Liver transplantation is the gold standard treatment for end stage liver disease, including patients with cirrhosis and hepatocarcinoma falling within Milan criteria. HCC is the sixth most common cancer around the world, and leading cause of death among cirrhotic patients. Diagnosis is based upon radiological characteristics and rarely biopsy results; the Barcelona Clinic Liver Cancer staging system is the most used guideline for treatment. With several treatment options available transplantation and resection continue to be the major curative therapeutic option for this patients. However treatment must be individualized to each patient to improve recurrences and outcomes. The aim of this paper is to review the present role of liver transplantation in the management of hepatocarcinoma.
El trasplante hepático es el estándar de oro en el tratamiento de enfermedad hepática avanzada, incluyendo pacientes cirróticos que han desarrollado hepatocarcinoma pero que se encuentran dentro de los criterios de Milán. El hepatocarcinoma es el sexto tumor más común alrededor del mundo y es la principal causa de muerte en pacientes cirróticos. El diagnóstico se basa principalmente en las características radiológicas del tumor y raras veces en resultados de patología. El sistema de estatificación desarrollado por el Clinic de Barcelona es la guía más usada para el tratamiento. Existen diferentes opciones terapéuticas para el hepatocarcinoma; sin embargo, el trasplante y la resección quirúrgica siguen siendo la opción curativa con mejores resultados. El tratamiento debe de ser individualizado para cada paciente con el fin de mejorar los resultados y minimizar recurrencias. El objetivo de este artículo es revisar el rol actual del trasplante hepático en el manejo del hepatocarcinoma.
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most frequent cause of cancer-related death.1 It's incidence is rising in western countries, usually occurs in the setting of chronic liver disease and cirrhosis. With more tight surveillance programs the diagnosis of this type of cancer has been earlier in the natural history of this disease, which means increased curative treatments and better survival rates.
There are several staging and treatment modalities for HCC although the mainstay of treatment is surgical resection; the majority of patients are not eligible because of tumor extent or underlying liver function. Underlying liver disease may limit any therapy; Nowadays hepato-pancreato-biliary surgery and liver transplantation programs choose de Barcelona Clinic Liver Cancer (BCLC) staging system to guide treatment decisions. Some selected group of patients may benefit from liver transplantation (LT).
The aim of this paper is to review the present role of liver transplantation in the management of HCC.
EtiologyChronic infections of hepatitis B virus (HVB) and hepatitis C virus (HVC) are responsible for nearly 78% of cases of HCC worldwide.2 However there are other potential risk factors for HCC such as alcoholic cirrhosis with an annual risk of 1%.3 Obesity and diabetes are also risk factor for HCC in patients with non-alcoholic steatohepatitis (NASH) and fatty liver disease (FLD); 20% of patients with NASH progress to liver fibrosis and ultimately in 3% cirrhosis.4
Other risks factors for HCC include: hemochromatosis, biliary disease such as primary cholangitis and primary biliary cholangitis, liver adenomas (risk for malignant transformation is 10%).5 Some toxins such as aflatoxin produced by Aspergilus flavus can be associated to HCC; finally tobacco use is an independent risk factor of HBV or alcohol abuse to develop HCC.6
Diagnosis, staging systems and therapeutic optionsDecades ago the diagnosis of HCC was made in patients with advanced stage disease, treatment was difficult and median survival rates were less than 3 months.7
In those days, morbidity associated therapy was high. In recent years with new technologies and surveillance strategies the diagnosis of HCC is done earlier and therefore we can offer curative treatments with 5-year survival rates from 50 to 75%.8
The diagnosis of HCC is based on imaging techniques and/or biopsy; the characteristic image on a single dynamic contrast-enhanced CT technique shows intense arterial uptake followed by “washout” of contrast in the venous-delayed phases makes the diagnosis.9 As the patient undergo for initial evaluation with contrast-enhanced TC, this also serves to rule out extrahepatic spread or macrovascular involvement, that could contraindicate transplantation.
