The results preceding this study documented that patients with early stage classical seminoma treated with radiotherapy in the Hospital General de Mexico, using doses of 30Gy in 15 fractions using the modified dog-leg technique have lower gastrointestinal toxicity than the conventional dog-leg technique, but with no difference in overall survival and disease-free survival, both resulting in 100% 60 month-survival.
ObjectiveTo determine the results of treatment in terms of relapse and gastrointestinal toxicity, comparing radiotherapy with conventional dog-leg, modified dog-leg and inverted-Y techniques.
Material and methodsretrospective, observational, comparative, analytical, retrolective study; 40 patients were analysed, all diagnosed with stage I seminoma treated at the RT Hospital General de Mexico between October 2009 and May 2016.
ResultsThe age of the patients was 33±8 years; 32 (80%) were treated in Accelerator linear and 8 patients (20%) in cobalt-60. The modified dog-leg technique was used in 24 patients (60%), conventional dog-leg technique in 12 patients (30%), and inverted-Y technique in 4 patients (10%). The radiation dose in 87% of patients was 25.2Gy. The most commonly found acute gastrointestinal toxicity was grade 2, present in 22% with modified dog-leg technique, 13% conventional dog-leg technique, and 3% for the inverted-Y technique (p=0.95). There was one relapse associated with the modified dog-leg technique; predicting factors for relapse including rete testis invasion, trans-scrotal violation, and lymphovascular invasion had no statistically significant impact.
ConclusionsRadiotherapy continues to be the treatment of choice in patients with early stage seminoma, with a low probability of relapse and acceptable gastrointestinal toxicity. There is no difference in relapse or gastrointestinal toxicity associated with the different radiation techniques in patients with stage I seminoma, therefore the modified dog-leg technique is recommended as the field of irradiation is already reduced without a negative impact on relapse.
Los resultados que preceden a este estudio documentan que pacientes con seminoma clásico en etapa temprana tratados con radioterapia en el Hospital General de México, usando dosis de 30Gy en 15 fracciones con técnica de tratamiento hemi Y modificada tienen menor toxicidad gastrointestinal frente a la técnica hemi Y convencional, ambas sin diferencia en sobrevida global ni sobrevida libre de enfermedad siendo ambas del 100%, a 60 meses.
ObjetivoDeterminar los resultados de tratamiento en términos de toxicidad gastrointestinal y recaída, al comparar la técnica de radioterapia con hemi Y convencional, hemi Y modificada e Y invertida.
Material y métodosEstudio retrospectivo, observacional, comparativo, analítico y retrolectivo; se analizaron 40 pacientes con diagnóstico de seminoma etapa I tratados en el Servicio de Radioterapia del Hospital General de México entre octubre 2009 y mayo 2016.
ResultadosLa edad de los pacientes fue de 33±8 años; treinta y dos (80%) fueron tratados en acelerador lineal y 8 pacientes (20%) en cobalto-60. La técnica hemi Y modificada se utilizó en 24 pacientes (60%), hemi Y convencional en 12 pacientes (30%), Y invertida en 4 pacientes (10%). La dosis de irradiación en el 87% de los pacientes fue de 25.2Gy. La toxicidad aguda gastrointestinal más frecuente encontrada fue grado 2, presente en el 22% con técnica hemi Y modificada, 13% hemi Y convencional y 3% Y invertida (p=0.95). Se presentó una recaída asociada a técnica de hemi Y modificada; los factores pronósticos para recaída como invasión a rete testis, invasión linfovascular y violación transescrotal no mostraron diferencias estadísticamente significativas.
ConclusionesLa radioterapia sigue siendo una opción de tratamiento en pacientes con seminoma etapa temprana, con baja probabilidad de recaída y con toxicidad gastrointestinal aceptable. No existe diferencia en cuanto toxicidad gastrointestinal ni recaída asociada a las diferentes técnicas de irradiación en pacientes con seminoma Etapa I, por lo que se recomienda la técnica de hemi Y modificada ya que el campo de irradiación en más reducido sin impacto negativo en recaída.
Testicular cancer has a low incidence, in 2016 an incidence of 8720 cases and mortality in 380 cases is estimated.1 In Europe, the incidence is 8%, Australia 6%, Asia and Africa <1%.2 In 2006 in Mexico, 1361 cases were recorded, corresponding to 1.2%.3 In a 2009 case study at our hospital, it was found that most patient presented in stage I (73%), stage IIA 13%, IIB 6%, and IIC 8%.4 The mean age at onset was 32 years ranging from 20 to 48 years.
Germinal testicular cancer accounts for 90–95% of the cases, of which seminoma has a frequency between 40% and 60%. There are three histological varieties: classical 85%, anaplastic 10–12%, and spermatocytic 4–6%,5,6 it mainly spreads through the lymph nodes.5 It has been documented worldwide that 80% of patients present in stage I,7 a result which is in agreement which what was found in our hospital.
The clearly identified risk factors are: cryptorchidism with a relative risk 5- to 10-fold the general population; immediate family history of testicular cancer (father relative risk 4, brother relative risk 9); chemical substances: naphthylamine, benzidine, and gasoline derivatives relative risk 5-fold5; contralateral intratubular neoplasia; infertility and Klinefelter syndrome.6
The diagnostic protocol uses the medical history, physical examination, testicular ultrasound, and tumour markers: alpha fetoprotein (AFP) which is raised in non-seminoma or mixed histology, its half-life is 5–7 days,5 a fraction β of human chorionic gonadotropin (β-HCG) is produced by the syncytiotrophoblast and is presented in 10–15% of seminomas, its half-life is 2–3 days, and lactate dehydrogenase (LDH) which is associated with tumours volume,8 which are important for diagnosing, staging, and predicting the response to treatment. The extension studies to complete the diagnostic protocol and to be able to stage it are chest X-ray and computed abdominal-pelvic tomography (CT). Brain imaging and bone scan studies are also ordered based on clinical suspicion.9
Positron emission tomography (PET/CT) with fluorodeoxyglucose (FDG) is a test to evaluate the residual tumour and its viability after seminoma treatment. It is recommended to order it at least 6 weeks after the end of chemotherapy (CTx), obtaining it before this period is associated with false positive results.9
The risk factors for relapse are: testicle size which has an impact on disease-free survival (DFS), a 5-year DFS of 87% is estimated in tumours ≤4cm vs. 76% in tumours >4cm (p=.003); rete testis invasion, in the absence of this invasion a 5-year DFS of 86% is estimated vs. 77% if it is present (p=.003); the absence if lymph node invasion is associated with a 5-year DFS of 86% vs. 77% if it is present (p=.038). In a multivariate analysis, it was demonstrated that the only factors impacting DFS are tumour size >4cm and rete testis invasion, with a relative risk of 2 and 1–2, respectively.10
The treatment for stage I seminoma consists of radical inguinal orchiectomy plus high spermatic cord ligation followed by three treatment strategies: surveillance, CTx, or radiotherapy (RTx).6,8
With surveillance, a relapse rate between 17 and 30% is estimated, and it can increase up to 50% when there is a vascular embolism. For relapse, the salvage therapy consists of RTx or CTx, obtaining a salvage rate of up to 100%. This method has been adopted in Canada and some European countries.6,11,12 However, surveillance involves a higher 10-year cost compared to giving definitive adjuvant treatment, therefore the population conditions should be evaluated.
