacologic measures, which will be described below.
Non-invasive ventilation and high-flow nasal cannulaHigh-flow nasal cannula (HFNC) is an oxygen device that can provide up to 100% of warm humidified oxygen through a high-calibre nasal cannula with a maximum flow rate of 60l/min. The pathophysiology of early-phase acute respiratory distress syndrome is a complex interaction between a high level of respiratory effort and redistribution of blood flow towards the respiratory muscles. Therefore, HFNC therapy offers the advantage of decreasing the complications associated with mechanical ventilation. It has been indicated that in patients with hypoxaemic acute respiratory failure, it significantly shortens the length of ICU stay, mortality at 90 days and need for intubation in patients with PaO2/FiO2<200mmHg. However, it has not been studied in patients with haemodynamic instability, and in patients with failure of HFNC therapy with late intubation (>48h after starting HFNC), it has been associated with greater mortality in the ICU and less success in extubation. Non-invasive ventilation (NIV) is useful in patients with chronic obstructive pulmonary disease and cardiogenic pulmonary oedema. However, it is less likely to have positive outcomes in patients with hypoxaemic respiratory failure. As in HFNC therapy, failure of NIV has been associated with increased mortality. Therefore, it is not recommended that its use be considered in patients with severe ARDS due to the course that these patients follow.17,29
Invasive mechanical ventilationThe lungs of many patients with ARDS are initially collapsed, poorly aerated, overdistended or entirely consolidated, and as a result, their ventilated lung volume is severely reduced. Such a lung is known as “baby lung” because only a third or a fourth of the normal lung is available for ventilation. The areas of lung tissue that are appropriately aerated are the size that they would be in a 5- or 6-year-old child (300–500g of aerated tissue), hence the name “baby lung”.
Ventilating this injured portion as if it were a normal-size lung may cause barotrauma, volutrauma or biotrauma. Thus alveolar protection strategies intended to minimise alveolar collapse and overdistension have been developed. Tidal volume refers to the quantity of gas delivered to the lungs during inspiration, and should be calculated from 4 to 6ml/kg of predicted body weight. It is calculated in men as ([height–152.4]×0.91)+50 and in women as ([height–152.4)×0.91+45.4, where the height unit is centimetres.17,29,30 In volume-controlled ventilation (VCV), Vt must be set in the ventilator by the physician, whereas in pressure-controlled ventilation (PCV), Vt will depend on lung distensibility.
Driving pressure (DP) is the change in pressure through the lung during the delivery of a tidal volume, measured as the difference between plateau pressure (Pplat) and PEEP. Therefore, this pressure is related both to the tidal volume and to the distensibility (C) of the respiratory system. The formula for driving pressure is as follows: DP=Vt/C. If Vt decreases or lung volume increases at the end of expiration, the pressure of stretching of the lung, commonly expressed as stress (internal forces per area unit that balance an external load and are equivalent to transpulmonary pressure in clinical practice), and tension (internal deformation due to applied pressure), is reduced. Therefore, low driving pressures are believed to be associated with decreased mortality in patients with ARDS.29,30 A meta-analysis by Amato et al. analysed 3562 patients with ARDS, from nine randomised studies, and reported that low driving pressure was correlated with a lower survival rate, to a greater extent than low tidal volume, Pplat and PEEP, and concluded that DP is the best risk stratifier in patients with ARDS.31 Plateau pressure is the pressure recorded in the airway during a pause at the end of inspiration; as there is no flow during this time and no resistance, Pplat is considered a substitute for alveolar pressure (PA).
Pplat below 27cm H2O has been observed to improve outcomes in these patients, since overdistension does not occur.30,32 Limiting plateau pressure is a strategy for protecting the lungs and right ventricle (RV), since the systolic pressure of the RV is 25mmHg. Pressures higher than this may compromise the RV due to increased afterload in the lungs.
Transpulmonary pressure is the pressure of actual distension of the lung, expressed as the pressure applied in the airway (Paw) minus the pressure required to exceed the pressure in the pleural space (TPP=Paw–Ppl). The actual pressure in the pleural space cannot be measured in clinical practice, but it can be estimated using oesophageal pressure as a substitute. When TPP prevents alveolar collapse during expiration, this means that it is equal in magnitude to the sum of all forces from the body surface, chest wall, pleura and elastic recoil of the lungs. The recommended value at the end of expiration is +2 to +5cm H2O, and the recommended value at the end of inspiration is +20 to +25cm H2O, with the objective of preventing overdistension.