Regarding on biopsies, their interpretation and distinction between high-grade dysplastic nodules and HCC is challenging, experienced pathologists tend to confirm their diagnosis by staining for glypican 3, heat shock protein 70 and glutamine synthetase, the positivity of two of these stains confirms HCC.10
Since the diagnosis is made on the basis of cirrhotic patients the aim of surveillance is to improve outcomes and decrease mortality rates, the most used tests for screening are alfa-fetoprotein (AFP) and liver ultrasound (US) the sensitivity is 87% and 75% respectively when used alone. Both are confirmatory tests. The current recommendation of the American Association for the Study of Liver Disease (AASLD) is an interval of 6 months surveillance in patients with high risk using US.11
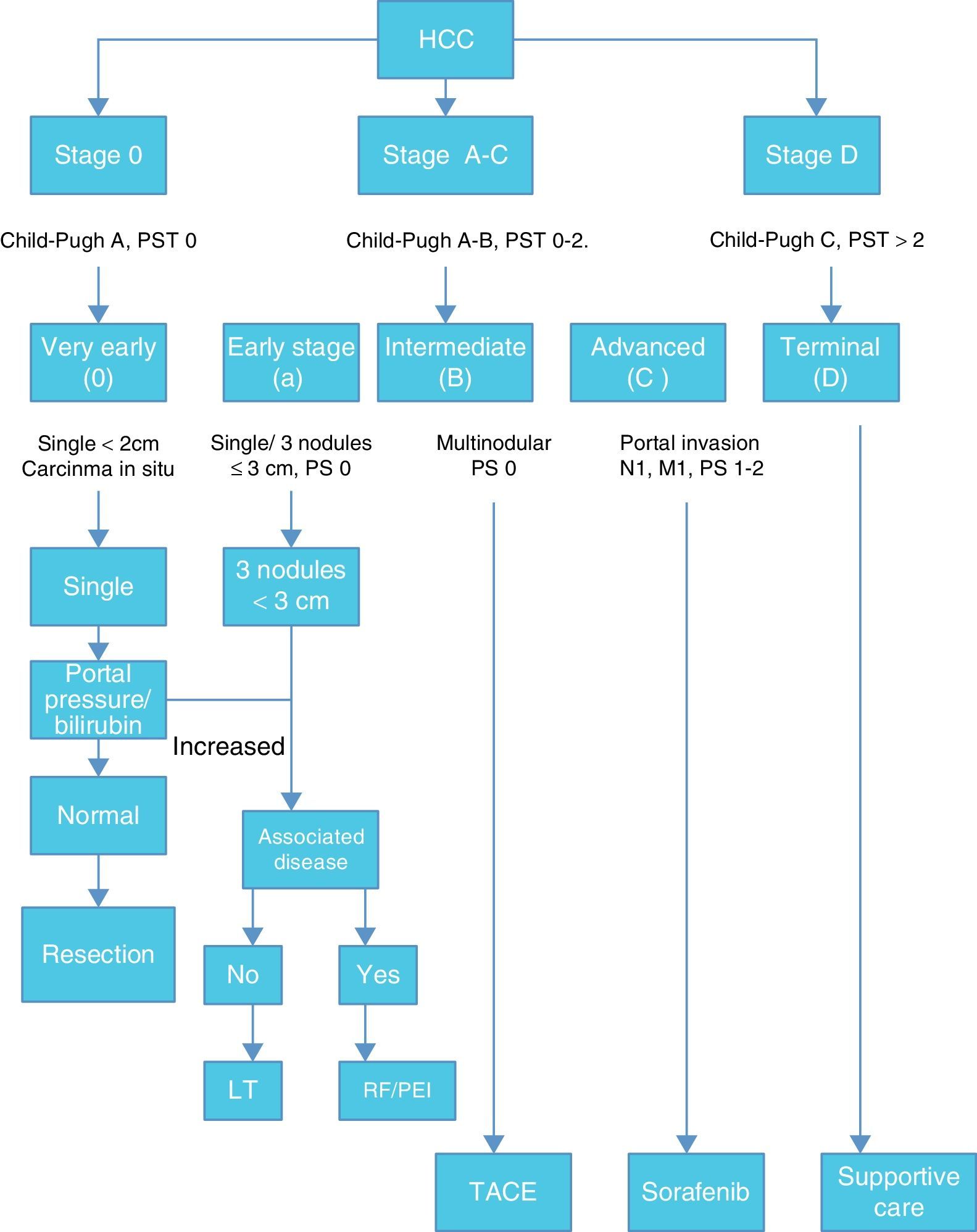
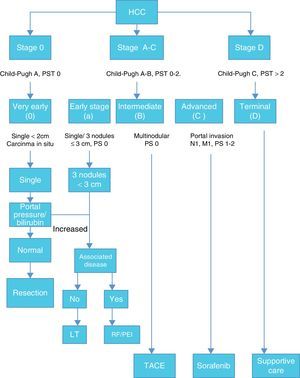
Once HCC is diagnosed the next step is staging. Among many staging systems developed worldwide the BCLC staging system is the most useful to guide treatment decision. (Fig. 1)
According with published results, this staging system allows an estimation of life expectancy. One of the most useful characteristics of this system is the identification of patients with early HCC potentially curable, those that may benefit from LT and those in terminal stage that could not benefit from any medical or surgical treatment.12,13
Therapeutic options for HCC includes: surgical resection, liver transplantation, radiofrequency ablation (RFA), microwave ablation, percutaneous ethanol or acetic acid ablation, trans arterial chemoembolization (TACE), radioembolization, cryoablation, radiation therapy and stereotactic radiotherapy, systemic chemotherapy and molecularly targeted therapies.