In the United States, radiation oncologists recommend adjuvant RTx,11 since the results are better than surveillance, estimating the 5- and 8-year overall survival (OS) around 99% and 98%, 5-year DFS 96%, and 5- and 8-year local control (LC) between 94 and 95%, respectively.12 With RTx, the probability is relapse is between 3 and 5%,6,13–15 with the main site being the para-aortic lymph nodes (70% of cases).
This study takes the 2009 study from our institution as its basis. The objective is to assess relapse and gastrointestinal (GI) toxicity by comparing RTx treatment techniques: 2D or 3D inverted-Y (IY), conventional dog-leg (CDL), and modified dog-leg (MDL).
Materials and methodsA retrospective (observational, comparative, analytical, retrolective) study was conducted.
The study population in this protocol was independent of that previously published in our hospital.
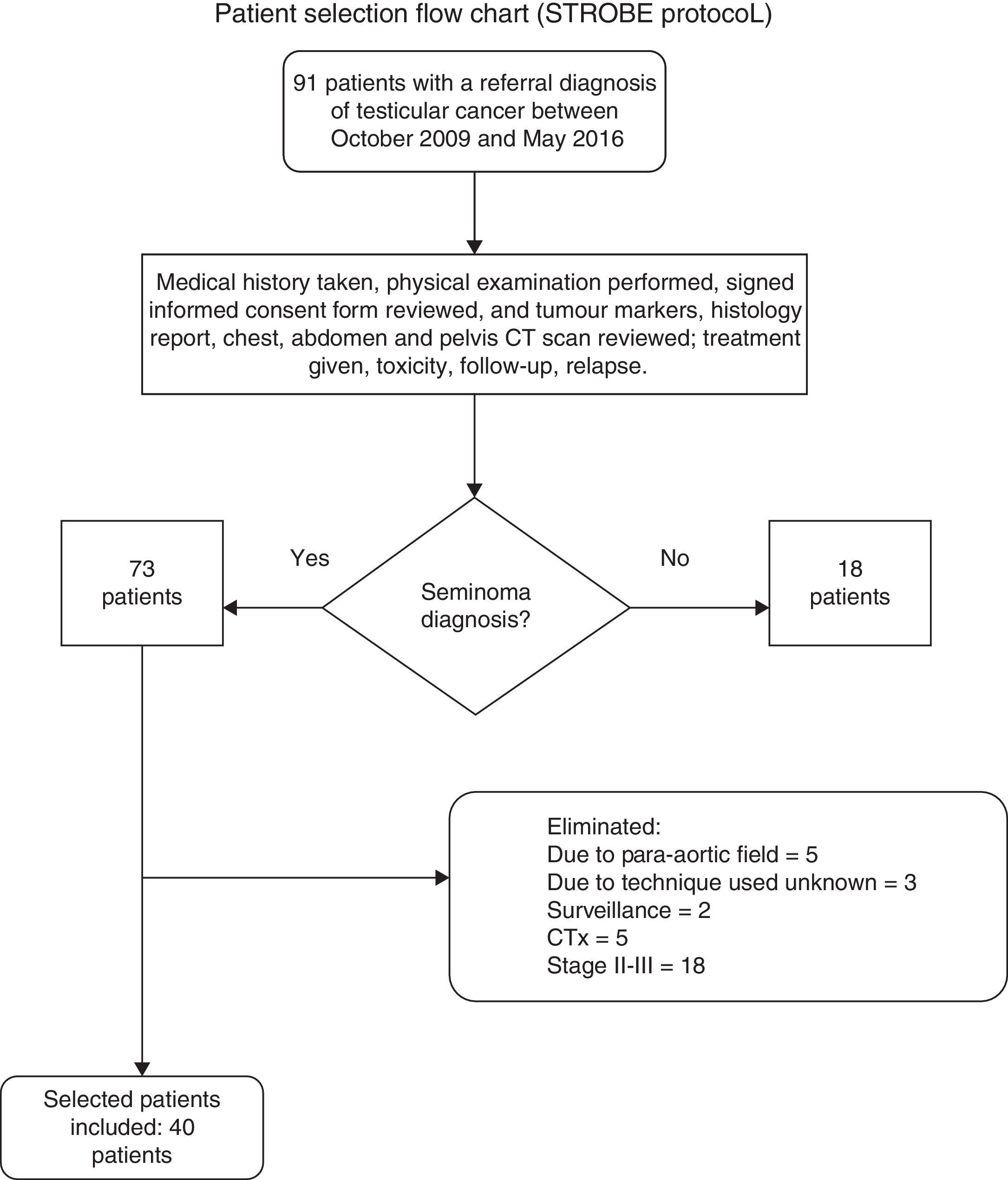
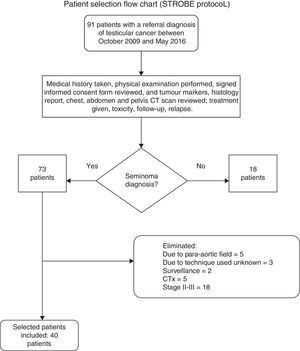
Ninety-one files were reviewed, of which 18 patients were excluded for having a histological diagnosis of non-seminoma or lymphoma; 33 patients were eliminated as they had not completed the scheduled RTx treatment, presented disease progression (therefore they were no longer stage I), or withdrew the informed consent form; in the end 40 clinical files were selected for patients with a stage I seminoma diagnosis according to the Royal Marsden and TNM (tumour size, lymph node involvement, and metastasis) classification treated with radical inguinal orchiectomy plus high spermatic cord ligation and adjuvant RTx, treated in the Radiation Oncology Department of the Hospital General de México with follow-up starting in October 2009 through May 2016 (Figure 1).