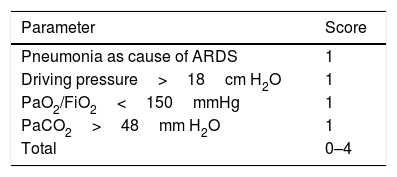
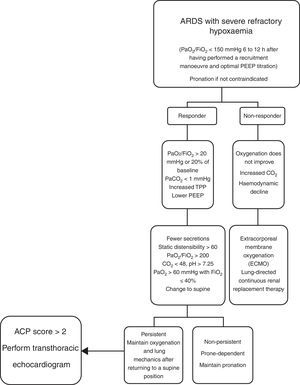
Up to a few years ago, “permissive hypercapnia”, wherein high levels of arterial pressure of carbon dioxide (PaCO2) were allowed without this modifying the pH, was considered acceptable. However, recent studies have found that increased PaCO2 causes greater pulmonary vasoconstriction and right ventricular dysfunction. PaCO2≥48mmHg is considered an independent risk factor for mortality, and PaCO2>60mmHg is associated with acute cor pulmonale.32 Mekontso et al. developed a score to assess the risk of acute cor pulmonale. It is known as the acute cor pulmonale score, or ACP score (see Table 2).33 Acute cor pulmonale is defined as septal dyskinesia with dilation of the RV (this is calculated as telediastolic area of RV/telediastolic area of LV, where >0.6 is abnormal and >1 represents severe dilation). In their study, they found a prevalence of acute cor pulmonale of 22% (164 of 752 patients).
Acute cor pulmonale score.
| Parameter | Score |
|---|---|
| Pneumonia as cause of ARDS | 1 |
| Driving pressure>18cm H2O | 1 |
| PaO2/FiO2<150mmHg | 1 |
| PaCO2>48mm H2O | 1 |
| Total | 0–4 |
The ACP score is shown, where a value of 1 is assigned to each variable. For a score ≥2, routine echocardiography should be performed; for scores 0–1, an echocardiogram must be requested from the treating physician. From Mekontso-Dessap et al.33
To improve gas exchange and prevent atelectrauma, PEEP should be used, since it increases functional residual capacity. Continuous alveolar recruitment, obtained through manoeuvres and optimal PEEP titration, has been used to improve hypoxaemia in patients with ARDS, in addition to preventing ventilator-induced lung injury, thereby minimising alveolar opening and collapse—i.e. atelectrauma—especially when high PEEP levels are associated with low Vt levels.22 The objectives of ventilation and strategies for alveolar protection are as follows: Vt 4–6ml/kg of predicted body weight, Pplat<27cm H2O, high PEEP if PaO2/FiO2 is lower than 200mmHg, DP<15cm H2O, PaCO2<48mmHg, PaO2>60mmHg and FiO2<60% (the lowest value sufficient for maintaining PaO2 60–100mmHg; values higher than 100mmHg are linked to ventilator-associated pneumonia).30,34
Recruitment manoeuvres are defined as a voluntary strategy to temporarily increase transpulmonary pressure in order to reopen alveolar units that are poorly ventilated or non-aerated, but may be opened or recruited. In the general population in the ICU, recruitment manoeuvres may improve the ratio of oxygenation (PaO2/FiO2) in 29%–50% of patients with ARDS; however, pulmonary oedema is associated with decreased recruitment manoeuvre capacity. Therefore, at present, it is believed that the efficacy of alveolar recruitment depends, at least in part, on extravascular lung water content in patients with ARDS.35
It is important to bear in mind that complete inactivity of the diaphragm results in diffuse atrophy and muscle weakness, which is known as ventilator-induced diaphragmatic dysfunction (VIDD) and appears after 18–24h of abolition of respiratory effort. This may contribute to problems in weaning from mechanical ventilation, fewer ventilation-free days, a longer intensive care stay and a longer hospital stay.