Surgical resection is the best curative treatment of HCC, offering a 5-year survival rate of 60–70%.14 However in this review we will focus on LT.
Liver transplantationLiver transplantation is an attractive option in patients with cirrhosis, end stage liver disease and HCC, because in the same procedure the tumor can removed meanwhile replacing the cirrhotic liver.
In the history of LT and HCC the initial experience with unresectable HCC was disappointing, elevated 90-days mortality, recurrence in nearly 80% of patients and long-term survival rates worst than patients transplanted for another indication made clinical and medical experts re-think this indication for LT. The use of LT for HCC evolved when as incidental findings of small HCC in explanted livers did not affect survival on patients who underwent LT for other condition when compared to patients without malignancy. In this subgroup of patients was the opportunity for LT. Mazzaferro et al.15 in 1996 showed that when LT is offered to a particular group of patients with single lesion <5cm, up to 3 different lesion separated but smaller than 3cm, without evidence of vascular invasion and localized disease, the 4 year survival rate could be up to 75% similar to those patients transplanted for other reason. These are now known as the Milan Criteria and have been accepted worldwide as guidance for LT on HCC. The 5-year and disease-free survival rates have been as high as 75% and 83%, respectively.
With this promising results other centers around the world started their own HCC – LT protocols, such as the University of California San Francisco (UCSF) that developed their own criteria: a single tumor 6.5cm or less or 3 or fewer, the largest of which is 4.5cm or less with a total diameter of 8cm or less. With a reported 1-year and 5-year survival rates were 90% and 75%, respectively.16
Years after, Mazzafferro et al. revisited the Milan criteria, incorporating 1556 patients to a new study where they developed the “Up to 7 criteria” this means up to 7 tumors in number the largest of which is less than 7cm with similar outcomes than the Milan Criteria.17
With the information obtained from studies in Italy and San Francisco, the researchers started using what they called “bridge therapy” consisting in down staging HCC using some other types of therapy such as RFA and TACE in patients with compensated liver disease. The results reported where similar in patients who underwent down staging than those who met Milan criteria.18
In 2010 the International Consensus Conference on liver transplantation for HCC concluded that bridging therapy is useful in patients that are likely to wait longer than 6 months on the transplantation list.19
It is important to mention that once the patient with HCC meets some criteria described previously then the patient could be evaluated and could complete the liver transplant protocol. Then the patient could be transplanted. Tumor biopsy is not mandatory.
Organ allocation for patients in the liver transplant waiting list, assignation is based upon the Model for End stage Liver Disease (MELD) a statistical model based on predicted survival in patients with cirrhosis. MELD criteria do not predict the risk of death among patients with chronic liver disease associated with HCC.