Flow chart for selecting medical records of seminoma patients, based on the guidelines from the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.16
The patients treated with adjuvant RTx received doses between 25.2 and 30.6Gy with conventional fractionation (1.8–2Gy per fraction), on the cobalt-60 equipment or linear accelerator and three different RTx techniques were used: IY, CDL, and MDL.
Radiotherapy treatmentTechniqueThere were three treatment groups according to the RTx technique used: 24 patients (60%) were treated with the MDL technique, 12 patients (30%) with the CDL technique, and 4 patients (10%) with the IY technique.
For the MDL treatment field, the upper limit was T10/T11 or T11/T12, the lower limit was the acetabular ceiling, and the field width was between 8 and 12cm (2cm from the vertebra body bilaterally). CDL differs in that the upper limit is the foramen obturatum, and in IY the upper and lower field border is the same as for CDL, but the irradiation towards the foramen obturatum is bilateral. All the patients were treated with two parallel opposed fields.
EquipmentThe irradiation was performed on two types of equipment.
Of the 40 patients, 32 were treated with photons on a 3D conformal linear accelerator, Varian 21Ex, energies 6 and 18MV (megavolts) and Elekta, energies 6 and 15MV. The other 8 patients were treated with a 2D cobalt-60 photon beam (Phoenix and Elite), energy 1.25MeV (Megaelectron-volts), maximum surface dose 0.5cm.
DoseThe dosage range went from 25.2 to 30.6Gy; it was given five times per week from Monday to Friday, one session per day. Thirty-five patients were treated with 25.2Gy in 14 fractions (treatment duration 3 weeks), 2 patients received 27Gy in 17 sessions (treatment duration 3.5 weeks), 2 patients 30Gy in 15 sessions (treatment duration 3 weeks), and one patient 30.6Gy in 17 sessions (treatment duration 3.5 weeks). All the RTx regimens used conventional fractionation (dosage per fraction between 1.8 and 2Gy).
Follow-upThe patients were evaluated to document the acute and chronic GI radiotoxicity, as well as relapses. Three appointments were scheduled during the RTx treatment and during the follow-up they were assessed every 4 months for the first 48 months, and subsequently every 6 months. During the follow-up appointments, the level of tumour markers was assessed, and every year a control imaging test with an abdominal and pelvic CT scan was performed.
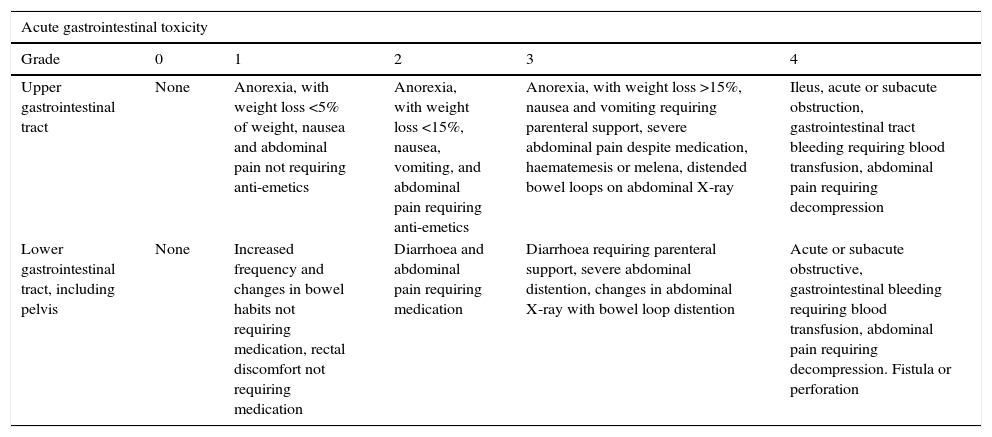
GI toxicity was evaluated using the Radiation Therapy Oncology Group (RTOG) criteria (Table 1).17
Gastrointestinal toxicity, measured by the RTOG toxicity scale.a
| Acute gastrointestinal toxicity | |||||
|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 4 |
| Upper gastrointestinal tract | None | Anorexia, with weight loss <5% of weight, nausea and abdominal pain not requiring anti-emetics | Anorexia, with weight loss <15%, nausea, vomiting, and abdominal pain requiring anti-emetics | Anorexia, with weight loss >15%, nausea and vomiting requiring parenteral support, severe abdominal pain despite medication, haematemesis or melena, distended bowel loops on abdominal X-ray | Ileus, acute or subacute obstruction, gastrointestinal tract bleeding requiring blood transfusion, abdominal pain requiring decompression |
| Lower gastrointestinal tract, including pelvis | None | Increased frequency and changes in bowel habits not requiring medication, rectal discomfort not requiring medication | Diarrhoea and abdominal pain requiring medication | Diarrhoea requiring parenteral support, severe abdominal distention, changes in abdominal X-ray with bowel loop distention | Acute or subacute obstructive, gastrointestinal bleeding requiring blood transfusion, abdominal pain requiring decompression. Fistula or perforation |
| Chronic gastrointestinal toxicity | ||||||
|---|---|---|---|---|---|---|
| Grade | 0 | 1 | 2 | 3 | 4 | 5 |
| Gastrointestinal tract | None | Mild diarrhoea, slight rectal discharge or bleeding, 5 bowel movements daily | Moderate diarrhoea and colic, >5 bowel movements daily, excessive rectal mucous or intermittent bleeding | Intestinal obstruction or bleeding requiring surgery | Necrosis, perforation, fistula | Death |
Association with secondary neoplasias was sought, defined according to Cahan's criteria18:
- •
The “radiation-induced” neoplasia must arise within the RTx treatment field.