Experimental studies in models with ARDS demonstrated that preserving spontaneous respirations was associated with decreased lung inflammation markers and epithelial damage; improved tidal volume, gas exchange and oxygen supply; and increased systemic blood flow.36 However, Yoshida et al. also demonstrated that allowing spontaneous respirations in patients with severe ARDS caused increased TPP and greater injuries due to shearing secondary to volutrauma which led to greater lung injury. Given these findings, it has been proposed that allowing spontaneous respiration should only be considered in patients with mild or moderate ARDS.37
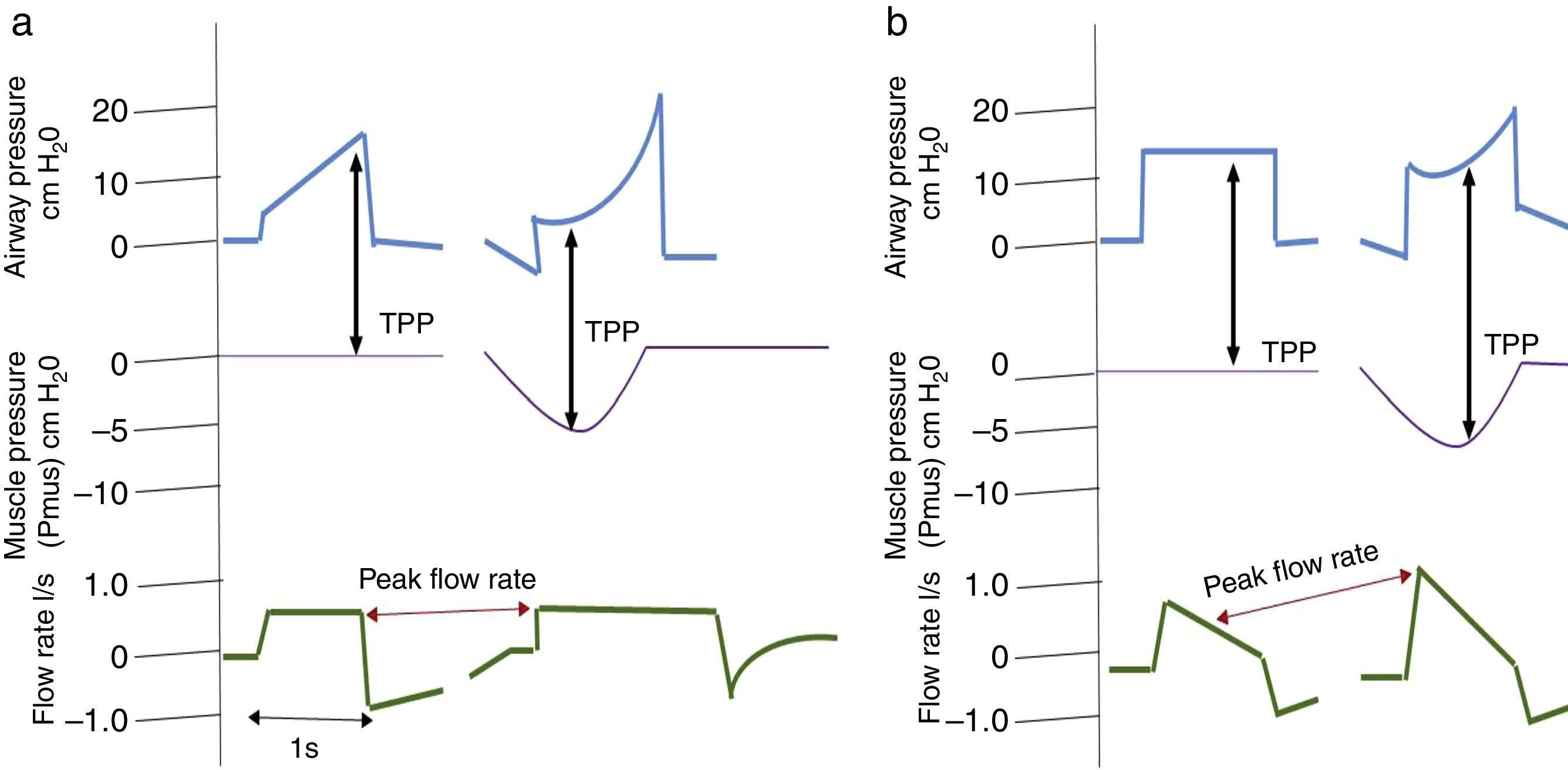
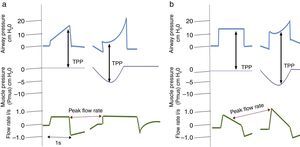
It is important to consider that when a patient generates spontaneous respiratory effort, there is an increase in TPP (Palv–Ppl), since the pressure generated by the ventilator and the pressure generated by the respiratory muscles are added. This should be taken into account when using pressure-assisted or pressure-controlled modes, including pressure-assisted/pressure-controlled ventilation, airway pressure release ventilation (APRV) and support pressure (SP), since, as mentioned above, both TPP and DP are higher than the airway pressure shown by the ventilator.38 In volume-controlled modes, this does not happen, since TPP and Vt remain constant compared to muscle pressure, as shown in Fig. 2.