This is the reason that patients with HCC meeting Milan or UCSF Criteria who are candidates for LT are assigned their calculated MELD with “exception points” for the fact of the HCC, according to the Criteria of the UNOS/OPTN after 2015, this exception score is of 6 points for the first 3 months and the first 3 month extension, for the second 3 months extension patients will receive a score of 28 points, and additional points for longer waiting times with a maximum of 34 points.20
Even though the adoption of this policies patients with HCC longer waiting time results in tumor growing which is one of the main causes of drop-out the waiting list.20,21 There has been some identified factors to drop-out including: multinodular tumors, failed neoadyuvant therapy, baseline AFP >200ng/mL, or increase of >15ng/mL month.21
As every other LT, inmmunossuppression is mandatory in order to reduce the risk of graft rejection but it is associated with a higher risk of tumor regrowth. The very first strategy design in these cases is reducing doses to the minimum but effective levels. Immunosuppression is divided into two different groups according to the time they are used: induction therapy consisting in one or two doses of basiliximab, monoclonal antibody Anti-CD-25, in this scenario; Toso et al.22 suggested that the use of basiliximab with sirolimus toward improved survival; however some other studies suggests that basiliximab induction therapy should be used with caution for the high risk of early HCC recurrence in high risk patients.23
The other group of drugs used for induction are steroids: methylprednisolone initially given intravenously for the first 6 days, then changed to prednisolone, progressively tapered to discontinuation by 3–6 months after LT.
For maintenance immunosuppression the most used scheme is based on the combination of steroids, calcineurin inhibitors (CNI) and mycophenolate mofetil (MMF).
CNI (tacrolimus and cyclosporine) both nephrotoxic, can induce diabetes mellitus or hypertension, serum levels need to be monitored frequently. Lee and collegues23 demonstrated in a study published in 2014 that average tacrolimus exposure is not an independent risk factor of HCC recurrence, which is different from what Vivarelli and colleagues24 described in 2008, they concluded that as in patients managed with cyclosporine, overexposure to tacrolimus increases the risk of HCC recurrence after LT. Therefore a careful management and close monitoring of CNI is recommended.
Sirolumus, mammalian target of rapamycin (mTor) is very attractive drug since it has antitumor and antiproliferative properties, as mentioned above the use of sirolimus-based immunosuppression can lead to improved survival, however in a recent prospective-randomized, open-label international trial by Geissler and colleagues concluded that the use of sirolimus in LT recipients with HCC does not improve long-term recurrence-free survival beyond 5 years. The benefit is evident in the first 3–5 years, especially in low risk patients.25 Everolimus, a semisynthetic form of sirolimus, shows significantly lower HCC recurrence rates compared with those on sirolimus or CNI.26
Recurrence and outcomesAfter LT for HCC patients are at risk of recurrence, 10–15% in patients transplanted within Milan criteria, because of extrahepatic disease occurred, the majority of patients recurrences occur in the first 2 years before LT. Some factors have been identified as risk factor for recurrence such as: bilobar disease, vascular invasion, portal thrombus, tumor grade, tumor size.27
Optimal post-treatment surveillance is needed; the international suggestions consist on imaging ever 3–6 months for 2 years, then annually. AFP every 3 months during the first 2 years, then every 6 months.27,28
Recently Chaiterrakij et al.29 at Mayo Clinic suggested that pretransplant SFP, Lens culinaris agglutinin-reactive alpha-fetoprotein (AFP-L3) and des-gamma-carboxyprothrombin DCP are useful for predicting the risk of HCC after transplantation in association with Milan criteria.
Post LT 5-year survival in patients transplanted in Milan criteria range from 70 to 75%, in patients with VHC associated infection survival range 60–65%.30
Some studies suggest resection for recurrent disease post-LT achieving 5-year survival near 66%, some others propose TACE with same results than those in primary HCC.31
ConclusionsLiver transplantation is an option for a selected group of patients with cirrhosis and hepatocarcinoma meeting Milan criteria. Imaging characteristics of the tumor is enough to make the diagnosis; prelisting biopsy is not mandatory. In some cases consider of “bridging” therapy is an option while waiting transplantation.
Patients with HCC tend to be beneficed from the actual organ allocation.
Once transplantation is done, immunosuppression to reduce risk of rejection often is associated recurrence. Post LT 5-year survival in this group of patients ranges from 70 to 75%. Surveillance after LT with imaging and AFP every 3–6 months is preferred.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe author declares no funding has been received for this paper.
Conflict of interestThe author declares there are no conflicts of interest.