- •
There must be a sufficient latency period, preferably more than 4 years.
- •
Both the primary tumour and the “radiation-induced” tumour must have differing histology.
- •
The tissue from which the “radiation-induced” tumour arose must be normal (metabolically and genetically) before exposure to the radiation.
Statistical analysis: the central tendency and dispersion measurements were used for quantitative variables and descriptive statistics (frequencies and percentages) were used for qualitative variables.
The correlations between GI toxicity and relapse with the treatment field were examined through non-parametric statistics using Spearman's rho. Survival curves were included using the Kaplan–Meier technique. A statistically significant difference for both was defined as p≤.05. The software programme SPSS V.23 (IBM, Chicago, Illinois, USA) was used.
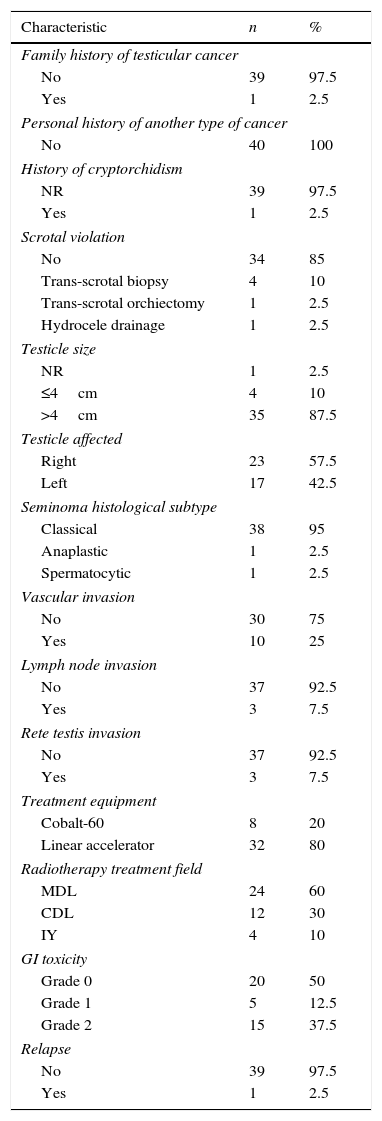
ResultsThe patients were recruited between October 2009 and May 2016. The mean age was 33±8 years (ranging from 18 to 57 years), with a follow-up of 30±8 months (ranging from 1 to 78 months) (Table 2).
Patient characteristics.
| Characteristic | n | % |
|---|---|---|
| Family history of testicular cancer | ||
| No | 39 | 97.5 |
| Yes | 1 | 2.5 |
| Personal history of another type of cancer | ||
| No | 40 | 100 |
| History of cryptorchidism | ||
| NR | 39 | 97.5 |
| Yes | 1 | 2.5 |
| Scrotal violation | ||
| No | 34 | 85 |
| Trans-scrotal biopsy | 4 | 10 |
| Trans-scrotal orchiectomy | 1 | 2.5 |
| Hydrocele drainage | 1 | 2.5 |
| Testicle size | ||
| NR | 1 | 2.5 |
| ≤4cm | 4 | 10 |
| >4cm | 35 | 87.5 |
| Testicle affected | ||
| Right | 23 | 57.5 |
| Left | 17 | 42.5 |
| Seminoma histological subtype | ||
| Classical | 38 | 95 |
| Anaplastic | 1 | 2.5 |
| Spermatocytic | 1 | 2.5 |
| Vascular invasion | ||
| No | 30 | 75 |
| Yes | 10 | 25 |
| Lymph node invasion | ||
| No | 37 | 92.5 |
| Yes | 3 | 7.5 |
| Rete testis invasion | ||
| No | 37 | 92.5 |
| Yes | 3 | 7.5 |
| Treatment equipment | ||
| Cobalt-60 | 8 | 20 |
| Linear accelerator | 32 | 80 |
| Radiotherapy treatment field | ||
| MDL | 24 | 60 |
| CDL | 12 | 30 |
| IY | 4 | 10 |
| GI toxicity | ||
| Grade 0 | 20 | 50 |
| Grade 1 | 5 | 12.5 |
| Grade 2 | 15 | 37.5 |
| Relapse | ||
| No | 39 | 97.5 |
| Yes | 1 | 2.5 |
| Mean | SD | |
|---|---|---|
| Age (years) | 33 | 8.5 |
| AFP levels (ng/mL) | 8.7 | 39.8 |
| β-HCG levels (mIU/mL) | 62 | 289.5 |
| LDH Levels (IU/L) | 313.5 | 274.3 |
| Treatment dose (Gy) | 25.6 | 1.3 |
| Size of dose per fraction (Gy) | 1.8 | 0.4 |
| Number of sessions | 14 | 0.54 |
| Performance status (%) | 97 | 4.2 |
| Follow-up (months) | 30 | 18 |
NR: not reported; MDL: modified dog-leg; CDL: conventional dog-leg; IY: inverted-Y; GI: gastrointestinal; SD: standard deviation; AFP: alpha fetoprotein; β-HCG: beta fraction of human chorionic gonadotropin; LDH: lactate dehydrogenase.
Present in 6 patients (15), which by definition are considered T4; of these one had a history of cryptorchidism.
Five patients were treated with the linear accelerator and one with cobalt-60; four patients were treated with the IY technique, one with CDL and one more with MDL. Four patients were given a dose of 25.2Gy in 14 sessions, the rest 30Gy in 15 sessions. The mean follow-up was 36 months (ranging from 25 to 60 months).
Tumour markers at diagnosisβ-HCG was over 10mIU/mL in 7 patients (17%), one of which was associated with lymphovascular invasion. One patient (2.5%) presented AFP over 10ng/dL (254ng/mL) and 27 patients (67%) presented elevated LDH >160IU/L.
Histology reportOf the patients with classical seminoma, one patient relapsed. There was no evidence of relapse in the other histological subtypes.