Changes in transpulmonary pressure (TPP) with active and passive effort are represented. Chart (a) shows a volume-controlled mode. The left side shows a patient with no respiratory effort represented by a respiratory muscle pressure (Pmus) of zero, whereas the right side shows that respiratory effort, represented by a decrease in Pmus, does not alter transpulmonary pressure. Part (b) shows a pressure-controlled mode. The left side shows no respiratory effort. The right side shows respiratory effort and represents an increase in transpulmonary pressure as well as an increase in flow rate.
Mechanical ventilation in a prone position may result in better oxygenation and prevents VILI, as it enables better distribution of Vt, stress and tension throughout the lung. Adding the prone position to mechanical ventilation with high levels of PEEP increases lung aeration while simultaneously reducing regional hyperinflation and decreasing events of closing/opening of the small airways during the respiratory cycle. Therefore, it has been suggested that the prone position decreases the risk of barotrauma and atelectrauma and prevents VILI. Improved survival has been observed in patients with ARDS, with a duration of more than 16h per day, if PaO2/FiO2 is <150mmHg.39 The main mechanisms are as follows: it increases functional residual capacity (FRC); it increases distensibility; it increases diaphragm mobility; it decreases the weight of the heart on the lungs (this promotes lung expansion, since the heart contributes an additional pressure of 3–5cm H2O to the lungs); it improves mobilisation and postural drainage of secretions of the posterior lung segments40; and it improves the haemodynamic profile as it reduces right ventricular overload, given that it increases transpulmonary pressure and relieves hypoxic pulmonary vasoconstriction, thereby allowing a lower PEEP requirement and, at the same time, maintaining the capacity for recruitment and protecting the function of the right ventricle. In addition, McAuley et al. documented the positive effects of a prone position on EVLWi; in their study, subsequent to a temporary initial increase, they observed a statistically significant decrease.
As EVLWi depends on lung perfusion, decreased EVLWi has been associated with the changes in lung perfusion that occur in the prone position. Reducing it helps to improve gas exchange and pulmonary shunts.41 Another mechanism, by which the prone position decreases EVLWi levels, to be considered is decreased pulmonary vascular resistance (PVR), which occurs through the following mechanisms: (1) decreased levels of carbon dioxide (CO2) and, in turn, improved reflex pulmonary vasoconstriction, (2) improved alveolar oxygenation, and (3) decreased Pplat and therefore DP.
Prior publications on time spent in a prone position by patients at HJM (Fig. 3) reported that the longest time spent was 7 straight days, with no contraindication in obese patients or patients who had just undergone abdominal surgery.42,43 Esteban reported patients with more than 16 days, but intermittently, i.e. no more than 16h in a prone position per day.
The criteria for response to a prone position are as follows:
- •
Increase in PaO2/FiO2 by more than 20mmHg.
- •
Decrease in PaCO2 by at least 1mmHg.
Patients with ARDS who respond may also be classified as “persistent” or “non-persistent”, if their arterial oxygenation is maintained or not maintained, respectively, when they return to the supine decubitus position. These patients may have any of the following responses:
- •
Showing improved oxygenation compared to the prone position
- •
Maintaining suitable oxygenation compared to before the prone position, but not as good as during the pronation manoeuvres,
- •
Showing a decline and returning to baseline oxygenation in a supine position.