Radiotherapy associated with GI toxicity and relapseThirty-two patients (80%) were treated with 3D conformal linear accelerator photons. Of these, MDL was used in 22 patients (69%), CDL in 7 patients (22%), and IY in 3 patients (9%).
Eight patients (20%) were treated with a 2D cobalt-60 beam of these, CDL was used in 5 patients (62%), MDL in 2 patients (25%), and one patient (12%) was treated with IY.
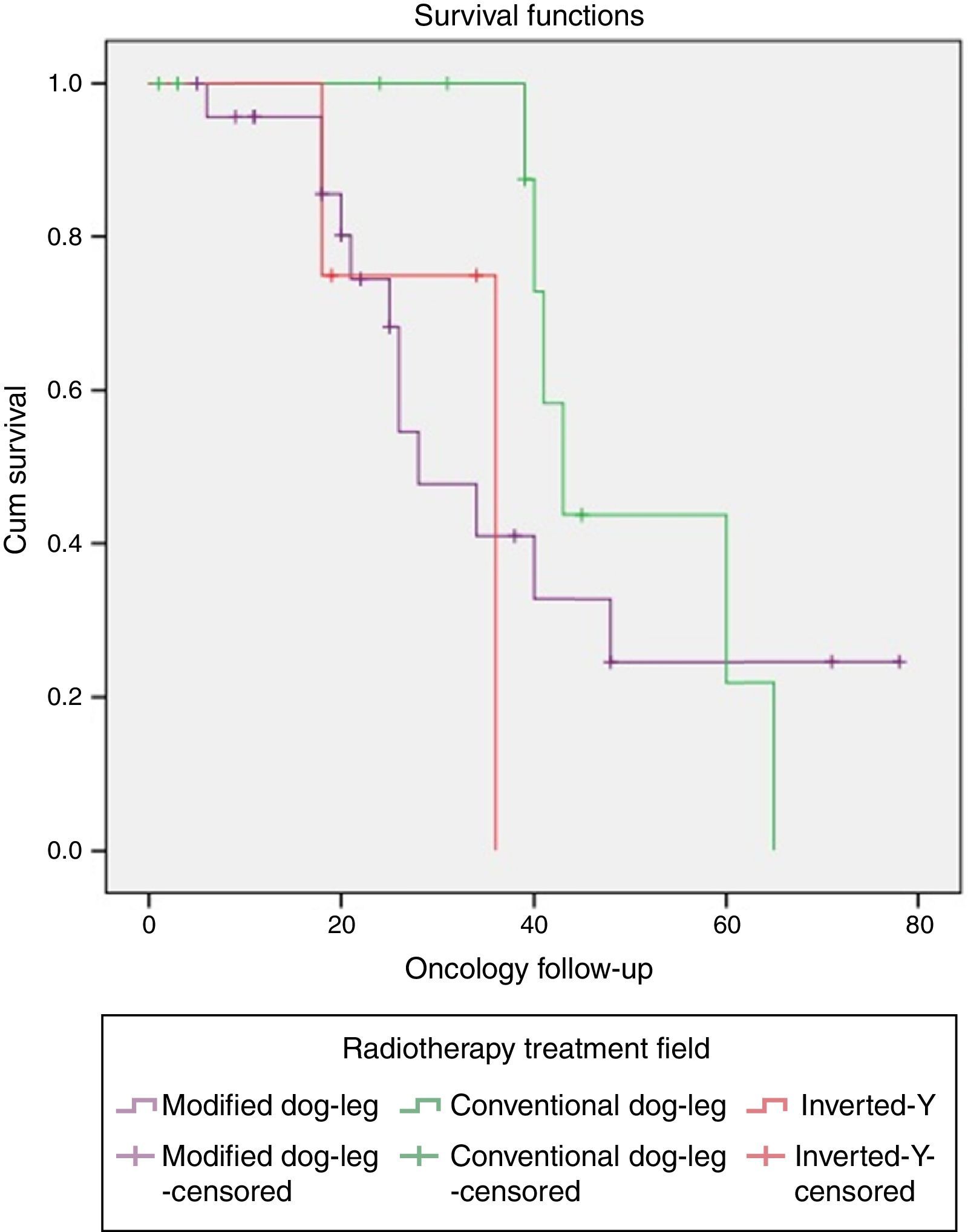
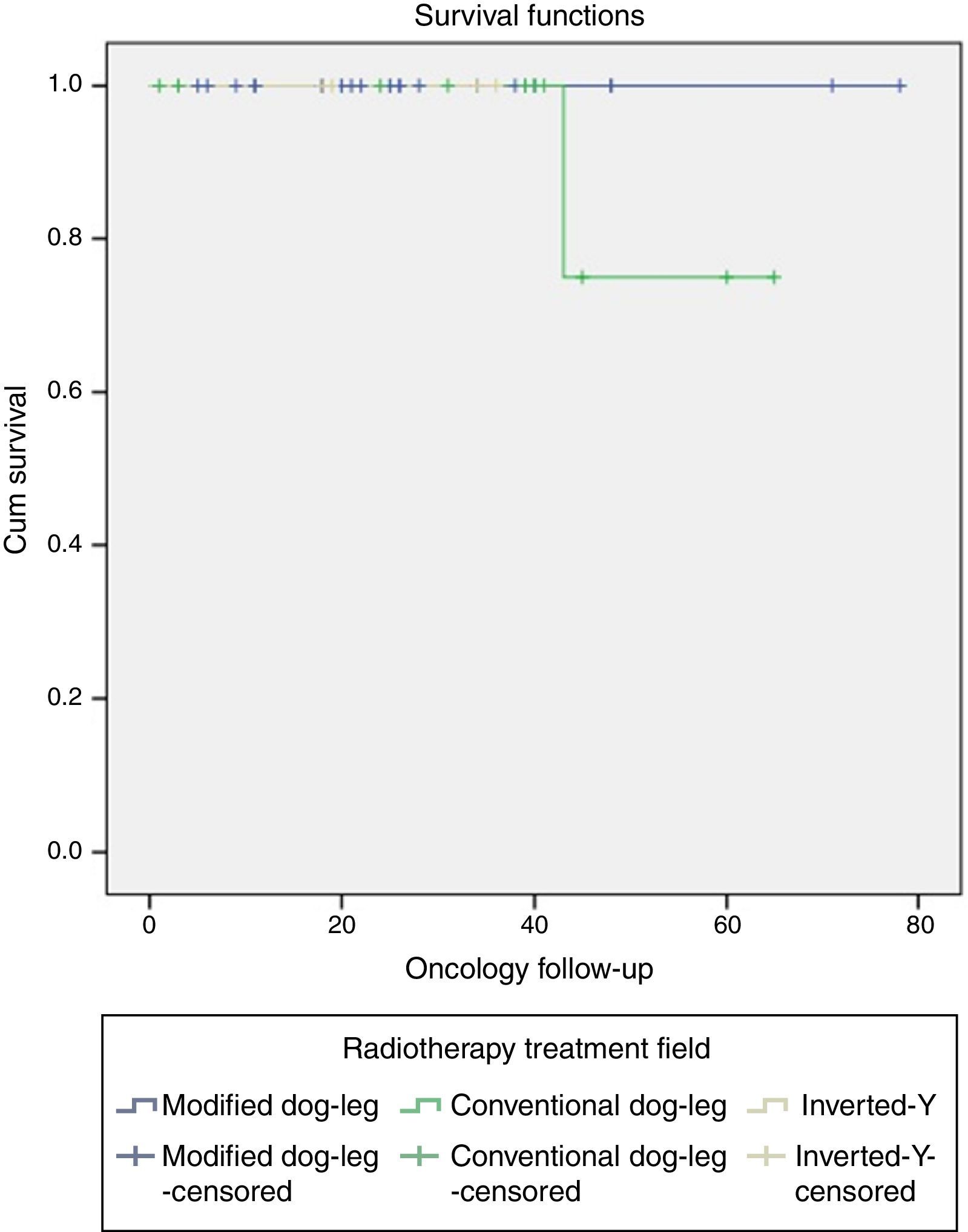
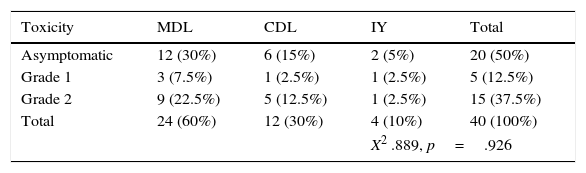
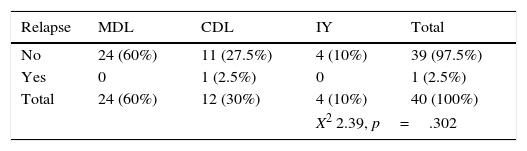
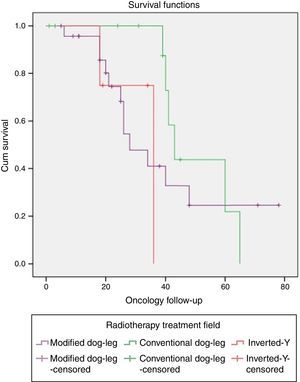
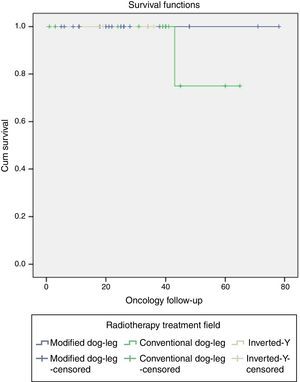
One patient with classical seminoma relapsed (2.5%) at the supraclavicular lymph node (outside the treatment field) 6 months after the end of treatment with RTx. The only unfavourable factor the patient presented was scrotal violation, treated with the CDL technique in the linear accelerator. No relapses were reported with the IY or MDL techniques (Figure 2 and Tables 3a and 3b).
Association of acute and chronic gastrointestinal toxicity with the treatment field.
| Toxicity | MDL | CDL | IY | Total |
|---|---|---|---|---|
| Asymptomatic | 12 (30%) | 6 (15%) | 2 (5%) | 20 (50%) |
| Grade 1 | 3 (7.5%) | 1 (2.5%) | 1 (2.5%) | 5 (12.5%) |
| Grade 2 | 9 (22.5%) | 5 (12.5%) | 1 (2.5%) | 15 (37.5%) |
| Total | 24 (60%) | 12 (30%) | 4 (10%) | 40 (100%) |
| X2 .889, p=.926 | ||||
The RTx dose in 35 patients (87%) was 25.2Gy in 14 fractions. Two patients (2%) were treated with 27Gy in 15 fractions, one each with the MDL and CDL technique, 2 patients (2%) were treated with 30Gy in 15 fractions, one with the IY technique and the other with MDL; 1 patient (2.5%) was treated with 30.6 GY in 17 fractions with the MDL technique.
Relapse associated with vascular invasion resulted in a X2 0.342, p=.559, associated with lymph node invasion X2 0.083, p=.773, and associated with rete testis invasion X2 0.83, p=.773 (Figure 3 and Table 4).
Testicular cancer has a low incidence, worldwide it is estimated to account for 1.5% of all tumours.19 In Mexico it accounts for 2% of neoplasias,20 which agrees with what was documented in this study.
Peak incidence is between 15 and 35 years of age. In our study we found a mean of 33±8 years (ranging from 18 to 57 years), which coincides with the world literature.
Seminoma falls within germinal testicular tumours, with an incidence of 40–60%. There are three histological subtypes: classical, anaplastic, and spermatocytic. This study included 40 patients diagnosed with stage I seminoma; 38 patients (95%) with the classical histological subtype, 1 patient (2.5%) with the anaplastic histological subtype, and the last patient (2.5%) at 33 years of age with the spermatocytic subtype. The affected testicle was predominantly the right (57%) vs. the left (43%).
It has been reported that the most significant risk factors is cryptorchidism associated with 2% of cases.20 In our cohort we found 2% of the sample was associated with this event.