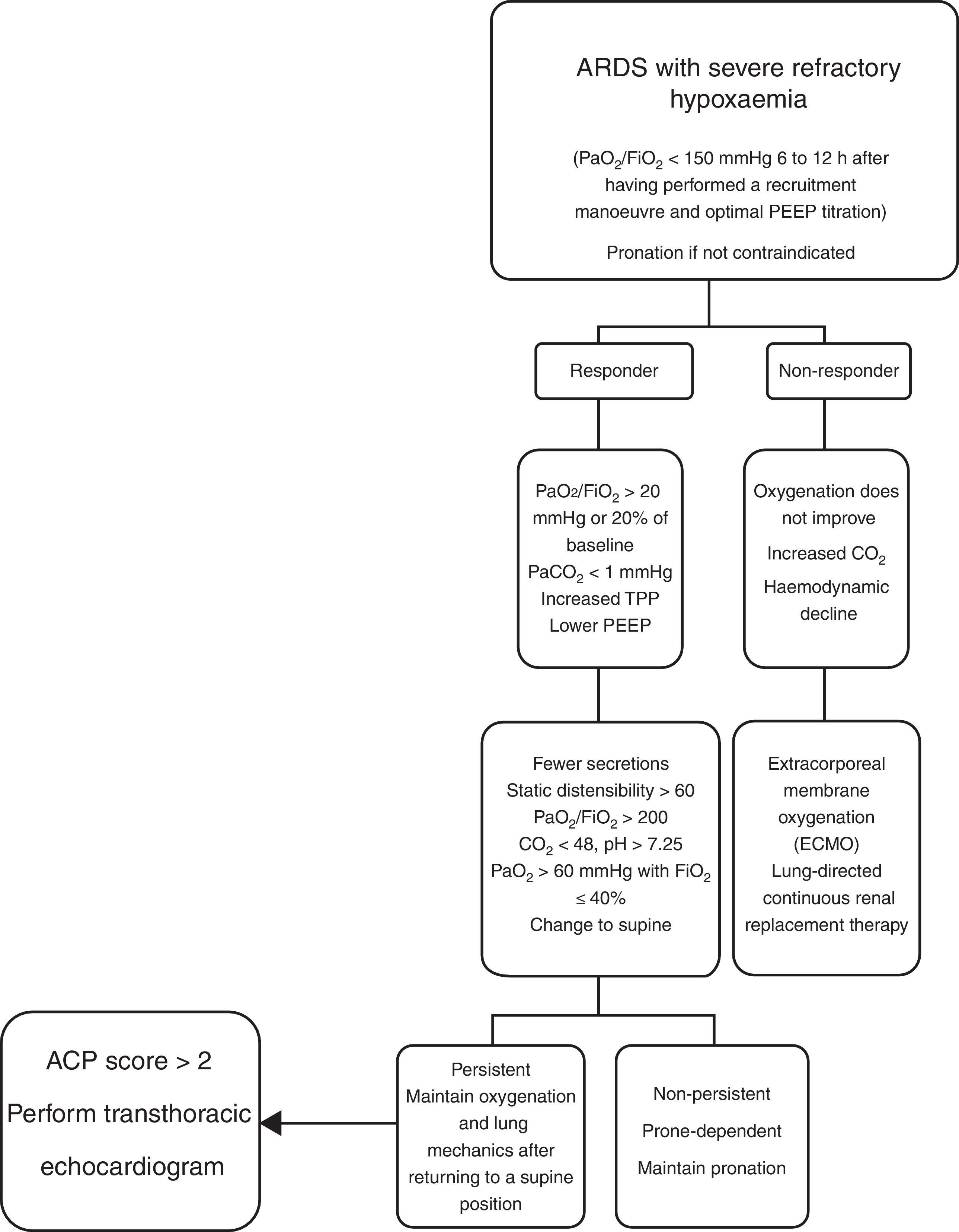
Contraindications for mechanical ventilation in a prone position are: cerebral oedema, intracranial hypertension, spinal fractures and severe haemodynamic instability.30,40,44,45 There are few publications on population subgroups to support other contraindications. At present, there is no internationally accepted algorithm on the management of the prone position in acute respiratory distress syndrome. The management of the prone position in the ICU at HJM is briefly described here (Fig. 4).