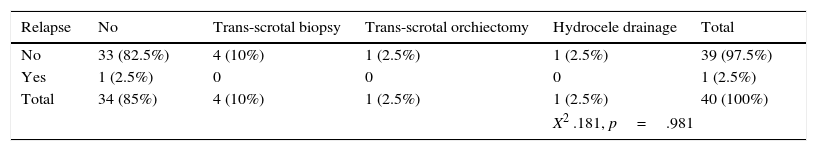
Furthermore, another significant factor is scrotal violation, defined as any trans-scrotal procedure such as open testicular biopsy, trans-scrotal orchiectomy, or fine needle aspiration biopsy in a patient diagnosed with testicular cancer. These patients have an additional 3% chance of local relapse (p=0.001),21 with no statistically significant impact on OS or distant metastasis. In another study, which recruited patients with non-seminoma histology, it was found that a history of scrotal violation is not an adverse prognostic factor, unless the surgical margin is positive, increasing the risk of local relapse.22 In our study 6 patients (15%) had this history, however none relapsed. This section covers the importance of planning RTx treatment, since for patients with a history of scrotal violation with stage I testicular cancer it is recommended in our Hospital that the treatment field include the para-aortic and iliac lymph nodes (CDL technique).
A tumour >4cm and rete testis invasion are considered risk factors for relapse; if both factors are present the probability of relapse is estimated to be 31%, if only one factor is present, the risk is 16%, if no adverse factors are present the probability is 12%.9,23 Likewise, if lymphovascular invasion is present, there is a 50% probability of relapse vs. a 15% probability without this risk factor.24
Nevertheless, these factors have not been validated in prospective studies or assessed in multivariate analyses,9 therefore it is currently controversial to consider them as adverse factors.
Of the patients evaluated in this study, 35 (87%) presented a tumour >4cm, and only one of them (3%) relapsed within 6 months of ending RTx treatment; lymphovascular invasion was documented in 13 patients (32%), and scrotal violation was documented in 6 patients (15%), neither being associated with relapse.
The correlation results between relapse and these prognostic factors are not statistically significant, although in our Hospital adjuvant therapy with CTx or RTx is considered advisable.
Surveillance is an option that allows unnecessary or additional treatment to be avoided, decreasing the possibility of secondary RTx- or CTx-associated neoplasms. Adjuvant treatment is preferred given the relapse scenario,25 with which DFS can be kept at 100% (95% confidence interval (CI), 99.24–99.93).9 It is considered that up to 80% of relapse cases are in the first 12 months, and the most common site is retroperitoneal with 70–90%.26
The indication for adjuvant treatment is based on the presence of risk factors, lymph node involvement, and personalised assessment that should take into account adherence to surveillance or the predicted treatment, as well as the protocols conducted in the benchmark medical centre.26
In our hospital, surveillance is considered in the context of the patient, adverse pathological factors, and lymph node involvement. Since there is a high incidence of patients lost during follow-up, which does not enable relapses or salvage therapy to be conveniently determined, it is recommended that the decision for surveillance be made on an individual basis.
Adjuvant treatment has an impact on disease-free survival (DFS). Nevertheless, no statistically significant benefit has been demonstrated for better OS or mortality.
Therefore they are considered therapeutic options, taking into account the morbidity they cause, percentage of relapses,27 and the probability of causing secondary neoplasias.
In a non-inferiority study comparing single-dose carboplatin (CB) versus RTx, it was documented that 5-year DFS with CB is 95% versus RTx 96%; a reduction in the probability of contralateral germinal testicular cancer was also proven, with better results for CB (2 patients affected) vs. RTx (15 patients affected) (p=.03).28
Two cycles of CB reduce the risk of relapse to a probability between 0 and 3%.22 Like in surveillance, the main site of relapse with CTx is retroperitoneal (interaortocaval, pre-aortic, para-aortic lymph nodes).29,30 Five patients were eliminated from our database since they preferred this adjuvant treatment strategy.
As for treatment with RTx, it has been modified over time leading to decreased acute cardiovascular, GI, dermatological, haematological, and reproductive toxicity. This was obtained by reducing the size of the field of irradiation and the dose.
Twenty years ago the treatment field was extensive, including the mediastinum, retroperitoneal space, and pelvic lymph nodes. Currently the only fields involving lymph nodes below the diaphragm use techniques such as the para-aortic (PA) field, in which the upper limit is T11/T12 and the lower border is L5/S1; Hockey stick, also called dog-leg or CDL, which includes the ipsilateral para-aortic and iliac lymph nodes with the same upper limit as for the PA field and using the foramen obturatum as the lower border;31 the IY technique includes the bilateral para-aortic and iliac lymph nodes; and the MDL technique in which the lower limit is the upper border or acetabulum ceiling.
Similarly the doses have been gradually reduced. Initially doses between 40 and 50Gy were considered, currently the recommended doses are between 20 and 25Gy.9,13,14,32 With this dose the probability of relapse is 1–3% and the main site is not retroperitoneal since the lymph nodes in this region are included in the field of irradiation with the different RTx techniques, which improves DFS.29,30
As for the treatment equipment, the literature backs the use of a linear accelerator as a better therapeutic option versus the cobalt-60 unit.
In our study, the treatment outcomes with three types of techniques (IY, MDL, CDL) were assessed, and it was found that no patient relapsed within the treatment field (para-aortic and pelvic lymph nodes); however, the relapse occurred in the supraclavicular and mediastinal region in one patient who 6 months prior was treated with the CDL technique using a linear accelerator at a dose of 25.2Gy. Given the early relapse, this patient received salvage CTx with 4 cycles of bleomycin, etoposide, and cisplatin (BEP). The patient had a complete response and has been disease free for 2 years 7 months.
The objective of the study by Hayoon Lee et al. was to assess OS, relapse-free survival (RFS), and acute toxicity in patients who received adjuvant RTx. The doses used were 25.5Gy, 25.05Gy, and 25.2Gy. It was documented in this study that the 5- and 10-year RFS was 97% and the 5-year OS was 100%.33
The international recommendations for virtual simulation are: patient in supine position, use the butterfly board and it is suggested to place a cushion under the patient's knees.31 In our study's patient sample, these recommendations were used for the cases treated with the 3D technique. For the patients treated with the 2D technique, they were not placed in the butterfly board.
Different studies have compared the results of irradiation in the PA field versus the CDL technique. In one of them, the dose given was 30Gy in 15 fractions for both treatment groups. The 3-year RFS was 96% for patients with the PA field and 97% for CDL; 3-year OS was 99% for PA field and 100% for CDL; for CDL grade 2 and 3 acute GI toxicity was reported in 51% of cases, with no patients with delayed toxicity.34
The use of techniques with a reduced field (PA field) offer advantages in terms of acute toxicity without negatively changing DFS. It also decreases the dose to at-risk organs such as the penis and contralateral testicle.4
Based on the above, we are currently recruiting the patient sample in our hospital intended for the PA field technique, who will receive between 20 and 25.2Gy of irradiation.
One study compared the treatment dose of 20Gy in 10 fractions versus 30Gy in 15 fractions. By comparing quality of life 1 month after having received the treatment, it was found that the patients who received 30Gy reported moderate-severe lethargy (20% versus 5%), and inability to perform their normal work (46% versus 28%); after 3 months the results were the same for both groups. The 2-year relapse rate was assessed with a difference of 0.7%.35
Another study compared doses of 30Gy versus 20Gy in relation to acute GI toxicity; 15% of the patients had grade 2 toxicity with the 30Gy dose versus 10% with those given 20Gy.35
In our study, the dose used was 25.2Gy in most of the patients (87%). With this dose there was no lethargy or decreased performance status, therefore we consider it to be an appropriate dose.
With the CDL technique it has been reported that grade 1 GI toxicity occurs in 46% of cases, grade 2 in 12%, grade 3 in 16%, and grade 4 only in 2% of cases. As for haematological toxicity, 29% of cases are associated with grade 1 toxicity, and only two cases of dermatological toxicity occurred.32
Hayoon Lee et al. documented that the CDL technique causes GI toxicity in 69% of cases (p=0.35)33 and that the 25.2Gy dose in 14 fractions was associated with acute GI toxic effects in 100% (p=0.001).33 Studies are currently being conducted to find the appropriate dose to decrease adverse effects with no negative impact on OS or DFS.
In our cohort, 20 patients (50%) remained asymptomatic regardless of the RTx technique used; the most commonly associated GI toxicity was grade 2, with 15 patients affected (38%), only 5 patients (12%) presented grade 1 toxicity. No patient showed signs of grade 3 or grade 4 toxicity.
The RTx technique associated with the most toxicity was MDL. However, it must be taken into account that most of the sample was concentrated in this study subgroup, and that in addition to having the highest percentage of GI toxicity (30%), it also has the highest percentage of asymptomatic patients (30%) compared to the other two RTx techniques.
Intensity-modulated radiotherapy (IMRT) is a sophisticated treatment technique for which the planning in patients with stage I seminoma and the CDL technique uses 7 treatment fields. This manages to reduce to dose to the bone marrow, small intestine, stomach, pancreas, and liver.31
In terms of RTx, the contralateral testicle is considered an at-risk organ when irradiating the pelvic region; generally the dose received with RTx treatment ranges between 30 and 180cGy. The risk of azoospermia with the CDL technique is estimated to be 30%.5 In order to decrease this effect, cerrobend blocks have been used, with a recommended thickness of 2cm. The RTx dispersion dose is greater in patients treated with cobalt.36
In a study in which the patients were treated with the CDL technique, it was demonstrated that the cerrobend block for the contralateral testicle reduced the dose of irradiation to 1.48cGy vs. no protection 3.89cGy (p=<0.001),37 therefore its use is justified, especially in patients wishing to preserve fertility.
The hormones that need to be evaluated in patients wishing to preserve fertility are follicle-stimulating hormone (FSH) and inhibin B. Likewise spermatobioscopy enables this condition to be more precisely evaluated. It is estimated that 44% of patients treated with CTx present oligospermia (<15 million per mL) and up to 15% azoospermia.38 In the two studies from our hospital on seminoma treated with RTx, these measurements were not performed since all the patients had these results.
Early recurrence is defined as occurring between 0 and 24 months, later recurrence after 2 years, and very late recurrent after 5 years. In seminoma patients the risk of early recurrence is 14%, late recurrence 5%, and very late recurrence 1%. OS for early recurrence is 90% and late recurrence 65%.39
Secondary neoplasias in testicular cancer patients occur in 1.5% of cases after orchiectomy, 4% in patients treated with RTx, 5% in those treated with CTx, and 4.4% with sequential CTx-RTx.40 These tumours have been reported 35 years after RTx treatment was given. However, with the decreased dose and treatment field, the incidence has fallen.
The relative risk of developing a secondary neoplasia in patients treated with RTx as the only treatment is 2 (95% CI 1.9–2.2), the relative risk for those who only received CTx is 1.8 (95% CI 1.3–2.5), and the relative risk increases to 2.9 for whose receiving both treatments (95% CI 1.9–4.2).41,42 The presentation site of the secondary neoplasias is gastrointestinal, gallbladder, thyroid, pancreas, bladder, kidney (relative risk 3.6), prostate, soft tissue sarcomas, and non-melanoma skin cancer (relative risk 1.77).42 Leukaemia has an incidence of 2.3% in patients with seminoma, compared to those with a non-seminoma histology where the percentage is higher (7%).43
Protons offer a suitable dosage distribution, with a decreased dose to at-risk organs, however more prospective, long-term studies are needed to confirm this.44
Today the RTx treatment field and the dose have been reduced, thus decreasing the incidence of radiation-induced secondary neoplasias. During the described follow-up in our patient cohort there were no cases of this event, but we will continue to monitor for it.
The main limitation of this study was the sample size, combined with its heterogeneity regarding the irradiation technique used. These three irradiation techniques had not previously been compared in any study since most only compare the CDL technique vs. the para-aortic field with more favourable results for the latter in terms of decreased gastrointestinal, dermatological, and haematological toxicity and excellent DFS.
ConclusionsRadiotherapy conditions continue to be a treatment option in early stage seminoma patients, with a low probability of relapse and acceptable gastrointestinal toxicity. Results are currently pending from treatment with the para-aortic field technique.
There is no difference in terms of gastrointestinal toxicity or relapse associated with the different irradiation techniques in patients with Stage I seminoma, therefore the modified dog-leg technique is recommended as the field of irradiation is smaller and there is no negative impact on relapse.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis article has received no funding.
Conflict of interestThe authors have no conflict of interest to declare.