Based on all the evidence available, the prone position has gone from being a rescue treatment for severe refractory hypoxaemia to a lung protection strategy which should be performed early, i.e. in the first 48h, since it has been associated with improved oxygenation and decreased mortality.
Neuromuscular blockIn patients with ARDS with PaO2/FiO2<150mmHg, early treatment with continuous infusion of a muscle relaxant (cisatracurium besilate) for 48h reduces mortality after 90 days and the risk of barotrauma, and increases the number of ventilator-free days and days outside of the ICU, without increasing the risk of neuropathy of the intensive-care patient. It improves patient–ventilator synchrony; generates greater uniformity in alveolar recruitment; and improves distensibility, gas exchange and systemic oxygenation. It results in better control of inspiratory volumes and pressures, thereby reducing the risk of volutrauma. It also results in better control of expiratory volumes and pressures, which decreases the risk of atelectrauma, thereby resulting in less pulmonary and systemic inflammation.17,22,46 Cisatracurium besilate itself has been proven to have an anti-inflammatory effect, through the nicotinic pathway of acetylcholine. Therefore, using muscle relaxants is believed to lead to more benign mechanical ventilation, with better synchrony between the patient and the ventilator and more uniform distribution during pressurisation during tidal ventilation. Administering an additional bolus of muscle relaxant when plateau pressure exceeds 32cm H2O has been reported to generate decreased pressures in the airway and therefore prevent ventilator-associated lung injury and decrease mortality in patients with ARDS.47
SedationSedation during early phases promotes suitable protective ventilation. No randomised studies have suggested advances in any sedation in particular; however, database analysis has suggested that benzodiazepine infusion was independently associated with increased mortality and a longer ICU stay compared to propofol.30 Propofol, for its part, has been associated with a higher rate of asynchrony due to ineffective infusion compared to dexmedetomidine. It should be noted that deep sedation, within the first 48h of admission to the ICU, has been identified as a predictor of delayed extubation time and increased risk of mortality. Therefore, many studies have demonstrated that deep sedation is not necessary in ventilated patients, as these patients tolerate strategies involving low tidal volumes and high PEEP levels, which are commonly used in patients with ARDS. However, deep sedation is necessary in patients with ARDS with neuromuscular block in order to ensure that patients do not consciously experience paralysis. The objectives of sedation should be to reduce discomfort, improve patient tolerance of mechanical ventilation and other adjuvant therapies, promote early mobilisation, and prevent oversedation, since it reduces the risk of delirium, length of ICU stay and mechanical ventilation time, and improves immunological effects. Experimental studies have shown that propofol may damage phagocytosis by neutrophils and alter the response of macrophages and release of cytokines induced by lipopolysaccharides. Dexmedetomidine may have anti-inflammatory effects and improve the function of macrophages. The choice of sedative and analgesia for the patient should be made with caution on a personalised basis.48
Fluid monitoringOedema in lung injury is hydrostatic and oncotic in nature (capillary leak syndrome). This suggests, based on Starling forces, that actively decreasing hydrostatic pressure (or at least avoiding unnecessary volume administration) should be beneficial. Administering fluids improves perfusion only if systolic volume increases, but only around 50% of patients in the ICU are preload responders. Once early tissue hypoperfusion has been corrected, a conservative fluid resuscitation strategy should be favoured.49 Therefore, it is recommended that a neutral to negative fluid balance be maintained. Fluid management during ARDS with the use of furosemide was associated with improved lung function and reduced mechanical ventilation time, without increasing non-lung organ failure. However, there was no significant difference in mortality after 60 days. It has been suggested that early use with haemofiltration as rescue treatment for patients with ARDS reduces cytokine levels and the systemic inflammatory response, improves cardiac function, and decreases the extravascular lung water index. In patients in whom combination therapy with furosemide and albumin was maintained, it demonstrated improved oxygenation, fluid balance and haemodynamic stability; no beneficial effect of albumin alone has been demonstrated in patients with ARDS.1,30
Transfusion of blood productsThe use of blood products is associated with progression of acute respiratory distress syndrome in patients with risk factors. The transfusion of blood products is associated with the transport of leukocytes, antibodies and pro-inflammatory cytokines to the patient. This plays an important role in the pathogenesis of transfusion-related acute lung injury (TRALI). Therefore, the transfusion of packed blood cells should be reserved for patients with haemoglobin <7mg/dl, with evidence of persistent tissue hypoperfusion, after a careful risk/benefit evaluation49; the transfusion of fresh plasma should be reserved for patients with evidence of bleeding with INR >1.5s; and the transfusion of platelet concentrates should be reserved only for severe thrombocytopenia <50,000mm3 in patients without brain injury and <100,000mm3 in patients with brain injury, in both situations with active bleeding, since unnecessary transfusions lead to positive balances and even transfusion-associated circulatory overload (TACO) leading to increased morbidity and mortality, as mentioned above.
Steroid therapyThe use of steroids in ARDS remains controversial, and the evidence available is contradictory. Multiple studies have handled different steroid start times in ARDS. In one study, Meduri et al. started management with methylprednisolone in the first 72h and, on day seven, observed greater success in extubation, a shorter ICU stay, fewer mechanical ventilation days, a lower mortality rate and a better PaO2/FiO2 ratio. However, a study by the ARDS Network started methylprednisolone from the 7th day of onset of ARDS and corroborated improved oxygenation, more ventilator-free days and decreased vasopressors; even so, muscle atrophy increased and mortality did not decrease after either 60 or 180 days, and even increased in those in whom methylprednisolone was started after day 14. Therefore, it is recommended that it be used early.34,48
It should be borne in mind that community-acquired pneumonia is the main cause of ARDS, and that treatment failure is associated with an excessive inflammatory response. Therefore, steroids may modulate cytokine release in these patients. Recently, Torres et al. conducted a controlled, multi-centre, randomised, double-blind study at 3 teaching hospitals in Spain, where they evaluated the use of steroids in patients with community-acquired pneumonia and a severe inflammatory response. They found that patients who were administered methylprednisolone 0.5mg/kg every 12h for 5 days, starting within 36h of their admission, had lower rates of treatment failure and a lower inflammatory response.51 Steroids have demonstrated a benefit in many infectious and non-infectious pulmonary lesions that lead to ARDS. Patients with pneumonia due to Pneumocystis may progress to ARDS and have evidence of diffuse alveolar damage. However, management with steroids for 21 days reduces mortality and improves hypoxaemia. Therefore, although the use of systemic steroids in the patient with ARDS is controversial, it should be noted that their use is justified as specific management of the triggering cause, not of ARDS per se.
ECMOThis is indicated in patients with severe hypoxaemia (PaO2/FiO2<80mmHg) with FiO2>80% for more than six hours, when other strategies have failed and there is still a chance of survival.30 The results obtained are contradictory with respect to improvement in mortality and are related to the study population, sample size, and start time. Its main complication is bleeding disorders.
Inhaled nitric oxide (iNO)Nitric oxide, administered intratracheally, selectively dilates the pulmonary vessels in well ventilated lung regions and redirects blood flow, resulting in better oxygenation and decreased pulmonary artery pressure. Some studies have not linked it to improved survival and even shown that it could adversely affect renal function. However, these studies were performed before the era of alveolar protection22,30 in limited populations.
ConclusionsARDS is a serious disease, with the maximum expression of inflammation in the lungs, which is life-threatening for patients. It has high healthcare costs and significant medium-term and long-term sequelae. The criteria for diagnosing ARDS have been simplified. Technology aids in diagnosis and helps the clinician to establish the severity and prognosis of the disease at the patient's bedside. Its many and varied treatments include preventive measures, alveolar protection, ventilation manoeuvres, ECMO and drugs, the benefits of which remain unclear. Healthcare staff must suitably identify the syndrome, start treatment wherever the patient may be and promptly refer to an intensive care unit, in order to improve the patient's prognosis, slow the progression of the syndrome and limit sequelae.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest with respect to this review.
The authors would like to thank Hospital Juárez de México for the facilities that it provided to conduct this study.